Abstract
Staphylococcus epidermidis infections are a common occurrence in hospitals, particularly in catheter-related bloodstream and surgical site infections and infective endocarditis. Higher daptomycin minimum inhibitory concentration (MIC) values may be associated with daptomycin treatment failure among patients with S. epidermidis infections. We therefore conducted a retrospective cohort study to determine the predictive value of daptomycin susceptibility. A retrospective study was undertaken in 1,337 patients with S. epidermidis infections. Data were collected from 1 January 2013 to 31 December 2016 at Ehime University Hospital, and included the following clinicopathological factors for evaluation: age, sex, resistance to vancomycin or teicoplanin, and history of antimicrobial therapy. Multiple analysis was performed using logistic regression to identify factors that independently and significantly affected the daptomycin resistance. Daptomycin-resistant S. epidermidis was identified in 38 (2.8%) patients. According to the multiple analysis, only higher MIC values (≥16 mg/L) for teicoplanin (P < 0.0001) were independently associated with an increased risk of developing daptomycin resistance. In conclusion, higher teicoplanin MIC values may predict resistance to daptomycin treatment in S. epidermidis infections.
Similar content being viewed by others
Introduction
Daptomycin, a cyclic polypeptide and a semisynthetic lipopeptide antibiotic, is derived from Streptomyces roseosporus1. The native molecule is anionic, although binding to the bacterial membrane and exploitation of the bactericidal activity that is typical of daptomycin require calcium. Daptomycin exhibits bactericidal activity by penetrating the bacterial cell wall and binding to the cytoplasmic membrane, thereby causing rapid depolarization of the membrane. This results in the loss of membrane potential and bacterial cell death2. Because of the unique mechanism of action, daptomycin-resistance has rarely been documented. However, treatment failure due to daptomycin resistance was observed in a patient with high-grade methicillin-sensitive Staphylococcus aureus (MRSA) bacteraemia3,4.
Staphylococcus epidermidis infections, particularly catheter-related bloodstream and surgical site infections and infective endocarditis, are common in hospitals5,6. S. epidermidis mostly engages beneficially in the host defence and immune maturation system but may show infective behaviour under certain circumstances7. Although a reduction in the susceptibility of S. aureus to daptomycin is well established, that of S. epidermidis is unknown. A high minimum inhibitory concentration (MIC) of daptomycin may contribute to treatment failure in patients with S. epidermidis, especially methicillin-resistant S. epidermidis (MRSE), infections. We, therefore, conducted a retrospective cohort study to determine the predictive value of daptomycin susceptibility.
Results
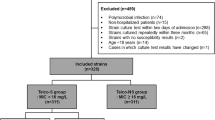
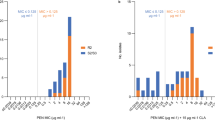
The data from 1,337 patients were included in this study, of whom, 1,299 (97.2%) had a lower daptomycin MIC (≤1 mg/L) for S. epidermidis and 38 (2.8%) had a higher daptomycin MIC (MIC >1 mg/L) for S. epidermidis. These patients were categorized into low (≤1 mg/L; sensitive) and high (>1 mg/L; resistant) MIC groups. A comparison of the demographic characteristics between the two groups is shown in Table 1. The groups were equivalent with respect to age, sex, implantation, vancomycin susceptibility, and prior use of linezolid, cephem, aminoglycoside, fluoroquinolone, and sulfamethoxazole/trimethoprim. However, there were significant differences in terms of prior use of glycopeptide (P = 0.003), daptomycin (P = 0.018), penicillin (P = 0.016), carbapenem (P = 0.014), and tetracycline (P = 0.016), as well as teicoplanin susceptibility (P < 0.0001).
