Abstract
Phospholipase A2 (PLA2) is one of the representative toxic components of snake venom. PLA2s are categorized into several subgroups according to the amino acid at position 49, which comprises either Asp49, Lys49, Arg49 or Ser49. Previous studies suggested that the Lys49-PLA2 assembles into an extremely stable dimer. Although the behavior on Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing or non-reducing conditions suggested the presence of intermolecular disulfide bonds, these bonds were not observed in the crystal structure of Lys49-PLA2. The reason for this discrepancy between the crystal structure and SDS-PAGE of Lys49-PLA2 remains unknown. In this study, we analyzed a Lys49-PLA2 homologue from Protobothrops flavoviridis (PflLys49-PLA2 BPII), by biophysical analyses including X-ray crystallography, SDS-PAGE, native-mass spectrometry, and analytical ultracentrifugation. The results demonstrated that PflLys49-PLA2 BPII spontaneously oligomerized in the presence of SDS, which is one of the strongest protein denaturants.
Similar content being viewed by others
Introduction
Snakebite induces acute myonecrosis as well as other biological effects including hemolytic, neurotoxic, cardiotoxic, anticoagulant and antiplatelet activities of multiple complex protein assemblies. These protein complexes are composed of various multi-locus gene families that underwent accelerated evolution1. Phospholipase A2 (PLA2, EC 3.1.1.4) is one of the representative toxic components of snake venom. Snake venom PLA2s are classified into groups I and II based on disulfide bond patterns. Group II PLA2s are further categorized into several subgroups based on the amino acid at position 49. These subgroups contain Asp49, Lys49, Arg49 or Ser49, among which Asp49 and Lys49 are the most common. Although PLA2s share high sequence similarity, their phospholipase activity is distinct. Asp49-PLA2 hydrolyzes the sn-2 ester bond of the membrane phospholipids to generate fatty acids and lysophospholipids in a Ca2+ dependent manner. The Asp49 coordinating on the Ca2+ ion acts as a catalytic residue for hydrolysis of the ester bond2,3. In contrast, Lys49-PLA2 lacks phospholipase activity, or exhibits minimal phospholipase activity because the catalytic Asp49 is substituted for Lys4,5,6. Despite the absence of phospholipase activity, Lys49-PLA2 exhibits myonecrotic activity due to the intrinsic function of its C-terminal region that is abundant in positive and hydrophobic residues7,8,9 which mediate caspase-independent apoptosis10.
Previous studies showed that the Lys49-PLA2 forms an extremely stable dimer which is not dissociated to monomers even in the presence of 0.1% (w/v) sodium dodecyl sulfate (SDS) and 2 M urea11. Even though each monomer shares a similar structure12, X-ray crystallography studies4,5,7,9,13,14,15,16,17,18,19,20,21,22 revealed two distinct dimer types: the conventional dimer and the alternative dimer. These dimers differ in their myotoxicity activity: the conventional dimer comprises the inactive state and the alternative dimer comprises the active form12. In the conventional dimer, two protomers interact via their β-wings and N-terminal α-helices. Compared with the conventional dimer, the alternative dimer contains a larger contact surface, generating a compact dimer conformation. The alternative dimer formation was also supported by the results of small angle X-ray scattering (SAXS)21,23. The gyration radius (Rg) calculated from SAXS showed good agreement with the alternative dimer rather than the conventional dimer, suggesting that the alternative dimer has a more stable conformation in solution21.
Previous studies have shown that when Lys49-PLA2 is subjected to non-reducing SDS-polyacrylamide gel electrophoresis (PAGE), a band is generated at the position corresponding to the oligomer, and the band shifts to the position of monomer in the presence of dithiothreitol11. These observations suggest that Lys49-PLA2 contains intermolecular disulfide bonds. However, intermolecular disulfide bonds were not observed in any solved crystal structure of Lys49-PLA2. The reason for this discrepancy between the crystal structure and SDS-PAGE of Lys49-PLA2 remains unknown. Clarification of these inconsistent results may provide insight into the physiological function of this protein.
