Abstract
The new Bi0.5Na0.5TiO3-SrMnO3−δ solid solution materials were fabricated via sol–gel method. The random incorporation of Sr and Mn cations into host lattice of Bi0.5Na0.5TiO3 resulted in structural distortion and influenced on the reduction of the optical band gap from 3.07 eV to 1.81 eV for pure Bi0.5Na0.5TiO3 and 9 mol% SrMnO3−δ solid solution into Bi0.5Na0.5TiO3. The magnetic properties of Bi0.5Na0.5TiO3 materials at room temperature were tuned via compensation of diamagnetic material with weak-ferromagnetism to ferromagnetism with low SrMnO3−δ content and combination of paramagnetism/antiferromagnetism-like and ferromagnetism with higher SrMnO3−δ content solid solution in Bi0.5Na0.5TiO3. The tunable magnetic and optical properties of lead-free ferroelectric materials was promising for their application to green electronic devices.
Similar content being viewed by others
Introduction
Bi0.5Na0.5TiO3 materials and their solid solution showed rapid development, especially in the search of high-performance lead-free piezoelectric materials to address human health and environmental protection concerns1,2. Bi0.5Na0.5TiO3 material which was firstly fabricated by Smolenskii et al. in 1961, is an A-site complex perovskite-structured material with random distribution of Bi and Na cation at A-site3. At room temperature, Bi0.5Na0.5TiO3 materials has a rhombohedral symmetry with Curie temperature (TC) ~320 °C, remanence polarization (Pr) ~38 μC/cm2, high dielectric constant (εr ~694 at 1 kHz) and low dielectric loss (tanδ ~0.103) while high coercive field ~7.3 kV/mm, thereby resulting in weak piezoelectric coefficient (d33) ~73–80 pC/N due to hard to polling treatment4,5. The Bi0.5Na0.5TiO3 materials displayed a direct transition optical band gap (Eg~3.01–3.18 eV), depending on the fabrication method6. However, the performance properties of lead-free ferroelectric Bi0.5Na0.5TiO3 materials are still not comparable with those of Pb(Ti,Zr)O3-based materials in terms of application requirements in electronic devices7,8. The high-performance properties of lead-free ferroelectric Bi0.5Na0.5TiO3 materials were recently greatly enhanced by using a solid solution with various compounds containing transition metal such as BiCoO3, Bi(Zn0.5Hf0.5)O3, Bi(Mn0.5Ti0.5)O3, Bi(Co0.5Ti0.5)O3 etc.4,9,10,11,12. Guo et al. reported that BiCoO3-modified Bi0.5Na0.5TiO3 materials were exhibited the increasing d33 values up to 107 pC/N, whereas and the coercive field were reduced to 5.25 kV/mm4. The Bi(Zn0.5Hf0.5)O3-modified Bi0.5Na0.5TiO3 materials increased the Pr and TC values to 43.5 μC/cm2 and 340 °C, respectively9. Bi(Mn0.5Ti0.5)O3 and Bi(Co0.5Ti0.5)O3 solid solution into Bi0.5Na0.5TiO3-based materials resulted in a display a giant electrical field-induced strain coefficient values (d*33) to 818 pm/V and 600 pm/V, respectively10,11,12. In addition, the A-site was modified Bi0.5Na0.5TiO3 materials via Sr as solid solution of SrTiO3 in Bi0.5Na0.5TiO3 materials, a large electrical field-induced strain of over 1000 pm/V for low-driving fields (less than 2 kV/mm) was achieved13. The solid solution with perovskite-type material containing the transition metal and alkaline cations possibly enhanced the performance of electrical properties of lead-free ferroelectric Bi0.5Na0.5TiO3-based materials.
The observation of room temperature ferromagnetism in pure Bi0.5Na0.5TiO3 materials was promising for the transfer of lead-free ferroelectric material to multiferroic applications in electronic devices14,15,16,17. Ju et al. obtained the room temperature ferromagnetism in nanocrystalline Bi0.5Na0.5TiO3 and its possible origin from Na vacancies located at/near surface of nanograins14. Thanh et al. also achieved the room temperature ferromagnetism versus diamagnetism in pure Bi0.5Na0.5TiO3 materials15,16. Zhang et al. predicted the ideal Bi0.5Na0.5TiO3 non-magnetic material; whereas Na or Ti vacancies can induce the magnetism rather than Bi or O vacancies by using ab initio calculations17. However, the main problem of magnetism in pure Bi0.5Na0.5TiO3 compounds is low magnetisation (less than 1 memu/g) and strong influence of diamagnetic components, which were raised from empty orbital of 3d°-Ti14,15,16. Therefore, for a new Bi0.5Na0.5TiO3-based compound is important for the transfer of the materials to industrial application in smart electronic devices. Simple ideas were used for investigating high ferromagnetism at room temperature; transition metal was used as impurities for substitution at Ti-site in perovskite structure. Room temperature ferromagnetism in Bi0.5Na0.5TiO3 was reported for Co-, Mn-, Ni-, Fe- and Cr-dopants15,16,18,19,20. However, the physical property of room temperature ferromagnetism ordering phenomenal was raised from various parameters, was not well understood. The room temperature ferromagnetism in Mn-, Ni- and Fe-doped Bi0.5Na0.5TiO3 compounds are intrinsic phenomena that resulting from the interaction between magnetic ions through the oxygen vacancies16,18,20. It is unlikely that the room temperature ferromagnetism in Cr-doped Bi0.5Na0.5TiO3 compound originated more from oxygen vacancies than the interaction of Cr ions, whereas ferromagnetism in Co-doped Bi0.5Na0.5TiO3 compound was exhibited from magnetic Co clusters15,19. The other method was used in tailoring ferromagnetic properties in lead-free ferroelectric Bi0.5Na0.5TiO3 materials that sintered the ferroelectric-ferromagnetic materials as composites, such as CoFe2O4/Bi0.5Na0.5TiO3 and MgFe2O4/Bi0.5Na0.5TiO321,22. However, the main problem of the other method is the pole under low-electrical field during high conductivity of spinel and/or interface effect, which results in large leakage current23. Recently, we proposed the new method for arching the room temperature of solid solution with alkaline-transition compounds such as MgFeO3−δ or SrFeO3−δ24,25. In the solid solution, both A- and B-sites of Bi0.5Na0.5TiO3 compounds were modified by alkaline cation and transition metal ions, respectively, thereby resulting display ferromagnetism with large magnetisation at room temperature and overcoming the single transition metal dopants.