The relationships between the study variables and daptomycin resistance are shown in Table 2. In the crude logistic regression analysis, a higher teicoplanin MIC value (P < 0.0001) and prior use of glycopeptide (P = 0.005), daptomycin (P = 0.023), penicillin (P = 0.018), carbapenem (P = 0.016), and tetracycline (P = 0.020) were associated with increased risk of developing daptomycin resistance. In the multiple analysis, only higher teicoplanin MIC value was independently associated with an increased risk of daptomycin resistance (adjusted OR = 8.01, 95% CI = 3.86–16.18, P < 0.0001). Additionally, the MIC of daptomycin was significantly positively correlated with that of teicoplanin (Pearson correlation coefficient (r) = 0.55, P < 0.0001).
Discussion
Our findings demonstrate that a lower susceptibility to teicoplanin was independently associated with an increased risk of lower susceptibility to daptomycin in patients with S. epidermidis infection. Teicoplanin, a glycopeptide, currently used for the treatment of potentially serious infections caused by β-lactam-resistant gram-positive pathogens, is not approved in the United States. However, in Europe, Japan, and Taiwan, it is commonly prescribed as vancomycin. Teicoplanin is considered as effective as vancomycin for the treatment of MRSA bacteraemia8. In cases of MRSE infection, glycopeptides or daptomycin is used for the treatment of MRSA.
Daptomycin resistance has been reported in cases of bacteraemia, endocarditis, osteomyelitis, skin and soft-tissue infections, and prosthetic joint infection. Where daptomycin non-susceptibility developed, other antibiotics were used previously (mainly vancomycin or teicoplanin)4. Thus, a vast majority of cases of daptomycin resistance were reported in patients previously treated with a glycopeptide antibiotic. The heterogeneous vancomycin-intermediate S. aureus (hVISA) with a vancomycin MIC of 1–2 mg/L were resistant to daptomycin. However, a higher MIC (≥2 mg/L) of vancomycin or other antimicrobials did not affect daptomycin resistance in this study. The majority of previous reports did not describe the mechanism of daptomycin resistance. A previous study on vancomycin resistance in VISA strains, however, indicated that a thickened cell wall was a common characteristic among the VISA strains, serving as a physical barrier against the penetration of vancomycin molecules and resulting in vancomycin resistance9,10. Furthermore, Garcia et al. reported that MRSE was the leading cause of coagulase-negative staphylococcal infective endocarditis, and patients receiving vancomycin had higher mortality rates than those receiving cloxacillin. The mortality was higher among patients having isolates with a vancomycin MIC of ≥2 mg/L11. Several mechanisms contributing to the reduced susceptibility of Staphylococci to daptomycin have been reported, many of which describe a common resistance mechanism of S. aureus and S. epidermidis to antibiotics4,12. The mechanism of S. epidermidis resistance is similar to that of VISA and hVISA strains13,14. A possible explanation of this correlation is that the thickened cell wall acts as a common barrier to daptomycin and teicoplanin penetration, although daptomycin does not bind to peptidoglycan to form a subsequent physical barrier within the cell wall15,16. As daptomycin (molecular weight 1620.67 Da) and teicoplanin (molecular weight 1564.25–1893.68 Da) are relatively larger in molecular size as compared to vancomycin (molecular weight 1485.7 Da), we suggest that daptomycin might not be able to efficiently penetrate the cell wall if it is as thick as that of S. epidermidis. In this case, daptomycin might be impeded by the thickened cell wall before reaching the cytoplasmic membrane resulting in ineffective bactericidal function in the target cells.
Furthermore, the ability of S. epidermidis to produce biofilms ensures significant resistance to antibiotics and impairs the host innate immune response6. In these situations, the use of an antimicrobial agent capable of crossing the biofilm enhances the treatment efficacy. In this context, the ability of daptomycin to penetrate rapidly into the biofilm produced by S. epidermidis is well known17. However, daptomycin treatment is not suitable for daptomycin-resistant S. epidermidis infection. In this study, patients with bloodstream infections caused by daptomycin resistant S. epidermidis were mainly treated with vancomycin. Hence, there was no difference in the treatment duration between the sensitive (12.2 days) and resistant (13.0 days) groups. There are some limitations to our study, including the retrospective design. Furthermore, genetic analysis of S. epidermidis was not performed. Due to the very small number of patients in the resistance group (n = 38), our confidence interval was wide which provides a lesser statistical certainty, and hence, the positive significant results might have happened by chance.