In this study, we comprehensively analyzed basic protein II (BPII), which is one of the Lys49-PLA2 homologues isolated from the venom of Protobothrops flavoviridis (PflLys49-PLA2 BPII)6,24,25. Our biophysical analyses included X-ray crystallography, SDS-PAGE, native-mass spectrometry (Native-MS), and analytical ultracentrifugation. The results demonstrated that PflLys49-PLA2 BPII spontaneously oligomerized in the presence of SDS.
Materials and Methods
Purification of PflLys49-PLA2 BPII
P. flavoviridis was collected at Amami Oshima Island, Kagoshima prefecture in Japan in accordance with Japanese guidelines and regulations under the law of humane treatment and management of animals as a dangerous animal. Collecting venoms was conducted after carbonic anesthesia or giving an electric shock for habu snake according to the experimental plan authorized by the Institute of Medical Science, the University of Tokyo. By this method, the pain of habu can be alleviated and accidental bite can be prevented. Snake venom was extracted from P. flavoviridis and was frozen quickly under liquid nitrogen and then lyophilized. The lyophilized venom was dissolved in Milli-Q water and was loaded onto a CM52 cation exchange column (15 mm i.d. x 870 mm) pre-equilibrated with 20 mM ammonium acetate buffer pH 6.8 containing 0.1 mM CaCl2. The bound PflLys49-PLA2 BPII (UniProt ID: P0DJJ9)6,24,25 was eluted with a linear gradient of 20–500 mM ammonium acetate. The fractions containing PflLys49-PLA2 BPII were collected and dialyzed against Milli-Q water three times utilizing a cellulose membrane (MWCO 10 kDa). The dialyzed sample was subsequently lyophilized, and stored until use.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis
The purity and electrophoretic characteristics of PflLys49-PLA2 BPII were analyzed by 15% (w/v) SDS-PAGE followed by Coomassie Blue staining. In order to confirm the presence of intermolecular disulfide bonds, the object protein was mixed with SDS-PAGE sample buffer [62.5 mM Tris-HCl pH 6.8, 2% (w/v) SDS and 5% (w/v) sucrose] with or without 5% (v/v) 2-mercaptoethanol (2-ME). To assess the conformation of the oligomerization states in the crystal, the protein crystals were picked up from the drop, and washed three times with crystallization buffer. Next, the crystals were dissolved in SDS-PAGE sample buffer without 2-ME. All samples were incubated at 95 °C for 5 min and loaded onto the SDS-PAGE gel.
Crystallization and X-ray crystallography
The concentration of the lyophilized protein was adjusted to 20 mg/ml with Milli-Q water. All crystallization attempts were performed using the sitting-drop vapor diffusion technique at 20 °C. Diffraction-quality crystals of the object protein were obtained with the optimized reservoir conditions [100 mM sodium acetate pH 4.2, 500 mM ammonium acetate, and 32.5% (w/v) PEG4000] after initial screenings using 96-condition crystallization screening kits (Qiagen, Hilden, Germany). All crystallization drops were prepared by mixing 1.0 µl of PflLys49-PLA2 BPII with an equal volume of reservoir solution followed by equilibration of the mixtures against 50 µl of reservoir solution. The PflLys49-PLA2 BPII crystals were added to a crystallization solution containing 20% (v/v) ethylene glycol as a cryoprotectant. After a few seconds, the crystals were picked up in a nylon loop and then flash-cooled to 100 K in a nitrogen gas stream. The X-ray diffraction experiments were performed at the Photon Factory (proposals 16G092 and 17G595) and SPring-8 (proposals 2015B6524, 2016A2565 and 2016B2565). X-ray diffraction data sets were collected on the BL-17A beamline at the Photon Factory (Tsukuba, Japan). The diffraction data of the crystals were processed and scaled with XDS26. The molecular replacements were performed with BsSP-7 (PDB code 5VFH)16 as the search model using Phaser27. The structure refinement of PflLys49-PLA2 BPII was performed using phenix.refine28, with the twin operators of (h, -h-k, –l) with a fraction of 0.49. The structure was modified manually with COOT29. The quality of the final models was assessed with MolProbity30. All crystallographic figures were prepared with PyMOL31. The crystallographic data and refinement statistics are summarized in Table 1. The coordinate and structure factor data of PflLys49-PLA2 BPII reported in this paper have been deposited under the accession number 6AL3.