To date, no reports on the use of Mn-based alkaline material as solid solution in Bi0.5Na0.5TiO3 materials are available. Alkaline-earth metal manganese double oxides are interesting materials because oxygen deficiency can be modulated according to their structural, electrical, and magnetic properties26,27,28,29,30. Kobayashi et al. reported that SrMnO3 and SrMnO2.5 compound exhibited the cubic and orthorhombic structure27. Hexagonal SrMnO3 was possibly transformed into the pseudocubic SrMnO3–δ by introducing oxygen vacancies and final reducing state to orthorhombic SrMnO2.5 structural27. The valence state of Mn near the interface gradually varied from Mn3+ to Mn4+ over an area of a few atomic layers27. Belik et al. reported that the polymorphous crystal structures of hexagonal 6H-SrMnO3, hexagonal 4H-SrMnO3, and cubic SrMnO3 have the same chemical composition as SrMnO328. Suescun et al. reported that oxygen vacancies ordering in oxygen-deficient perovskites SrMnO3–δ compounds were possibly reduced to SrMnO2.6 and SrMnO2.74 with tetragonal and monoclinic properties, respectively28. The 6H-SrMnO3, 4H-SrMnO3, and cubic-SrMnO3 exhibited the antiferromagnetic property with Neel temperatures (TN) of 235 K, 280 K, and 240 K, respectively27. The cubic-SrMnO3 exhibited a G-type antiferromagnetic structure with TN in the range 230–260 K probably because of the small variations in oxygen stoichiometry28,30. Rahman et al. predicted that SrMnO2 is a tetragonal lattice structure with an A-type antiferromagnetic conductor31. Given the well solid solution of SrMnO3–δ into host Bi0.5Na0.5TiO3 crystal, we expected that the Sr and Mn cations were diffused to randomly incorporate with the host lattice of Bi0.5Na0.5TiO3 crystal to form a solid solution. Thus, the interaction between random magnetic Mn cations at the B-site and co-modified by Sr cations at the A-site in host Bi0.5Na0.5TiO3 materials was expected to be of phenomenal interest. In this work, the solid solution of (1−x)Bi0.5Na0.5TiO3 + xSrMnO3−δ compounds was fabricated using sol–gel method. The solid solution SrMnO3−δ in Bi0.5Na0.5TiO3 compound reduced the optical band gap and tunable magnetic properties of host materials.
Results
Chemical compositions
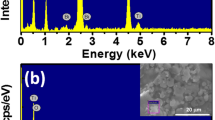
The chemical composition of pure Bi0.5Na0.5TiO3 and SrMnO3-δ-modified Bi0.5Na0.5TiO3 materials were confirmed, as shown in Fig. 1(a,b) for the energy dispersive X-ray spectra of pure and 5 mol% SrMnO3−δ-modified Bi0.5Na0.5TiO3 compounds, respectively, wherein which the selected area for the characterisation element was shown in the inset of each figure. The results showed that the all expectations of elements such as Bi, Na, Ti and O were obtained in pure Bi0.5Na0.5TiO3 as shown in Fig. 1(a). The EDX spectra of SrMnO3−δ-modified Bi0.5Na0.5TiO3 compounds showed the spectral addition elements such as Sr and Mn tailoring with elements Bi, Ti, Na and O host Bi0.5Na0.5TiO3 compounds.
Surface morphologies
The solid solution of SrMnO3−δ into host Bi0.5Na0.5TiO3 materials resulted in modification of the surface morphologies of samples where the surface morphologies were exhibited inhomogeneous as increasing the SrMnO3−δ amounts solution. Figure 2(a–g) show the surface morphology of pure Bi0.5Na0.5TiO3 samples and SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples with 0.5, 1, 3, 5, 7, and 9 mol%, respectively. The surface morphology of pure Bi0.5Na0.5TiO3 materials exhibited cubic-like shape with grain size of approximately 3–4 μm, as shown in Fig. 2(a). The minimal addition of 0.5 and 1 mol% SrMnO3−δ into host Bi0.5Na0.5TiO3 materials resulted in inhomogeneous structure with grain size ranging from 1 μm to 4 μm, as shown in Fig. 2(b,c), respectively. Further addition of SrMnO3−δ into host Bi0.5Na0.5TiO3 materials with SrMnO3-δ up to 9 mol% as solid solution reduced the grain size and inhomogeneous distribution of the grain in a wide range from several hundred nanometers to few micrometers, as shown in Fig. 2(d–g). Normally, the presentation of impurities near the grain boundaries resulted in decreasing their mobility substantially as densification occurs. Therefore, small grain size is formed because of the reduction in the mobility of the grain boundary weakens the mass transport, resulting in obviously inhibited grain growth32,33. However, the grain size possibly increased because the presentation of oxygen vacancies is beneficial to mass transport during sintering32,33. Therefore, we suggested that the combination of both impurities and oxygen vacancy parameter affected the grain growth of SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples, wherein Sr and Mn impurities cations showed inhibited grain growth, whereas oxygen vacancies promoted grain growth.
