Abstract
Hysterectomy has been associated with metabolic change and cardiovascular risk for women after removing the uterus, but inconclusive. This large retrospective cohort study evaluated the hyperlipidemia risk for women with a hysterectomy and/or oophorectomy. From claims data of one million people in the National Health Insurance (NHI) database of Taiwan, we established a cohort consisting of 5887 women newly received a surgery of hysterectomy from 2000–2013, 563 women had a hysterectomy and a oophorectomy, and 556 women had a oophorectomy. From the claims data, 28024 women without any of the surgeries were identified to form the comparison cohort, frequency matched by birth year and surgery year of the women with hysterectomy. By the end of 2013, the incidence of hyperlipidemia was 1.3 times greater in women with a hysterectomy than in comparison women (3.43 vs. 2.65 per 100 person-years), with an adjusted hazard ratio (aHR) of 1.27 (95% CI = 1.19–1.35) for hysterectomy women after controlling for age, oophorectomy, hormone therapy and comorbidities. The incidence of hyperlipidemia increased to 4.93 per 100 person-years in women with both a hysterectomy and an oophorectomy. The relative risk of hyperlipidemia was higher for young women than the elderly women with the surgery. Women with comorbidity of obesity, hypertension or diabetes had a higher incidence of hyperlipidemia. In conclusion, the risk of developing hyperlipidemia could be elevated for women who had a hysterectomy and/or an oophorectomy. Women with hysterectomy should routinely monitor their metabolic status, particularly for young women and those with comorbidity of metabolic symptoms.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is a leading cause of death in women, and serum cholesterol is one of the well-known risk factors of CVD1. Hyperlipidemia and hypertension increase with age and are more prevalent in women after middle age. Hyperlipidemia refers to an increased level of plasma lipids, including triglycerides, cholesterol, cholesterol esters, phospholipids, and plasma lipoproteins2. In addition to genetic caused hyperlipidemia, disorders such as diabetes, alcohol consumption, and some medications may lead to metabolic disorders and the development of acquired hyperlipidemia3,4. The National Cholesterol Education Panel’s Adult Treatment Program III released in 2001 set the standard lipid levels, commonly used guideline for clinicians3.
Hysterectomy is a surgery to remove the uterus, which is one of common gynecologic surgeries for women in both developed and developing areas5,6. Hysterectomy is generally recommended for benign clinical indications, such as uterine leiomyoma, abnormal uterine bleeding and endometriosis, in addition to for reproductive cancers7. It has been a concern for the potential of leading to hormone changes after hysterectomy and increasing the risk of CVD and other comorbidities8,9,10. The Norwegian health study found women had an adjusted hazard ratio (aHR) of 1.92 (95% CI: 1.51–2.38) to develop CVD after a hysterectomy10.
The effect of hysterectomy on the metabolic status, such as lipid profile, has not been well investigated. We assumed that changes in the hormone level post hysterectomy might change the serum lipid profile. We, therefore, investigated the risk of developing hyperlipidemia for women with hysterectomy, using the Taiwan National Health Insurance (TNHI) Database of one million randomly sampling cohort.
Materials and Methods
Data source
The TNHI program covers more than 99% of the entire 23.4 million population of Taiwan. Data of registration files and medical claims for all beneficiaries can be linked through encrypted identification numbers in The National Health Insurance Research Database (NHIRD) (details available at: http://nhird.nhri.org.tw/en/index.htm). A sub-dataset of NHIRD containing 1 million people randomly selected from all beneficiaries was used in this study.
Diseases in the claims data were coded with the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The insurance system required the disease diagnosis with valid supporting clinical findings to prevent a medical fraud. This study was approved by the Research Ethics Committee, China Medical University and Hospital (IRB permits number: CMUH-104-REC2-115). Our research was performed in accordance with relevant guidelines/regulations.