In conclusion, a higher teicoplanin MIC may predict the resistance of S. epidermidis to daptomycin. A more effective therapeutic strategy against daptomycin-resistant S. epidermidis bacteraemia, such as initial regimens that combine different classes of antimicrobial agents or early transition to an alternative treatment, is required. The true significance of the clinical activity of daptomycin against teicoplanin-resistant S. epidermidis warrants further explanation and investigation in future clinical studies.
Patients and Methods
Ethical approval of the study protocol
The study was carried out in accordance with the guidelines on human studies adopted by the ethics committee of Ehime University Hospital (Ehime, Japan; review board approval no. 1602008), the Declaration of Helsinki, and the Ethical Guidelines on Medical and Health Research Involving Human Subjects by the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare of Japan. The Japanese law does not mandate obtaining individual informed consent from participants in non-invasive observational trials, such as the present study. Therefore, we used our official pharmacy website as an opt-out method rather than obtaining written or verbal informed consent from each patient.
Patients and study design
The data were collected from 1 January 2013 to 31 December 2016 at Ehime University Hospital. In this study, a total of 1699 patients with S. epidermidis infection were included and 362 patients were excluded due to missing data on daptomycin MIC values. The data of 1337 patients with S. epidermidis infection available for analysis in a retrospective study (participation rate, 78.7%). As compared to the excluded patients, the study patients were more likely to use glycopeptide, daptomycin, and cephem, and less likely to show a higher teicoplanin susceptibility (MIC ≥16 mg/L) or use fluoroquinolone and sulfamethoxazole/trimethoprim. The following clinicopathological factors were obtained from the medical charts: age, sex, implantation, resistance to vancomycin or teicoplanin, and history of antimicrobial therapy. Where one or more samples were determined as resistant, the patients were included in the resistant group. The criteria for MIC value were according to the CLSI M100 S28 guidelines. According to the CLSI guidelines, an MIC of ≤4 mg/L signifies sensitivity to vancomycin. However, a low success rate of vancomycin therapy with a vancomycin MIC of ≥2 mg/L has previously been reported18,19. Therefore, patients with a vancomycin MIC of ≥2 mg/L were included in the lower susceptibility group. The determination of MIC was carried out using the standard broth microdilution method.
Statistical analysis
χ2-test and Fisher’s exact test were used to compare the categorical variables. Using logistic regression analysis, we calculated the crude and adjusted odds ratios (ORs) with their 95% confidence intervals (CIs). Parameters with a P-value of < 0.01 in the crude analyses were considered for inclusion in the multivariate model. All statistical analyses were performed using the SAS software package version 9.4 (SAS Institute, Inc., NC, USA).
References
Tally, F. P. et al. Daptomycin: a novel agent for Gram-positive infections. Expert opinion on investigational drugs 8, 1223–1238, https://doi.org/10.1517/13543784.8.8.1223 (1999).
Schriever, C. A., Fernandez, C., Rodvold, K. A. & Danziger, L. H. Daptomycin: a novel cyclic lipopeptide antimicrobial. Am. J. Health Syst. Pharm. 62, 1145–1158 (2005).
Mangili, A., Bica, I., Snydman, D. R. & Hamer, D. H. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 40, 1058–1060, https://doi.org/10.1086/428616 (2005).
Stefani, S. et al. Insights and clinical perspectives of daptomycin resistance in Staphylococcus aureus: A review of the available evidence. Int. J. Antimicrob. Agents 46, 278–289, https://doi.org/10.1016/j.ijantimicag.2015.05.008 (2015).
Piette, A. & Verschraegen, G. Role of coagulase-negative staphylococci in human disease. Vet. Microbiol. 134, 45–54, https://doi.org/10.1016/j.vetmic.2008.09.009 (2009).