Room temperature structure
The structural studied in SrMnO3−δ-modified Bi0.5Na0.5TiO3 compound with SrMnO3−δ concentration up to 9 mol.% were provided that the SrMnO3−δ were well solute into host Bi0.5Na0.5TiO3 crystal. Figure 3(a) shows the XRD of pure Bi0.5Na0.5TiO3 samples and SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples with various SrMnO3−δ concentrations. The peak position and relative intensity of peaks of pure Bi0.5Na0.5TiO3 samples were indexed as perovskite structure with rhombohedral symmetry. No diffraction peaks of SrMnO3−δ materials were observed, as shown in the XRD spectra. Besides, the impurity phase was not obtained in the XRD spectra under the resolution of XRD method. The results indicated that the SrMnO3−δ phase was a good solid solution for host Bi0.5Na0.5TiO3 materials. The influence of solid solution SrMnO3−δ on the lattice of Bi0.5Na0.5TiO3 materials is shown in Fig. 3(b), wherein the XRD spectra were enlarged in 2θ range from 30°–35° for (012)/(110) peaks. The peak position was overlapped together. Therefore, the peak positions were distinguished using the Lorentzian fitting with r-square of over 0.99. The peak position clearly shifted to a high angle for 3 mol% SrMnO3−δ-modified Bi0.5Na0.5TiO3 materials. Furthermore, addition of SrMnO3−δ into Bi0.5Na0.5TiO3 materials as solid solution which SrMnO3-δ over 3 mol.% were resulted in the shrinkage of the lattice parameter as evidence by the shifting of the peak position to a low diffraction angle. The distortion of the lattice parameter was strong evidence for the random substitution of Sr and Mn cations into the host lattice of Bi0.5Na0.5TiO3 materials. The lattice constant of pure Bi0.5Na0.5TiO3 materials and SrMnO3–δ-modified Bi0.5Na0.5TiO3 materials as a function of SrMnO3–δ concentration is calculated and presented in Fig. 3(c). The results further indicated that the lattice parameter of Bi0.5Na0.5TiO3 compounds was complex in distortion via addition of the different SrMnO3−δ concentrations. The distorted lattice parameters were possibly understood when the radius of cations in host lattice was identified and compared with substitution impurities. The radius of Sr2+ (1.44 Å) cations is larger than that of average A-site (Bi3+/Na+) of 1.28 Å (Bi3+ (1.17 Å)/Na+ (1.39 Å))34. However, the complex substitution of Sr2+ cations for Bi3+ or Na+ cations in host lattice resulted in different behaviour, wherein Sr2+ cations substituted for Bi3+ cations generated the oxygen vacancies, whereas Sr2+ cations replacing for Na+ cations created the Na-vacancies35. Therefore, the Sr substitution in A-site was complex in distorted the lattice parameter. In addition, the Mn cations have multivalence states, e.g. as Mn2+, Mn3+ and Mn4+. Moreover, the spin state of each valence state influences the radius radii of cation Mn. Mn2+ cations have radii of 0.67 Å and 0.830 Å for low-spin and high-spin states, respectively34. The Mn3+ cations at low-spin and high-spin states have radii of 0.58 Å and 0.645 Å, respectively, whereas the Mn4+ cations with only high spin state have radii of 0.530 Å34. The Ti4+ cations in coordination number of VI have radii of 0.605 Å34. Therefore, if Mn cations exist at Mn3+ with low-spin state and Mn4+, then substitution for Ti-octahedral structure results in compressor lattice parameter and otherwise expands the lattice parameter of host Bi0.5Na0.5TiO3 materials. Thus, the distortion lattice parameter has a major influence on valence state and spin-state of Mn cations. However, the valence and spin state of Mn cations are very complex and strongly dependent on the chemical environment around the impurities of host materials and on the fabrication condition. Erdem et al. reported that Mn2+ and Mn3+ state existed in Bi0.5Na0.5TiO3-BaTiO3 materials36. Meanwhile, Li et al. reported that the mix Mn2+/Mn4+ valence states are obtained in Mn-doped Bi0.5Na0.5TiO3-BaTiO3-based material37. Hejazi et al. obtained the multivalence states of Mn such as Mn2+, Mn3+ and Mn4+ in Mn-doped Bi0.5Na0.5TiO3-based thin films38. Anthoniappen et al. reported that the Mn3+ possibly substituted at Ti-site, whereas Mn2+ was locally at the grain boundary39. Aksel et al. reported that the valence state of Mn changed from Mn3+ to Mn2+ while increasing the sintering temperature obtained in Mn-doped Bi0.5Na0.5TiO3 materials40. In addition, due to a lower valence state compared with Ti4+, the incorporation of Mn2+ and/or Mn3+ into the octahedral site of the structure produces excess negative charges, resulting thereby creating oxygen vacancies maintaining compensate for the maintenance of the overall electrical neutrality. Notably, the radius of oxygen vacancies (1.31 Å), which shrunk the lattice parameter, was smaller than that of oxygen anion (1.4 Å)41. The result indicated the XRD peak of host Bi0.5Na0.5TiO3 materials shifted after carrier SrMnO3−δ was used as the solid solution, thereby providing evidence for the incorporation of Sr and Mn into the host lattice. In other words, the SrMnO3−δ materials were good solid solutions in Bi0.5Na0.5TiO3 materials.
(a) X-ray diffraction spectra of pure Bi0.5Na0.5TiO3 and SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples with various concentrations of SrMnO3-δ, (b) magnification and deconvolution of X-ray diffraction spectra of pure Bi0.5Na0.5TiO3 and SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples in the 2θ range of 31°–35° with various concentrations, and (c) the dependent of lattice parameter of pure Bi0.5Na0.5TiO3 and SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples as function of SrMnO3-δ as solid solution.