Study subjects
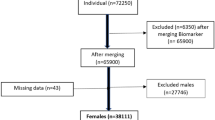
We established a hysterectomy related case group, including women who had received only a hysterectomy, both a hysterectomy and an oophorectomy, and only an oophorectomy from 2000 to 2013. Cases were defined by the procedure codes of 65.5, 65.6, 68.3–5, 68.97 and diagnoses codes of uterine myoma (ICD9 code: 218.9), adenomyosis (ICD9 code: 617.0), uterine prolapse (ICD9 code: 618), and ovarian tumor ICD9 code: 220. Women who had been diagnosed with hyperlipidemia (ICD9 code: 272) and/or cancer at baseline, or with cancer within 1 year after hysterectomy were excluded (Fig. 1). Similar exclusion criteria were applied for establishing the comparison group, which was randomly selected from women without the history of hysterectomy and oophorectomy, with a sample size 4-fold of the case group, frequency matched by the year of birth and the year of surgery. The validity of hysterectomy and hyperlipidemia in the claims data of NHIRD has been reported in previous reports5,6,7.
Women in both the hysterectomy case group and the comparison group were followed up until hyperlipidemia identified, death, withdrawal from the NHI program, or the end of 201311.
Baseline comorbidities, including obesity (ICD9 codes: 278, 783.1, V77.8), diabetes (ICD9 code: 250), coronary arterial disease (CAD) (ICD9 code: 410–414), hypothyroidism (ICD9 code: 244), and hypertension (ICD9 codes: 401–405), were identified as related factors. We also included hormone therapy (ATC code: G03C, estrogens) before or after the baseline into account as a potential confounder. Women who received hormone therapy more than 30 days were considered as hormone therapy users.
Statistical analysis
We applied Chi-square test to determine the differences of baseline characteristics for categorical variables and used Wilcoxon rank sum test to examined continuous variables between hysterectomy and comparison groups. Incidence of hyperlipidemia was estimated for both groups by the end of 2013. We used the Kaplan-Meier method to measure fractions free of hyperlipidemia during the follow-up period in two cohorts by the type of surgery, and used the Log-rank test to examine the difference. Incidence rates of hyperlipidemia, per 100 person-years, were calculated for women with only a hysterectomy, with both a hysterectomy and with only an oophorectomy, and for the comparison group, by age group (<45, 45–64 and ≥65 years), hormone therapy and comorbidities. Cox proportional hazards regression analysis was used to calculate each surgery group to the comparison group hazard ratio (HR) of hyperlipidemia and 95% confidence interval (CI). Adjusted hazard ratio (aHR) was estimated after controlling for covariates. All statistical analyses were performed using SAS software version 9.4 (SAS Institute INC., Carey, NC). A two-tailed p-value below 0.05 was considered as significant.
Results
Table 1 shows that near a half of study population were less than 45 years old with a mean age of 46.9 years. The baseline comorbidities were more prevalent in the hysterectomy case groups than in the comparison group. The prevalence rate of hormone therapy was also higher in the hysterectomy case groups than in comparisons.
By the end of follow-up, the Kaplan-Meier analysis showed that the overall incident hyperlipidemia was 7.5% greater in the hysterectomy group than in the comparison group (p < 0.0001) (Fig. 2). The incidence density rate of hyperlipidemia was near 1.3 times greater in the hysterectomy group than in the comparison group (3.43 vs. 2.65 per 100 person-years), with an adjusted HR of 1.27 (95% CI = 1.19–1.35) for the hysterectomy group after controlling for age, oophorectomy, hormone therapy and comorbidities (Table 2). The hyperlipidemia incidence increased further for women had both hysterectomy and oophorectomy. The overall incident hyperlipidemia in women with oophorectomy alone was approximately similar to women with only hysterectomy. Those who had both a hysterectomy and an oophorectomy, and underwent hormone therapy, had an incidence of 5.40 per 100 person-years. The age stratified analysis showed that incidence rates of hyperlipidemia were higher in women aged 45–64 years than in youngers and the elderly. However, the age-specific surgery group to comparison group HRs were the highest in the young women for the three types of surgery. Obesity, hypertension and diabetes at the baseline were also associated with the elevated incidence of hyperlipidemia after the surgeries.
Discussion
Our study population encompassed mainly middle-aged women with a mean age of 46 years, which indicates that hysterectomy is a surgery prevalent in women in the middle ages instead of in the elderly. After a median follow-up time of 6.0 years, approximately one fifth of women with the hysterectomy developed hyperlipidemia. The risk of hyperlipidemia initiates in young women and reaches a peak in their 45–64 years.