Native-MS analysis
Native-MS analysis was performed as described previously32. Briefly, 50 µM of BPII aqueous solution was measured by nanoflow electrospray ionization mass spectrometry using gold-coated glass capillaries made in house with approximately 2–5 µL of sample loaded per analysis. The spectra were recorded on a SYNAPT G2-Si HDMS mass spectrometer (Waters, Milford, Massachusetts, USA) in positive ionization mode at 1.33 kV with a 150 V sampling cone voltage and source offset voltage, 0 V trap and transfer collision energy, and 5 mL/min trap gas flow. The spectra were calibrated using 1 mg/mL cesium iodide and analyzed using MassLynx software (Waters).
Sedimentation velocity analytical ultracentrifugation (SV-AUC)
SV-AUC experiments were conducted with 1 mg/mL of PflLys49-PLA2 BPII in the presence or absence of 1% (w/v) SDS. Data collection was performed at 20 °C in a ProteomeLab XL-I analytical ultracentrifuge (Beckman Coulter, Brea, California, USA) at 60,000 rpm using UV detection. The collected data were analyzed using the continuous c(s) distribution from the program SEDFIT (version 15.01b)33 with fitting for the frictional ratio, meniscus, and time-invariant noise with a regularization level of 0.68. The partial specific volume of PflLys49-PLA2 BPII was calculated as 0.728 cm3 g−1 using the program SEDNTERP 1.09. The buffer density and viscosity of each condition were determined using a DMA 5000 densitometer (Anton Paar, Graz, Austria) and a Lovis 2000 M viscometer (Anton Paar), respectively. The resulting data were analyzed with the program SEDNTERP 1.09. The partial specific volume of PflLys49-PLA2 BPII in the presence of 1% (w/v) SDS was determined experimentally by using density contrast SV-AUC34,35 where the samples with identical protein concentrations but different H2O/D2O ratios (H2O 80%/D2O 20% and H2O 40%/D2O 60%) were subjected to a sedimentation velocity run. The data were analyzed by setting the partial specific volume as a floated parameter in the “Hybrid Global Continuous Distribution and Global Discrete Species” model in the program SEDPHAT (version 12.1b)36. The partial specific volume and molecular mass of the SDS micelle were assumed to be 0.870 cm3 g−1 and 14,000 Da, respectively37,38.
Results
Crystal structure of PflLys49-PLA2 BPII
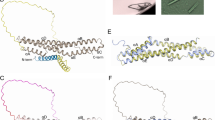
The crystal structure of PflLys49-PLA2 BPII was determined at a resolution of 2.57 Å. The asymmetric unit contained four molecules of PflLys49-PLA2 BPII (Fig. 1a). These four molecules were superimposed well relative to each other with root-mean-square-deviation (r.m.s.d.) values less than 0.5 Å for the Cα-atoms (Fig. 1b). All 14 Cys residues formed the following seven intramolecular disulfide bonds (Fig. 1c): Cys26–Cys116, Cys28–Cys44, Cys43–Cys96, Cys49–Cys122, Cys50–Cys89, Cys57–Cys82, and Cys75–Cys87. Ca2+ ions, which are important for the phospholipase activity of other Lys49-PLA2s was not bound on the catalytic site of PflLys49-PLA2 BPII due to the substitution of Asp49 for Lys4,5,7,13,14,15,17,20,22,39.
X-ray crystal structure of PflLys49-PLA2 BPII. (a) Crystal structures of four molecules in an asymmetric unit. (b) Superimposition of the four molecules. (c) Disulfide bond network. The sulfur atoms are indicated as orange spheres. (d) Superimposition of molecules A and B on molecules C and D. (e) Superimposition of an alternative dimer (gray, PDB ID: 2Q2J) on molecule A of PflLys49-PLA2 BPII. (f) Superimposition of the conventional dimer (wheat PDB ID: 2Q2J) on molecule A of PflLys49-PLA2 BPII. (g) Surface charge distributions of the surface between molecules A and B. Surfaces comprising positive and negative charges are depicted as blue and red colors, respectively.