The solute solution of SrMnO3−δ into host Bi0.5Na0.5TiO3 materials was further confirmed by using Raman scattering studies
Figure 4(a) shows the Raman scattering spectra of pure Bi0.5Na0.5TiO3 and SrMnO3−δ-modified Bi0.5Na0.5TiO3 as solid solution at various SrMnO3−δ concentrations. The pure Bi0.5Na0.5TiO3 sample exhibited broad band Raman scattering, which resulted from the random distribution of Bi and Na cations at A-site in perovskite structure42. However, the Raman scattering spectra of Bi0.5Na0.5TiO3 samples can be devised into three main regions in the wavenumber range of 300–1000 cm−1. The addition of SrMnO3−δaddition into Bi0.5Na0.5TiO3 materials caused to the occurrence of new vibration modes. We used the Lorentz fitting to estimate each vibration peak for pure Bi0.5Na0.5TiO3 and SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples. The results of distinguished vibration modes of pure Bi0.5Na0.5TiO3 and SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples with selected SrMnO3−δ concentration are shown in Fig. 4(b). The nice vibration modes were obtained for pure Bi0.5Na0.5TiO3 samples, and this finding was consistent with the theoretical prediction of Niranjan et al.42. The addition of the vibration modes at approximately 670 cm−1 was obtained for SrMnO3−δ-modified samples, and the intensity of peak increased with the increase of increasing SrMnO3−δ concentration. The appearance of new vibration modes was suggested for Mn substitution of Ti site to induce the MnO6 cluster vibration43,44. The band observed at approximately 528 cm−1 was relative to the breathing modes of TiO6 octahedral structure, which shifted to a high wavenumber for 5 mol% SrMnO3−δ dopants then shifted back to low wavenumber for 9 mol% SrMnO3−δ dopants. This result was consistent with the distorted XRD study for structural distortion of Bi0.5Na0.5TiO3 solid solution with SrMnO3−δ. The Bi/Na–O vibration at low vibration modes was not recorded due to the limitations of our experimental measurement setup. The structural XRD and Raman scattering study of SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples indicated that Mn possible substituted for the Ti site.
Optical properties
The solute solution of SrMnO3−δ into host Bi0.5Na0.5TiO3 materials results in reduction of optical band gap energy. Figure 5(a) shows the absorbance coefficient as function of absorption photon wavelength for pure Bi0.5Na0.5TiO3 and SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples with various SrMnO3−δ amounts. The single absorbance edge at approximately 400 nm with a small tail was obtained for pure Bi0.5Na0.5TiO3 samples. The small tail shown in the absorbance spectra of pure Bi0.5Na0.5TiO3 samples was related to self-defect and/or surface defect45. The addition of SrMnO3−δ-addition into host Bi0.5Na0.5TiO3 materials changed the optical properties of Bi0.5Na0.5TiO3 materials. The absorbance edge of SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples tended to shift to high wavelength and was not clearly shown due to contribution of absorbance peaks of impurity cations. In addition, the various absorbance peaks were obtained in the absorbance spectra of SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples, which were suggested for various transitions of the transition level energy of impurity cations. Bi0.5Na0.5TiO3 materials with direct transition, wherein in which the electronic band structures were constructed from Bi-6s, Ti-4s and O-2p for conduction band, and the bottom valence band mainly consisting of O-2p, Na-3s and Ti-3d orbitals were theoretically predicted46,47. Thus, we used Wood–Tauc method to estimate the value of optical band gap of pure Bi0.5Na0.5TiO3 and SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples. The optical band gap Eg can be obtained from the intercept of (αhν)1/n versus photon energy (hν), where α, ν and h are coefficient absorbance, wavelength and Plank constant, respectively. The band gap values were estimated with n = 1/2 for direct transition. The dependence of (αhν)1/2 to (hν) for pure Bi0.5Na0.5TiO3 and SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples is shown in Fig. 5(b). Eg of pure Bi0.5Na0.5TiO3 was estimated at approximately 3.07 eV, which were consistent with the recently reported optical band gap of that materials in the range 3.00–3.14 eV6,48. SrMnO3−δ addition into Bi0.5Na0.5TiO3 materials reduced the optical band gap to 1.81 eV for 9 mol% SrMnO3−δ. The details of dependent optical band gap values of Bi0.5Na0.5TiO3 as a function of SrMnO3−δ amount solid solution into Bi0.5Na0.5TiO3 are shown in the inset of Fig. 5(b). The reduction of optical band gap of Bi0.5Na0.5TiO3 was consistent with the recently reported Mn- or Cr-doped Bi0.5Na0.5TiO3 materials, which resulted from the occurrence of local energy state of transition metal in the middle of the electronic band structure of Bi0.5Na0.5TiO3 materials15,16. In addition, the created oxygen vacancies caused by unbalance charges between Mn2+/3+ with Ti4+ and/or Sr2+ with Bi3+ were flexible enough to reduce the optical band gap, because the oxygen vacancy state was locally near the conduction band49. The substitution of Sr2+, possibly acting as a donor, for Na+ at A-site created the Na-site vacancies.
(a) Absorbance spectra of pure Bi0.5Na0.5TiO3 and SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples with 0.5, 1, 3, 5, 7, and 9 mol.%, and (b) plot of (αhγ)2 values as a function of absorbance photon energy (hγ) for pure Bi0.5Na0.5TiO3 and SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples with various SrMnO3-δ amounts. Optical band gap energy as a function of SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples as solid solution is shown the inset of (b).
The addition of SrMnO3−δ into host Bi0.5Na0.5TiO3 materials as solid solution were suppressed the photoluminescence
Figure 6(a) shows the photoluminescence (PL) emission spectra of pure Bi0.5Na0.5TiO3 and SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples at room temperature. The spectra of pure Bi0.5Na0.5TiO3 samples clearly showed a broad blue emission band within 476–510 nm relative with various transitions. The addition of SrMnO3−δ-addition into Bi0.5Na0.5TiO3 materials as solid solution decreased the intensity of PL emission. In addition, new emission peaks appearing at approximately 489 nm, and the intensity were increased with the increase of increasing SrMnO3−δ amounts, as shown in the inset of Fig. 6(a) after the standard unit. We tried to distinguish each contribution PL peak via the Lorentz fitting. The results were shown in the examples for undoped Bi0.5Na0.5TiO3 and 9 mol% SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples, as exhibited in Fig. 6(b) for down and up separation figures, respectively. The contribution of various PL peaks is unclear and requires further theoretical investigation. Normally, the PL of ferroelectric materials was not simply from band-to-band transition. The combination of photo-electron–hole pairs was difficult to combine due to the separation of the nature polarisation of electrical domain in materials. However, the surface states are often regarded as the dominant cause of luminescence in perovskites. A large number of unsaturated atoms exist on the surface of the perovskites, thereby forming localised levels within the forbidden gaps of the materials. Recently, Bac et al. suggested that the observation of PL of Bi0.5K0.5TiO3 materials was related to the disorder coupled with the tilt of TiO6-TiO6 adjacent octahedral structure, thereby resulting in structural distortion and generation of localised electronic levels above the valence band45. The re-appearance of new photoluminescent peaks was suggested reliving with the new vibration of MnO6-TiO6 or MnO6-MnO6 adjacent octahedral structure, as exhibited in the Raman scattering results. The replacement of Mn2+/3+ cations for Ti4+ cations at B-site and Sr2+ cation for Bi3+ cations at A-site created oxygen vacancies, which trapped the electron generated from absorbance photon energy and prevented the recombination of the electron–holes to generate photon, suppressing the PL intensity of SrMnO3−δ-modified Bi0.5Na0.5TiO3 samples. In addition, the substitution of Sr2+ cations for the resulting Na+ cations created the Na vacancies, which also acted as chapping for electrons, thereby reducing the combination of the photon-electron–hole pair.