Several cohort studies have reported the relationship between hysterectomy and risk of developing hyperlipidemia8,12,13,14. An Iran study found increased levels of triglyceride, total cholesterol and low density cholesterol in 31 women six months after their hysterectomy and bilateral salpingo-oophorectomy14. By comparing women with natural and surgical menopause, hysterectomy women may experience increased low-density lipoprotein and reduced high density cholesterol12,13. These findings are all fragmental and can not make a conclusion.
The Women’s Health Initiative Observational Study, a very large cohort study of white, black, Hispanic, and American Indian women in the USA, has shown that the increased cardiovascular disease risk in women with a hysterectomy is likely associated with baseline comorbidities8. In our study, the baseline comorbidities were more prevalent in the hysterectomy cohort than in the comparison cohort. The baseline hypertension and diabetes are associated with a further increase in hyperlipidemia, near 2-fold greater than women without these comorbidity.
After following-up 3,302 pre-menopausal women for 11 years, the Study of Women’s Health across the Nation revealed that levels of high density lipoprotein cholesterol, low density lipoprotein cholesterol and apolipoprotein B were similar for those with natural menopause and hysterectomy women with or without ovarian conservation over time15. The sample size of women with a hysterectomy (n = 183) in the Study may not be enough to observe significant difference15. The CARDIA Study followed up 1,045 young women ages 15–30 for 25 years, after their hysterectomy or natural menopause, and found a small increase in high-density lipoprotein cholesterol from their baseline values16. On the other hand, our study showed an increased incident hyperlipidemia in women post hysterectomy. The population size and ethnic characteristics of this nationwide cohort study in Taiwan were different from previous studies. Race-specific factors may have an impact on the lipid and lipoprotein changes characteristic of menopause and hormonal statuses17. In addition, our follow-up study included women of younger ages, when the decline in ovarian hormones would be less prominent. The incidence of hyperlipidemia in women of <45 years old was near 1.6 folds greater in those with a hysterectomy than in comparisons. The relative impact of hysterectomy on the hyperlipidemia risk is stronger in young women than in the elderly women.
Our study showed that hysterectomy alone or oophorectomy alone was associated with approximately 1.3-fold greater risk of developing hyperlipidemia than comparison women. Women with both hysterectomy and oophorectomy had an incidence of hyperlipidemia increased to 4.93 per 100 person-years, near 1.9-fold greater than comparison women. Age, hysterectomy alone, and the effect of bilateral oophorectomy are factors to be considered for the CVD risks. Hysterectomy without oophorectomy has been shown to interfere ovarian blood flow, resulting in premature ovarian failure and hormone-related effects on the vascular bed18. Alternatively, a bilateral oophorectomy can result in an abrupt fall in circulating estrogens and testosterone levels to cause menopause immediately19 and accelerate the increase in lipids and lipoproteins levels20.
Hysterectomy and/or oophorectomy can cause a surgical menopause. Following the menopausal transition, the estradiol level declines and testosterone progressively dominates the hormonal level, affecting the lipid metabolism15. Studies have also reported that metabolic conditions lead to the possible occurrence of the indications for hysterectomy and surgical menopause15,16,21,22. Adverse lipid levels tend to develop during and after menopause23,24,25. The Study of Women’s Health Across the Nation showed changes of lipid peaked in the late phase of menopause25. Women who have hysterectomy tend to reach physiological menopause earlier than women who do not have the surgery26,27. The average menopause age in Taiwan is 49.5 years28. The oophorectomy and comorbidity status may affect hormone and other disorders. A recent US cohort study with 113679 hysterectomy women found a greater hazard of mortality in those who had bilateral ovarian removed29. In our study, the hormone therapy status was adjusted in the data analysis.
Strengths and Limitations of the Study
The novelty of this research lies in the population based assessment. This study composed of a large size of study population after a median follow-up period of 6 years containing all events of hysterectomy for benign diseases. This population based data could minimize selection bias. Our study also adjusts for some independent variables, such as diabetes and hypertension, known to influence the risk of CVD. Moreover, our data are representative for the general population and the comparison group is drawn from the same population by collected data from nationwide healthcare registers.
The potential limitation in the study is that hysterectomy women were more prevalent with comorbidities and thus were less healthy than general female population. In our baseline data, among 8942 women underwent hysterectomy, 1879 women (21.0%) had the history of hyperlipidemia and were excluded from the study. The baseline prevalence of hyperlipidemia was 1.27-fold greater in women with hysterectomy than in women without hysterectomy (16.6%) (data not shown). The baseline prevalence ratio was approximately similar to the hysterectomy cohort to the comparison cohort ratio of the newly developed incident hyperlipidemia (3.43 to 2.65 per 100 person-years, or 1.29). It is possible that hyperlipidemia appeared after the hysterectomy in this case would be a mere continuation of these underlying metabolic derangement rather than a result of the hysterectomy.