The relative orientation between molecules A and B was similar to that between molecules C and D (Fig. 1d). The r.m.s.d. between molecules B and D after superimposing molecules A and C was 1.3 Å, which suggested the possibility that this dimer is an intrinsic dimer structure of PflLys49-PLA2 BPII. However, this dimer was superimposed neither on the conventional dimer nor the alternative dimer previously reported (Fig. 1e,f). Furthermore, basic residues were located on both sides of the interface, and the surface charges of both interfaces were positive (Fig. 1g). The area of the interface among four molecules was less than 350 Å2. Based on these observations, we concluded that the conserved relative orientation between molecules is merely preferable packing in the crystal and PflLys49-PLA2 BPII does not form any oligomers in the crystal.
The mobility of PflLys49-PLA2 BPII in SDS-PAGE
The mobility of PflLys49-PLA2 BPII was analyzed by SDS-PAGE (Fig. 2a). PflLys49-PLA2 BPII without 2-ME treatment migrated at approximately 27 kDa when subjected to SDS-PAGE. On the other hand, PflLys49-PLA2 BPII treated with 5% (v/v) 2-ME shifted to the position of approximately 14.4 kDa (Fig. 1a, lane 2). In general, the 2-ME-mediated band shift of PflLys49-PLA2 BP II subjected to SDS-PAGE suggested the presence of intermolecular disulfide bond formation. However, all cysteine residues formed intramolecular disulfide bonds in the crystal structure (Fig. 1c), and thus, intermolecular disulfide bond formation is highly unlikely. These observations suggested that PflLys49-PLA2 BPII may exhibit unusual behavior when subjected to SDS-PAGE. Therefore, we analyzed the mobility of PflLys49-PLA2 BPII under a variety of conditions (Fig. 2b,c).
SDS-PAGE of PflLys49-PLA2 BPII under various conditions. (a) PflLys49-PLA2 BPII with and without 2-ME treatment. Lane 1, without 2-ME; lane 2, with 2-ME. (b) PflLys49-PLA2 BPII treated with 6 M urea. Lane 1, without 95 °C treatment; lane 2, after 95 °C treatment. (c) Resuspension of PflLys49-PLA2 BPII crystals with SDS-PAGE sample buffer.
First, we evaluated the effect of urea, a typical protein denaturation reagent, on oligomerization. PflLys49-PLA2 BPII was still oligomerized when subjected to SDS-PAGE in the presence of 6 M urea (Fig. 2b, lane 1). However, heat treatment in the presence of 6 M urea resulted in the dissociation of PflLys49-PLA2 BPII to monomers (Fig. 2b, lane 2). It should be noted that a reducing reagent was not added to these samples. These results showed that the stable oligomer formation of PflLys49-PLA2 BPII subjected to SDS-PAGE is not due to disulfide bond formation.
Next, we loaded crystals of PflLys49-PLA2 BPII dissolved in the SDS-PAGE sample buffer containing 2% (w/v) SDS. Surprisingly, PflLys49-PLA2 BPII, which adopted a monomeric form with no intermolecular disulfide bonds in the crystals, oligomerized after being subjected to SDS-PAGE (Fig. 2c). These observations suggested that treatment with the SDS-PAGE sample buffer induced oligomerization of this protein.
Oligomerization state analyzed by native-MS and SV-AUC
To identify the oligomerization state of PflLys49-PLA2 BPII in an aqueous condition with neither detergent nor denaturant, the molecular mass distributions of 50 µM of the purified PflLys49-PLA2 BPII dissolved in water were analyzed by native-MS. The molecular mass of PflLys49-PLA2 BPII in water was determined to be 13,753 Da, which is in good agreement with the estimated molecular mass of the deduced amino acid sequence (13,872 Da) (Fig. 3). It should be noted that native-MS analysis detected no oligomer. These results revealed that PflLys49-PLA2 BPII exists as a monomer in an aqueous solution.