(a) Photoluminescence of pure Bi0.5Na0.5TiO3 and SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples with various SrMnO3-δ concentrations at room temperature, and (b) deconvolution photoluminescence peaks for pure Bi0.5Na0.5TiO3 and 9 mol% SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples. Inset of (a) shows the comparison of the photoluminescence peak positions of pure Bi0.5Na0.5TiO3 and SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples as solid solution with various concentrations of SrMnO3-δ after substrate photoluminescence intensity to the unit.
Magnetic properties
The complex magnetic properties at room temperature of Bi0.5Na0.5TiO3 materials were obtained as function of SrMnO3−δ solute solution. Figure 7 show the magnetization as function of applied magnetic field (M–H) for pure Bi0.5Na0.5TiO3 samples and SrMnO3−δ-modified Bi0.5Na0.5TiO3 sample with various SrMnO3−δ amounts from 0.5 to 9 mol.%. The pure Bi0.5Na0.5TiO3 materials exhibited the anti-S-shape in M–H curves, which resulted from the combination of diamagnetic and weak ferromagnetic properties. The diamagnetic property of Bi0.5Na0.5TiO3 materials behaviour originated from the empty state of Ti4+ cations with 3d° states15,16. The weak-ferromagnetism observation in pure Bi0.5Na0.5TiO3 materials was possibly related with self-defect and/or surface effects, which were well explained by the first-principle theoretical prediction and experimental achievement15,16,17,50. The anti-S-shape in M–H curve was obtained for 0.5 mol% SrMnO3−δ solid solution into Bi0.5Na0.5TiO3 sample. The saturation trend in magnetisation for SrMnO3−δ-doped Bi0.5Na0.5TiO3 with 1 mol% SrMnO3−δ. Further addition of the SrMnO3−δ concentration into Bi0.5Na0.5TiO3 materials resulted in unsaturation of magnetisation with low applied magnetic field. The slight addition of SrMnO3−δ into Bi0.5Na0.5TiO3 induced the ferromagnetism due to the interaction of Mn2+/3+ through oxygen vacancies (□)49. Furthermore, the solid solution of SrMnO3−δ into Bi0.5Na0.5TiO3 samples enhanced the ferromagnetic ordering due to several favourable Mn2+/3+-□-Mn2+/3+. In addition, the vacancies of such Na-vacancies, which causes the substitution of Sr2+ for Na+ at A-site in perovskite structure, influenced the ferromagnetism in samples14,17. Moreover, if Sr2+ cations substituted for Bi3+ cations were possible resulted in changing the valence state of Ti4+ to Ti3+ state cause of enhancement the number of oxygen vacancies35. Our recently predicted that the Ti3+-defects state in Bi0.5K0.5TiO3 materials was strong induced the ferromagnetism51. However, the unsaturation in magnetisation as a function of low applied magnetic field via further addition of SrMnO3−δ (over 3 mol%) into Bi0.5Na0.5TiO3 materials was possibly related to the isolation of Mn cations, which favoured of paramagnetic property or/and interaction of polaron (Mn2+/3+-□-Mn2+/3+) vs. (Mn2+/3+-□-Mn2+/3+), which favoured the antiferromagnetic-like materials52. The magnetisation of 9 mol%-doped Bi0.5Na0.5TiO3 samples was achieved at approximately 12.5 memu/g at room temperature, which was greatly enhanced compared with pure Bi0.5Na0.5TiO3 materials15,16. The values were also larger than that of Mn-, Fe-, Cr- and Co-doped Bi0.5Na0.5TiO3 materials15,16,18,19. Thus, we suggested that the co-modification at A-site and B-site via alkalize and transition metals, respectively, displayed higher performance magnetic properties than single transition metal-doped Bi0.5Na0.5TiO3 materials. However, the role of A-site-modification on the magnetic properties of Bi0.5Na0.5TiO3-dope-material with transition metal needs further theoretical calculated investigation.