The other limitation was the potential misclassification in establishing study cohorts. We used hysterectomy procedure codes to identify the hysterectomy group. However, our further data analysis revealed that approximately 30% of women identified from the procedure codes lack of the diagnosis code. These women were thus mistakenly excluded from this study. Otherwise, this study should be highly reliable because the claims data have been peer reviewed by specialists. Women living in Taiwan are relatively homogenous, which may increase the internal validity and reliability to the findings. But, the ability to generalize our findings to other population is unknown. We were also unable to trace the surgical history before 1996.
Conclusion
We concluded that women with a hysterectomy are at an elevated risk of hyperlipidemia compared to women of the same age without the surgery. The risk is increased further for those who have both a hysterectomy and an oophorectomy. Hysterectomy is indicated for a variety of benign disorders, providing reproductive and perimenopausal women with a definitive surgical solution to gynecological disorders. Women with these surgeries should be aware of the potential risk of developing hyperlipidemia. Oophorectomy could play another important role and future research that focuses on the oophorectomy status should be conducted.
Availability of data and material
The data that support the findings of this study are disclosed in the paper. The raw data should be requested from the National Health Insurance Department.
References
Walsh, J. M. E. & Pignone, M. Drug treatment of hyperlipidemia in women. JAMA 291, 2243–2252 (2004).
Shattat, G. F. A Review Article on Hyperlipidemia: Types, Treatments and New Drug Targets. Biomed. Pharmacol. J. 7, 399–409 (2014).
Williams, L. & Wilkins Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 106, 3143–3143 (2002).
Nelson, R. H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care 40, 195–211 (2013).
Ding, D.-C., Chu, T.-Y. & Chang, Y.-H. Trend changes in the proportion of minimal invasive hysterectomies over a five-year period: A single-center experience. Tzu Chi Med. J. 24, 136–138 (2012).
Wu, M.-P. & Lee, C.-L. The trends of minimally invasive surgery for benign gynecologic lesions, 1997-2007 in Taiwan. Gynecology and Minimally Invasive Therapy 1, 3–8 (2012).
Lai, J. C.-Y. et al. Decreasing trend of hysterectomy in Taiwan: A population-based study, 1997–2010. Taiwan. J. Obstet. Gynecol. 54, 512–518 (2015).
Howard, B. V. et al. Risk of Cardiovascular Disease by Hysterectomy Status, With and Without Oophorectomy. Circulation 111, 1462–1470 (2005).
Lobo, R. A. Surgical menopause and cardiovascular risks. Menopause 14, 562–566 (2007).
Michelsen, T. M., Dørum, A., Cvancarova, M., Liavaag, A. H. & Dahl, A. A. Association between hysterectomy with ovarian preservation and cardiovascular disease in a Norwegian population-based sample. Gynecol. Obstet. Invest. 75, 61–67 (2013).
Huang, C.-I. et al. Hyperlipidemia and statins use for the risk of new-onset anxiety/depression in patients with head and neck cancer: A population-based study. PLoS One 12, e0174574 (2017).
Kabir, F., Jahan, N., Sultana, N. & Akter, R. Lipid Profile Status In Surgical Menopause. Journal of Bangladesh Society of Physiologist 6, 127–133 (2011).
Tuna, V. et al. Variations in blood lipid profile, thrombotic system, arterial elasticity and psychosexual parameters in the cases of surgical and natural menopause. Aust. N. Z. J. Obstet. Gynaecol. 50, 194–199 (2010).
Yazdani, S., Sharbatdaran, M., Abedi Samakoosh, M., Bouzari, Z. & Masoudi, Z. Glucose tolerance and lipid profile changes after surgical menopause. Caspian J Intern Med 5, 114–117 (2014).
Matthews, K. A., Gibson, C. J., El Khoudary, S. R. & Thurston, R. C. Changes in Cardiovascular Risk Factors by Hysterectomy Status With and Without Oophorectomy: Study of Women’s Health Across the Nation. J. Am. Coll. Cardiol. 62, 191–200 (2013).