Next, the effect of SDS on the oligomerization state of PflLys49-PLA2 BPII was analyzed by analytical ultracentrifugation. Purified PflLys49-PLA2 BPII dissolved in 20 mM Tris-HCl pH 8.0 and 200 mM NaCl at a concentration of 1 mg/mL distributed as a monomer with a molecular mass of 13.1 kDa (Fig. 4a, Table 2). This result is in good agreement with the native-MS observations described above (Fig. 3). In contrast to the monomeric state of PflLys49-PLA2 BPII dissolved in 20 mM Tris-HCl pH 8.0 and 200 mM NaCl, the sedimentation coefficients of PflLys49-PLA2 BPII in the presence of SDS exhibited a bimodal distribution. The sedimentation coefficients significantly increased compared to those obtained in the absence of SDS (Fig. 4b).
The density contrast SV-AUC analysis revealed that the partial specific volume of PflLys49-PLA2 BPII in the presence of SDS was 0.771 cm3 g−1. Three possible protein-SDS complexes that have a partial specific volume of 0.771 cm3 g−1 are as follows: (PflLys49-PLA2 BPII)(SDS)21, (PflLys49-PLA2 BPII)2(SDS)42, and (PflLys49-PLA2 BPII)3(SDS)63 of which the calculated molecular masses were 19.9 kDa, 39.9 kDa, and 59.8 kDa, respectively (Fig. 4c,d). The molecular masses obtained from c(s) analysis of the two peaks (under the assumption that the three complexes have a similar molecular shape) were 33.1 kDa for the 2.6 S peak and 48.3 kDa for the 3.4 S peak (Fig. 4b, Table 2). These results lead to the conclusion that PflLys49-PLA2 BPII forms SDS bound dimers and SDS bound trimers in the presence of 1% (w/v) SDS.
Discussion
Previous studies reported that Lys49-PLA2s exist as either conventional or alternative dimers, based on the results of X-ray crystallography, electrophoresis, dynamic light scattering, and spectroscopy11,12,14,15,19,23,39,40,41,42. In contrast to these previous reports, our present crystal structure showed that PflLys49-PLA2 BPII exists as a monomer in the crystal (Fig. 1). This was consistent with the results of native-MS (Fig. 3) and analytical ultracentrifugation analyses (Fig. 4), both of which showed that monomeric PflLys49-PLA2 BPII was detected in the absence of SDS. These results indicated that PflLys49-PLA2 BPII exists as a monomer in both solution and crystal states.
Although the behavior of PflLys49-PLA2 BPII in the crystal and solution states was distinct from Lys49-PLA2s from other species (i.e., PflLys49-PLA2 BPII is a monomer while other Lys49-PLA2s assemble to dimers), the behavior upon SDS-PAGE was similar between PflLys49-PLA2 BPII and other Lys49-PLA2s. Specifically, both proteins oligomerized upon SDS-PAGE. The dissolved crystals of PflLys49-PLA2 BPII formed oligomers upon SDS-PAGE, which demonstrated that during sample preparation and/or electrophoresis the oligomerization of PflLys49-PLA2 BPII occurred spontaneously (Fig. 2c). Moreover, analytical ultracentrifugation indicated that 1% (w/v) SDS induces dimerization and trimerization of PflLys49-PLA2 BPII (Fig. 4). Taking these observations together, we concluded that PflLys49-PLA2 BPII assembles to form SDS-resistant stable oligomers in the presence of 1% (w/v) SDS. Kilby and coworkers also reported a similar phenomenon, i.e., the interaction of SDS micelles with bovine PLA2 induced trimer formation, in which approximately three molecules of bovine PLA2 was predicted to bind one SDS micelle43,44. The results of our analytical ultracentrifugation showed that PflLys49-PLA2 BPII assembles to form dimers and trimers. PflLys49-PLA2 BPII may assemble to form oligomers in a similar manner as bovine PLA2. Notably, this characteristic of PflLys49-PLA2 BPII in SDS-PAGE did not change even in the presence of crude snake venom (Supplementary Fig. 1), indicating no significant synergy effect from another venom components to the behavior against SDS.