Discussion
The new system Bi0.5Na0.5TiO3-SrMnO3−δ solid solution materials were fabricated via sol–gel method. X-ray diffraction and Raman scattering were used to study the structure of pure Bi0.5Na0.5TiO3 and SrMnO3−δ-modified Bi0.5Na0.5TiO3 materials with various SrMnO3-δ amount, providing that all samples followed the crystal structural symmetry of host rhombohedral structure of Bi0.5Na0.5TiO3 materials. This phenomenon indicated that the SrMnO3−δ materials were good solid solution in host Bi0.5Na0.5TiO3 crystal structure. The Sr and Mn cations were diffused to random incorporation with host lattice of Bi0.5Na0.5TiO3 crystal to form as solid solution, resulting in complex-distorted structure. The random distribution of Sr2+ cations into A-site of Bi0.5Na0.5TiO3 materials was possibly different, indicating that Sr2+ cations substituted for Bi3+ cations generate the oxygen vacancies, whereas the Sr2+ cations replaced for Na+ create the Na vacancies. The presentation of complex defects in Bi0.5Na0.5TiO3 materials during solid solution of SrMnO3−δ reduced the optical band gap values from approximately 3.07 eV to 1.18 eV for 9 mol% SrMnO3−δ solid solution. The absorbance spectroscopy of SrMnO3-modified Bi0.5Na0.5TiO3 materials exhibited the multi-absorbance peaks in visible absorbance range, presenting the multivalence state of Mn cations, such as Mn2+, Mn3+, and Mn4+. Thus, the modified B-site by multivalence state of Mn cations also possibly exhibited the different interactions, wherein the Mn2+/3+ cation interaction through the oxygen vacancies (Mn2+/3+-□-Mn2+/3+) resulted in ferromagnetic ordering, whereas Mn4+ cation interaction through oxygen (Mn4+-O2−-Mn4+) was favourable to antiferromagnetic ordering. Mn cation isolates incorporated with host lattice displayed the paramagnetic behaviour. The possible antiferromagnetic-like structure started to occur when the Mn cations were rich enough to bind together the superinteraction of pair (Mn2+/3+-□-Mn2+/3+) vs. (Mn2+/3+-□-Mn2+/3+). Therefore, by controlling the SrMnO3−δ concentration doping in host lattice Bi0.5Na0.5TiO3 materials, the magnetic properties of Bi0.5Na0.5TiO3 materials were tuned from compensationof diamagnetic and weak ferromagnetic property of pure materials to typical ferromagnetism behaviour and at the end of combination of paramagnetism/antiferromagnetism-like versus ferromagnetism with the increase of the SrMnO3−δ amount solid solution into host Bi0.5Na0.5TiO3 materials. We expected that co-modification at the A-site and B-site in lead-free ferroelectric perovskite ABO3 materials via alkali earth and transition metals, respectively, resulted in great enhancement of the ferromagnetism than that of contribution of self-defect and/or surface effects, or by using single transition metal dopants. We also expected that our method opened the new way to develop injection ferromagnetism in lead-free ferroelectric materials, such as BaTiO3-based and (K,Na)NbO3-based family, by using solid solution method. The observation of tuneable magnetic and optical properties of lead-free ferroelectric material was promising for application to green electronic devices.
Methods
Sample preparation
The pure Bi0.5Na0.5TiO3 compounds were fabricated from material source of bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), sodium nitrate (NaNO3) and tetraisopropoxytitanium (IV, C12H28O4Ti). The Bi(NO3)3·5H2O and NaNO3 were weighed and distinguished in acetic acid and de-ions water H2O (VH2O:VCH3COOH = 5: 1). The acetylacetone was added dropwise before the addition of C12H28O4Ti. The solution was magnetically stirred at approximately 3 h and heated followed by at100 °C to prepare the gel. The (x)SrMnO3−δ + (1−x)Bi0.5Na0.5TiO3 (x = 0.5, 1, 3, 5, 7, 9 mol%) compounds were fabricated by using a similar method of fabrication with pure Bi0.5Na0.5TiO3. However, the starting materials were Sr(NO3)2 and a solution of Mn(NO3)3 (60%). These materials were weighed, and the solution was added by estimating the dopant concentrations. The gels were ground and annealed for 5 h at 800 °C and then naturally cooled to room temperature. Extra sodium was added at approximately 50 mol% to prevent sodium loss during gelling and annealing processing24,25.
Sample characterization
The surface morphology and presentation of the elements in samples was measured by Energy Dispersive X-ray analysis (EDX, S-4800 Hitachi). The chemical composition of pure Bi0.5Na0.5TiO3 samples and SrMnO3−δ-modified Bi0.5Na0.5TiO3 compounds was further confirmed by using an electron probe micro-analyzer (EPMA, Shimadzu EPMA 1600). The sample powder was ground for characterisation via X-ray diffraction (XRD, Brucker D8 Advance) and Raman scattering (with a 475 nm LASOS laser and a DU420A-Oe defector) to analyse the crystal structure and vibration mode of the atom, respectively. The absorbance spectroscopy and photoluminescent properties of pure and SrMnO3−δ-modified Bi0.5Na0.5TiO3 compounds were measured by using Ultraviolet–Visible spectroscopy (UV–Vis, Jasco V-670) and photoluminescence (exciter with 475 m LASOS laser and a DU420A-Oe defector), respectively. The magnetic properties were studied using a Vibrating Sample Magnetometry (VSM, Lakeshore 7404) at room temperature, respectively. Finally, we used X-ray photoelectron spectroscopy (XPS, Thermofisher, a twin anode X-ray source (Al Kα, hν =1686.6 eV) gun and monochromatic gun) to determine the valence state of the cations in the pure Bi0.5Na0.5TiO3 and 9 mol.% SrMnO3-δ-modified Bi0.5Na0.5TiO3 samples, as shown in Figure S1 and Figure S2, respectively, in suplimental data. The X-ray diffraction peaks, Raman scattering peaks, XPS peaks and photoluminescence peaks were distinguished by using Lorentzian fitting with r-square over 0.99.
Change history
22 January 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Reichmann, K., Feteira, A. & Li, M. Bismuth sodium titanate based materials for piezoelectric actuato. Materials 8, 8467–8495 (2015).
Quan, N. D., Bac, L. H., Thiet, D. V., Hung, V. N. & Dung, D. D. Current development in lead-free-based piezoelectric materials. Adv. Mater. Sci. Eng. 2014, 365391 (2014).
Smolenskii, G. A., Isupov, V. A., Agranovskaya, A. I. & Krainik, N. N. New ferroelectrics of. complex composition. Sov. Phys. Solid State 2, 2651–2654 (1961).
Guo, F. F. et al. Morphotropic phase boundary and electric properties in (1−x)Bi0.5Na0.5TiO3-xBiCoO3 lead-free piezoelectric ceramics. J. Appl. Phys. 111, 124113 (2012).
Pattipaka, S., Mahesh, P. & Pamu, D. Structural and dielectric properties of lead free Bi0.5Na0.5TiO3 ceramics. AIP Conf. Proc. 1728, 020352 (2016).
Thanh, L. T. H. et al. Influence of sintering temperature on phase formation and optical properties of lead-free ferroelectric Bi0.5Na0.5TiO3. materials. J. Sci. Tech. 54, 104–111 (2016).