Uckay, I. et al. Foreign body infections due to Staphylococcus epidermidis. Ann. Med. 41, 109–119, https://doi.org/10.1080/07853890802337045 (2009).
Otto, M. Staphylococcus epidermidis–the ‘accidental’ pathogen. Nature reviews. Microbiology 7, 555–567, https://doi.org/10.1038/nrmicro2182 (2009).
Lin, S. H., Lai, C. C., Tan, C. K., Liao, W. H. & Hsueh, P. R. Comparative efficacy of vancomycin and teicoplanin in the treatment of hospitalised elderly patients with persistent meticillin-resistant Staphylococcus aureus (MRSA) bacteraemia. Int. J. Antimicrob. Agents 37, 179–181, https://doi.org/10.1016/j.ijantimicag.2010.10.018 (2011).
Cui, L., Tominaga, E., Neoh, H. M. & Hiramatsu, K. Correlation between Reduced Daptomycin Susceptibility and Vancomycin Resistance in Vancomycin-Intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50, 1079–1082, https://doi.org/10.1128/aac.50.3.1079-1082.2006 (2006).
Cui, L. et al. Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 50, 428–438, https://doi.org/10.1128/aac.50.2.428-438.2006 (2006).
Garcia de la Maria, C. et al. Epidemiology and prognosis of coagulase-negative staphylococcal endocarditis: impact of vancomycin minimum inhibitory concentration. PLoS One 10, e0125818, https://doi.org/10.1371/journal.pone.0125818 (2015).
Ibrahem, S. et al. Carriage of methicillin-resistant Staphylococci and their SCCmec types in a long-term-care facility. J. Clin. Microbiol. 47, 32–37, https://doi.org/10.1128/jcm.01085-08 (2009).
Sieradzki, K., Roberts, R. B., Serur, D., Hargrave, J. & Tomasz, A. Heterogeneously vancomycin-resistant Staphylococcus epidermidis strain causing recurrent peritonitis in a dialysis patient during vancomycin therapy. J. Clin. Microbiol. 37, 39–44 (1999).
Sieradzki, K., Villari, P. & Tomasz, A. Decreased susceptibilities to teicoplanin and vancomycin among coagulase-negative methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 42, 100–107 (1998).
Laganas, V., Alder, J. & Silverman, J. A. In vitro bactericidal activities of daptomycin against Staphylococcus aureus and Enterococcus faecalis are not mediated by inhibition of lipoteichoic acid biosynthesis. Antimicrob. Agents Chemother. 47, 2682–2684 (2003).
Cui, L. et al. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 41, 5–14 (2003).
Stewart, P. S., Davison, W. M. & Steenbergen, J. N. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 53, 3505–3507, https://doi.org/10.1128/aac.01728-08 (2009).
Soriano, A. et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46, 193–200, https://doi.org/10.1086/524667 (2008).
Watanabe, S. et al. Treatment with linezolid in a neonate with meningitis caused by methicillin-resistant Staphylococcus epidermidis. Eur. J. Pediatr. 172, 1419–1421, https://doi.org/10.1007/s00431-013-1978-7 (2013).
Acknowledgements
No specific funding was received for this study, and the data were generated as a part of routine work of our organisation.
Author information
Authors and Affiliations
Contributions
S.W., A.T. and H.A. conceived and designed the study; Y.K., H.K., S.M. and H.M. performed the study and the analyses; K.T. reviewed the statistical analysis; S.W. took the lead in writing the manuscript with support from S.T., K.S., M.T., H.T., J.M. and T.Y. All authors provided critical feedback and helped in shaping the research, analysis, and drafting the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watanabe, S., Kawakami, Y., Kimura, H. et al. Association between daptomycin susceptibility and teicoplanin resistance in Staphylococcus epidermidis. Sci Rep 9, 18533 (2019). https://doi.org/10.1038/s41598-019-55149-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55149-z