It is important to note that SDS, one of the strongest protein denaturants, induces oligomerization of PflLys49-PLA2 BPII. Thus, PflLys49-PLA2 BPII exhibits outstandingly strong stability against chemical denaturation. Indeed, oligomers of PflLys49-PLA2 BPII did not dissociate even in the presence of 6 M urea, and further heat treatment was necessary for the dissociation to monomers (Fig. 2). PflLys49-PLA2 BPII harbors seven intramolecular disulfide bonds, thus 12% of the total 122 amino acids (14 Cys residues) contributes to covalent bond formation. Such a large number of intramolecular disulfide bonds may contribute to this unusual behavior of this protein, i.e., oligomerization by a protein denaturant.
In the present study, we demonstrated that SDS induces oligomerization of PflLys49-PLA2 BPII. SDS can be regarded as a simple phospholipid analogue. These oligomers may also be induced by phospholipids, and the oligomer may interact with the membrane and function as a myotoxic agent to disrupt the cell membrane. Although the physiological significance of SDS-induced oligomerization of PflLys49-PLA2 BPII is still unclear, it may play a key role in its myotoxic function.
References
Shibata, H. et al. The habu genome reveals accelerated evolution of venom protein genes. Sci. Rep. 8, 11300 (2018).
Verheij, H. M. et al. Methylation of Histidine-48 in Pancreatic Phospholipase A2. Role of Histidine and Calcium Ion in the Catalytic Mechanism. Biochemistry 19, 743–750 (1980).
Scott, D. L. et al. Interfacial catalysis: The mechanism of phospholipase A2. Science 250, 1541–1546 (1990).
Holland, D. R. et al. The Crystal Structure of a Lysine 49 Phospholipase A2 from the Venom of the Cottonmouth Snake at 2.0 Å Resolution. J. Biol. Chem. 265, 17649–17656 (1990).
Fernandes, C. A. H. et al. Comparison between apo and complexed structures of bothropstoxin-I reveals the role of Lys122 and Ca2+-binding loop region for the catalytically inactive Lys49-PLA2s. J. Struct. Biol. 171, 31–43 (2010).
Liu, S. Y. et al. Purification and amino acid sequence of basic protein II, a lysine-49-phospholipase A2 with low activity, from Trimeresurus flavoviridis venom. J. Biochem. 107, 400–408 (1990).
Arni, R. K., Ward, R. J., Gutierrez, J. M. & Tulinsky, A. Structure of a calcium-independent phospholipase-like myotoxic protein from Bothrops asper venom. Acta Crystallogr. - Sect. D Biol. Crystallogr. 51, 311–317 (1995).
Chioato, L. et al. Mapping of the structural determinants of artificial and biological membrane damaging activities of a Lys49 phospholipase A2 by scanning alanine mutagenesis. Biochim. Biophys. Acta - Biomembr. 1768, 1247–1257 (2007).
Murakami, M. T. et al. Inhibition of myotoxic activity of Bothrops asper myotoxin II by the anti-trypanosomal drug suramin. J. Mol. Biol. 350, 416–426 (2005).
Murakami, T. et al. A [Lys49]-phospholipase A2 from Protobothrops flavoviridis Venom Induces Caspase-Independent Apoptotic Cell Death Accompanied by Rapid Plasma-Membrane Rupture in Human Leukemia Cells. Biosci. Biotechnol. Biochem. 75, 864–870 (2011).
Francis, B., Gutierrez, J. M., Lomonte, B. & Kaiser, I. I. Myotoxin II from Bothrops asper (terciopelo) venom is a lysine-49 phospholipase A2. Arch. Biochem. Biophys. 284, 352–359 (1991).
Fernandes, C. A. H., Borges, R. J., Lomonte, B. & Fontes, M. R. M. A structure-based proposal for a comprehensive myotoxic mechanism of phospholipase A2-like proteins from viperid snake venoms. Biochim. Biophys. Acta - Proteins Proteomics 1844, 2265–2276 (2014).