Appiah, D. et al. Is Surgical Menopause Associated With Future Levels of Cardiovascular Risk Factor Independent of Antecedent Levels? The CARDIA Study. Am. J. Epidemiol. 182, 991–999 (2015).
Phan, B. A. P. & Toth, P. P. Dyslipidemia in women: etiology and management. Int. J. Womens Health 6, 185–194 (2014).
Siddle, N., Sarrel, P. & Whitehead, M. The effect of hysterectomy on the age at ovarian failure: identification of a subgroup of women with premature loss of ovarian function and literature review. Fertil. Steril. 47, 94–100 (1987).
Ingelsson, E., Lundholm, C., Johansson, A. L. V. & Altman, D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur. Heart J. 32, 745–750 (2011).
Yoshida, T., Takahashi, K., Yamatani, H., Takata, K. & Kurachi, H. Impact of surgical menopause on lipid and bone metabolism. Climacteric 14, 445–452 (2011).
Kok, H. S. et al. Heart disease risk determines menopausal age rather than the reverse. J. Am. Coll. Cardiol. 47, 1976–1983 (2006).
Boynton-Jarrett, R., Rich-Edwards, J., Malspeis, S., Missmer, S. A. & Wright, R. A prospective study of hypertension and risk of uterine leiomyomata. Am. J. Epidemiol. 161, 628–638 (2005).
Zhou, J.-L. et al. Serum lipid profile changes during the menopausal transition in Chinese women: a community-based cohort study. Menopause 17, 997–1003 (2010).
Saha, K. R. et al. Changes in lipid profile of postmenopausal women. Mymensingh Med. J. 22, 706–711 (2013).
Derby, C. A. et al. Lipid changes during the menopause transition in relation to age and weight: the Study of Women's Health Across the Nation. Am. J. Epidemiol. 169, 1352–1361 (2009).
Trabuco, E. C., Moorman, P. G., Algeciras-Schimnich, A., Weaver, A. L. & Cliby, W. A. Association of Ovary-Sparing Hysterectomy With Ovarian Reserve. Obstet. Gynecol. 127, 819–827 (2016).
Moorman, P. G. et al. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet. Gynecol. 118, 1271–1279 (2011).
Chen, S. C., Lo, T. C., Chang, J. H. & Kuo, H. W. Variations in aging, gender, menopause, and obesity and their effects on hypertension in taiwan. Int. J. Hypertens. 2014, 515297 (2014).
Mytton, J., Evison, F., Chilton, P. J. & Lilford, R. J. Removal of all ovarian tissue versus conserving ovarian tissue at time of hysterectomy in premenopausal patients with benign disease: study using routine data and data linkage. BMJ 356, j372 (2017).
Acknowledgements
This work was supported by grants from the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-123004), China Medical University Hospital; Academia Sinica Stroke Biosignature Project (BM10701010021); MOST Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005-); Tseng-Lien Lin Foundation, Taichung, Taiwan; and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Author information
Authors and Affiliations
Contributions
Pei-Chen Li: manuscript preparation; I.-Ju Tsai: data analysis, manuscript preparation; Chung Y. Hsu: study design; Jen-Hung Wang: study design; Shinn-Zong Lin: study design; Dah-Ching Ding: study concepts, design and manuscript preparation; Fung-Chang Sung: study design and manuscript preparation and revision. Ding and Sung have equal contributions.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, PC., Tsai, IJ., Hsu, C.Y. et al. Risk of Hyperlipidemia in Women with Hysterectomy-A Retrospective Cohort Study in Taiwan. Sci Rep 8, 12956 (2018). https://doi.org/10.1038/s41598-018-31347-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31347-z
This article is cited by
-
Association of hysterectomy with nonalcoholic fatty liver disease among US women
Lipids in Health and Disease (2024)
-
Prevalence and determinants of hysterectomy in India
Scientific Reports (2023)
-
The relationship between sufficient leisure time physical activity and happiness: An age stratification perspective
Current Psychology (2023)
-
Association of laparoscopy and laparotomy with adverse fetal outcomes: a retrospective population-based case–control study
Surgical Endoscopy (2021)
-
Increased risk of knee osteoarthritis in patients using oral N-acetylcysteine: a nationwide cohort study
BMC Musculoskeletal Disorders (2020)