Hong, C. H. et al. Lead-free piezoceramics – Where to move on? J. Materomics 2, 1–24 (2016).
Koruza, J. et al. Requirements for the transfer of lead-free piezoceramics into application. J. Materomics 4, 13–26 (2018).
Liu, F., Wahyudi, O. & Li, Y. A new Bi0.5Na0.5TiO3 based lead-free piezoelectric system with calculated end-member Bi(Zn0.5Hf0.5)O3. J. Appl. Phys. 115, 114101 (2014).
Ullah, A. et al. High strain response in ternary Bi0.5Na0.5TiO3–BaTiO3–Bi(Mn0.5Ti0.5)O3 solid solutions. RSC Adv. 6, 63915–63921 (2016).
Bai, W. et al. Structure and electromechanical properties in Bi0.5Na0.5TiO3-based lead-free piezoceramics with calculated end-member Bi(Ni0.5Ti0.5)O3. J. European Ceram. Soc. 35, 3457–3466 (2015).
Bai, W. et al. Phase evolution and correlation between tolerance factor and electromechanical properties in BNT-based ternary perovskite compounds with calculated end-member Bi(Me 0.5Ti0.5)O3 (Me = Zn, Mg, Ni, Co). Dalton Trans. 45, 14141–14153 (2016).
Li, H. L. et al. Grain size dependent electrostrain in Bi1/2Na1/2TiO3-SrTiO3 incipient piezoceramics. J. European Ceram. Soc. 36, 2849–2853 (2016).
Ju, L. et al. Room-temperature magnetoelectric coupling in nanocrystalline Na0.5Bi0.5TiO3. J. Appl. Phys. 116, 083909 (2014).
Thanh, L. T. H. et al. Origin of room temperature ferromagnetism in Cr-doped lead-free ferroelectric Bi0.5Na0.5TiO3 materials. J. Electron. Mater. 46, 3367–3372 (2017).
Thanh, L. T. H. et al. Making room-temperature ferromagnetism in lead-free ferroelectric Bi0.5Na0.5TiO3 materials. Mater. Lett. 186, 239–242 (2017).
Zhang, Y., Hu, J., Gao, F., Liu, H. & Qin, H. Ab initio calculation for vacancy-induced magnetism in ferroelectric Na0.5Bi0.5TiO3. Comput. Theor. Chem. 967, 284–288 (2011).
Wang, Y. et al. Room-temperature ferromagnetism in Fe-doped Na0.5Bi0.5TiO3. crystals. Mater. Sci. Poland 27, 471–476 (2009).
Wang, Y. et al. Room-temperature ferromagnetism in Co-doped Na0.5Bi0.5TiO3: diluted magnetic ferroelectrics. J. Alloys Compound. 475, L25–L30 (2009).
Wu, X. et al. Luminescent–electrical–magnetic performances of sol–gel-derived Ni2+-modified Bi0.5Na0.5TiO3. J. Mater. Sci.: Mater. Electron. 28, 12021–12025 (2017).
Kumari, M., Singh, A., Gupta, A., Rrahash, C. & Chatterjee, R. Self-biased large magnetoelectric coupling in co-sintered Bi0.5Na0.5TiO3 based piezoelectric and CoFe2O4 based magnetostrictive bilayered composite. J. Appl. Phys. 116, 244101 (2014).
Manjusha, Y. K. L., Adhlakha, N., Shah, J. & Kotnala, R. K. Strain mediated magnetoelectric coupling induced in (x)Bi0.5Na0.5TiO3-(1−x)MgFe2O4 composites. Physica B 514, 41–50 (2017).
Zhang, R. F., Deng, C. Y., Ren, L., Li, Z. & Zhou, J. P. Ferroelectric, ferromagnetic, and magnetoelectric properties of multiferroic Ni0.5Zn0.5Fe2O4–BaTiO3 composite ceramics. J. Electron. Mater. 43, 1043–1047 (2014).
Hung, N. T. et al. Room-temperature ferromagnetism in Fe-based perovskite solid solution in lead-free ferroelectric Bi0. 5Na0.5TiO3 materials. J. Magn. Magn. Mater. 451, 183–186 (2018).
Hung, N. T. et al. Structural, optical, and magnetic properties of SrFeO3-δ-modified Bi0.5Na0.5TiO3 materials. Physica B 531, 75–78 (2018).
Kuroda, K., Shinozaki, K., Uematsu, K., Mizutani, N. & Kato, M. Oxygen-deficiency-induced polymorphohs and electrical conductivity of SrMnO3-x. J. American Ceram. Soc. 63, 109–110 (1980).
Kobayashi, S. et al. Quantitative analyses of oxidation states for cubic SrMnO3 and orthorhombic SrMnO2.5 with electron energy loss spectroscopy. J. Appl. Phys. 108, 124903 (2010).
Belik, A. A. et al. Crystal structure and magnetic properties of 6H-SrMnO3. Phys. Rev. B 84, 094438 (2011).
Suescun, L., Chmaissem, O., Mais, J., Daborwski, B. & Jorgensen, J. D. Crystal structures, charge and oxygen-vacancy ordering in oxygen deficient perovskites SrMnOx (x < 2.7). J. Solid State Chem. 180, 1698–1707 (2007).
Kamba, S. et al. Strong spin-phonon coupling in infrared and Raman spectra of SrMnO3. Phys. Rev. B 89, 064308 (2014).
Rahman, M., Zhou, K. C., Nie, Y. Z. & Guo, G. H. Electronic structure and magnetism of layered compounds SrBO2 (B = Ni, Co, Mn): A theoretical investigation. Solid State Commun. 266, 6–10 (2017).
Zhou, C., Liu, X., Li, W. & Yuan, C. Structure and piezoelectric properties of Bi0.5Na0.5TiO3-Bi0.5K0.5TiO3-BiFeO3 lead-free piezoelectric ceramics. Mater. Chem. Phys. 114, 832 (2009).
Tuan, N. H. et al. Structural, optical, and magnetic properties of lead-free ferroelectric Bi0.5K0.5TiO3 solid solution with BiFeO3 materials. J. Electron. Mater. 46, 3472–3478 (2017).