Fernandes, C. A. H. et al. Structural bases for a complete myotoxic mechanism: Crystal structures of two non-catalytic phospholipases A2-like from Bothrops brazili venom. Biochim. Biophys. Acta - Proteins Proteomics 1834, 2772–2781 (2013).
Ambrosio, A. L. B. et al. A molecular mechanism for Lys49-phospholipase A2 activity based on ligand-induced conformational change. J. Biol. Chem. 280, 7326–7335 (2005).
Murakami, M. T., Melo, C. C., Angulo, Y., Lomonte, B. & Arni, R. K. Structure of myotoxin II, a catalytically inactive Lys49-phospholipase A2 homologue from Atropoides nummifer venom. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 62, 423–426 (2006).
de Lima, L. F. G., Borges, R. J., Viviescas, M. A., Fernandes, C. A. H. & Fontes, M. R. M. Structural studies with BnSP-7 reveal an atypical oligomeric conformation compared to phospholipases A2-like toxins. Biochimie 142, 11–21 (2017).
Watanabe, L., Soares, A. M., Ward, R. J., Fontes, M. R. M. & Arni, R. K. Structural insights for fatty acid binding in a Lys49-phospholipase A2: Crystal structure of myotoxin II from Bothrops moojeni complexed with stearic acid. Biochimie 87, 161–167 (2005).
Toyama, D. et al. Umbelliferone induces changes in the structure and pharmacological activities of Bn IV, a phospholipase A2 isoform isolated from Bothrops neuwiedi. Toxicon 57, 851–860 (2011).
Salvador, G. H. M. et al. Structural and functional studies with mytoxin II from Bothrops moojeni reveal remarkable similarities and differences compared to other catalytically inactive phospholipases A2-like. Toxicon 72, 52–63 (2013).
Magro, A. J., Soares, A. M., Giglio, J. R. & Fontes, M. R. M. Crystal structures of BnSP-7 and BnSP-6, two Lys49-phospholipases A2: Quaternary structure and inhibition mechanism insights. Biochem. Biophys. Res. Commun. 311, 713–720 (2003).
Murakami, M. T. et al. Interfacial surface charge and free accessibility to the PLA2-active site-like region are essential requirements for the activity of Lys49 PLA2 homologues. Toxicon 49, 378–387 (2007).
dos Santos, J. I., Soares, A. M. & Fontes, M. R. M. Comparative structural studies on Lys49-phospholipases A2 from Bothrops genus reveal their myotoxic site. J. Struct. Biol. 167, 106–116 (2009).
Salvador, G. H. M. et al. Structural and Phylogenetic Studies with MjTX-I Reveal a Multi-Oligomeric Toxin - a Novel Feature in Lys49-PLA2s Protein Class. PLoS One 8, e60610 (2013).
Murakami, T. et al. Island specific expression of a novel [Lys49]phospholipase A2 (BPIII) in Protobothrops flavoviridis venom in Amami-Oshima, Japan. Toxicon 54, 399–407 (2009).
Ohno, M., Chijiwa, T., Oda-Ueda, N., Ogawa, T. & Hattori, S. Molecular evolution of myotoxic phospholipases A2 from snake venom. Toxicon 42, 841–854 (2003).
Kabsch, W. Xds. Acta Crystallogr. D. Biol. Crystallogr. 66, 125–132 (2010).
McCoy, A. J. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. D. Biol. Crystallogr. 63, 32–41 (2007).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D. Biol. Crystallogr. 68, 352–367 (2012).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D, Biol. Crystallogr. 60, 2126–2132 (2004).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. Biol. Crystallogr. 66, 12–21 (2010).
Schrödinger, L. L. C. The JyMOL Molecular Graphics Development Component, Version 1.0 (2010).
Ishii, K. et al. Disassembly of the self-assembled, double-ring structure of proteasome α7 homo-tetradecamer by α6. Sci. Rep. 5, 18167 (2015).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Brown, P. H., Balbo, A., Zhao, H., Ebel, C. & Schuck, P. Density contrast sedimentation velocity for the determination of protein partial-specific volumes. PLoS One 6, 18167 (2011).