Shannon, R. D. & Prewitt, C. T. Effective ionic radii in oxides and fluorides. Acta Cryst. B 25, 925–946 (1969).
Qiao, Y. et al. Local order and oxygen ion conduction induced high-temperature colossal permittivity in lead-free Bi0.5Na0.5TiO3-based systems. ACS Appl. Energy Mater. 1, 956–962 (2018).
Erdem, E. et al. analysis of MnO2-doped [Bi0.5Na0.5]TiO3-BaTiO3 piezoelectric ceramics – manganese oxidation states and materials ‘hardening’. Ferroelectrics 428, 116–121 (2012).
Li, F., Zhai, J., Shen, B., Liu, X. & Zeng, H. Simultaneously high-energy storage density and responsivity in quasi-hysteresis-free Mn-doped Bi0.5Na0.5TiO3-BaTiO3-(Sr0.7Bi0.2□0.1)TiO3 ergodic relaxor ceramics. Mater. Res. Lett. 6, 345–352 (2018).
Hejazi, M. H., Taghaddos, E. & Safari, A. Reduced leakage current and enhanced ferroelectric properties in Mn-doped Bi0.5Na0.5TiO3-based thin films. J. Mater. Sci. 48, 3511–3516 (2013).
Anthoniappen, J. et al. Raman spectra and structural stability in B-site manganese doped (Bi0.5Na0.5)0.925Ba0.075TiO3 relaxor ferroelectric ceramics. J. European Ceram. Soc. 35, 3495–3506 (2015).
Aksel, E. et al. Processing of manganese‐doped [Bi0.5Na0.5]TiO3 ferroelectrics: reduction and oxidation reactions during calcination and sintering. J. Am. Ceram. Soc. 94, 1363–1369 (2011).
Chatzichristodoulou, C., Norby, P., Hendriksen, P. V. & Mogensen, M. B. Size of oxide vacancies in fluorite and perovskite structured oxides. J. Electroceram. 34, 100–107 (2015).
Niranjan, M. K., Karthik, T., Asthana, S., Pan, J. & Waghmare, U. V. Theoretical and experimental investigation of raman modes, ferroelectric and dielectric properties of relaxor Na1/2Bi1/2TiO3. J. Appl. Phys. 113, 194106 (2013).
Guowei, Z., Youngsoo, K., Tianduo, L. & Guiying, X. Sol-gel preparation and spectroscopic study of the pyrophanite MnTiO3 nanoparticles. Sci. China Ser. B. Chem. 48, 210–215 (2005).
Awan, M. Q. et al. limit and its effects on physical properties of lead-free Bi0.5Na0.5TiO3 ceramics. Ceram. Inter. 44, 12767–12773 (2018).
Bac, L. H. et al. Tailoring the structural, optical properties and photocatalytic behavior of ferroelectric Bi0.5K0.5TiO3 nanopowders. Mater. Lett. 164, 631–635 (2016).
Baedi, J. & Mircholi, F. The study of the electronical properties of BiTiO3 crystal by substitution of Na atom. Optik 127, 1503–1506 (2016).
Baedi, J., Gholampur, S. & Mircholi, F. The study of the electronic properties of Bi0.5Na0.5TiO3, Bi0.5Na0.5Ti0.5Zr0.5O3 and Bi0.5Na0.5ZrO3 compounds and comparing their structures. Optical Mater. 67, 44–51 (2017).
Thanh, L. T. H., Tuan, N. H., Bac, L. H. & Dung, D. D. Influence of fabrication condition on the microstructural and optical properties of lead-free ferroelectric Bi0.5Na0.5TiO3 materials. Commun. Phys. 26, 51–57 (2016).
Tuan, N. H. et al. Defect induced room temperature ferromagnetism in lead-free ferroelectric Bi0.5K0.5TiO3 materials. Physica B 532, 108–114 (2018).
Ju, L. et al. Room-temperature magnetoelectric coupling in nanocrystalline Na0.5Bi0.5TiO3. J. Appl. Phys. 116, 083909 (2014).
Tuan, N. H. et al. Theoretical and experimental studies on the influence of Cr incorporation on the structural, optical, and magnetic properties of Bi0.5K0.5TiO3 materials. J. Sol-Gel Sci. Tech. 87, 528–536 (2018).
Tuan, N. H., Linh, N. H., Odkhuu, D., Trung, N. N. & Dung, D. D. Microstructural, optical, and magnetic properties of BiCoO3-modified Bi0.5K0.5TiO3. J. Electron. Mater. 47, 3414–3420 (2018).
Acknowledgements
This work was financially supported by The Ministry of Science and Technology, Viet Nam, under project number ĐTĐLCN.29/18.
Author information
Authors and Affiliations
Contributions
D.D.D. and D.O. conceived the idea and designed the experiments. N.T.H. performed the experiments and measurements. D.D.D. wrote the paper. D.O. reviewed and commented on the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dung, D.D., Hung, N.T. & Odkhuu, D. Structure, optical and magnetic properties of new Bi0.5Na0.5TiO3- SrMnO3−δ solid solution materials. Sci Rep 9, 18186 (2019). https://doi.org/10.1038/s41598-019-54172-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54172-4
This article is cited by
-
Enhanced room-temperature ferromagnetism and tuned band gap in AlCoO3-modified lead-free Bi0.5Na0.5TiO3
Journal of Sol-Gel Science and Technology (2025)
-
Optical, Magnetic, and Electrical Properties of New Binary CoTiO3-Modified Ba(Zr0.2Ti0.8)O3 System as Solid Solution
Journal of Electronic Materials (2024)
-
Fabrication and Optical Properties of (1−x)Bi½Na½TiO3−xEr½Na½TiO3 Solid Solution System
Journal of Electronic Materials (2023)
-
Enhanced room temperature ferromagnetism in YMnO3-modified lead-free ferroelectric Bi0.5Na0.5TiO3 materials
Applied Physics A (2023)
-
Magnetic properties of new (1−x)Bi1/2Na1/2TiO3+xBaNiO3−δ solid solution materials
Applied Physics A (2022)