Nango, E. et al. Taste substance binding elicits conformational change of taste receptor T1r heterodimer extracellular domains. Sci. Rep. 6, 18167 (2016).
Schuck, P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal. Biochem. 320, 104–124 (2003).
Tanford, C., Nozaki, Y., Reynolds, J. A. & Makino, S. Molecular Characterization of Proteins in Detergent Solutions. Biochemistry 13, 2369–2376 (1974).
Miyabe, K., Takahashi, R. & Shimazaki, Y. Kinetic Study of Interaction between Solute Molecule and Surfactant Micelle. Anal Sci 31, 1019–1025 (2015).
Liu, Q. et al. The crystal structure of a novel, inactive, lysine 49 PLA2 from Agkistrodon acutus venom: An ultrahigh resolution, ab initio structure determination. J. Biol. Chem. 278, 41400–41408 (2003).
Arni, R. K. et al. Crystal structure of myotoxin II, a monomeric Lys49-phospholipase A2 homologue isolated from the venom of Cerrophidion (Bothrops) godmani. Arch. Biochem. Biophys. 366, 177–182 (1999).
Da Silva Giotto, M. T. et al. Crystallographic and spectroscopic characterization of a molecular hinge: Conformational changes in Bothropstoxin I, a dimeric Lys49- phospholipase A2 homologue. Proteins Struct. Funct. Genet. 30, 442–454 (1998).
Ullah, A., Souza, T. A. C. B., Betzel, C., Murakami, M. T. & Arni, R. K. Crystallographic portrayal of different conformational states of a Lys49 phospholipase A2 homologue: Insights into structural determinants for myotoxicity and dimeric configuration. Int. J. Biol. Macromol. 51, 209–214 (2012).
Kilby, P. M., Primrose, W. U. & Roberts, G. C. Changes in the structure of bovine phospholipase A2 upon micelle binding. Biochem. J. 305, 935–44 (1995).
Duplâtre, G., Ferreira Marques, M. F. & da Graça, M. M. Size of Sodium Dodecyl Sulfate Micelles in Aqueous Solutions as Studied by Positron Annihilation Lifetime Spectroscopy. J. Phys. Chem. 100, 16608–16612 (1996).
Acknowledgements
The X-ray diffraction experiments were performed at the Photon Factory (proposals 16G092, 18G060, and 17G595) and SPring-8 (proposals 2015B6524, 2016A2565 and 2016B2565). This work was supported in part by the following sources: the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to T.Matsui and Y.T.), the PRESTO (to Y.T.), the Joint Research by Exploratory Research Center on Life and Living Systems (ExCELLS) (to SU and KK), and the Platform Project for Supporting Drug Discovery and Life Science Research [Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)] from the Japan Agency for Medical Research and Development (AMED) under Grant Number JP18am0101095 (to T.Matsui). This research was also supported by a JSPS Grants-in-Aid for Scientific Research on Innovative Areas entitled “Dynamical Ordering of Biomolecular Systems for Creation of Integrated Functions” (25102008, 16H00770, and 16H00748), NEDO, and JICE.
Author information
Authors and Affiliations
Contributions
T. Matsui, T.O. and Y.T. designed the experiments. T. Matsui, S.K., K.I., T. Maruno, N.G. and A.S. performed the experiments. T. Matsui, S.K., K.I., T. Maruno, N.G., S.U. and Y.T. analyzed the data. T. Matsui, S.K., S.U., K.K., N.O.-U. and Y.T. wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsui, T., Kamata, S., Ishii, K. et al. SDS-induced oligomerization of Lys49-phospholipase A2 from snake venom. Sci Rep 9, 2330 (2019). https://doi.org/10.1038/s41598-019-38861-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-019-38861-8
This article is cited by
-
SDS-induced hexameric oligomerization of myotoxin-II from Bothrops asper assessed by sedimentation velocity and nuclear magnetic resonance
European Biophysics Journal (2023)
-
The allosteric activation mechanism of a phospholipase A2-like toxin from Bothrops jararacussu venom: a dynamic description
Scientific Reports (2020)