Abstract
Bacteria of the genus Elizabethkingia are emerging infectious agents that can cause infection in humans. The number of published whole-genome sequences of Elizabethkingia is rapidly increasing. In this study, we used comparative genomics to investigate the genomes of the six species in the Elizabethkingia genus, namely E. meningoseptica, E. anophelis, E. miricola, E. bruuniana, E. ursingii, and E. occulta. In silico DNA–DNA hybridization, whole-genome sequence-based phylogeny, pan genome analysis, and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed, and clusters of orthologous groups were evaluated. Of the 86 whole-genome sequences available in GenBank, 21 were complete genome sequences and 65 were shotgun sequences. In silico DNA–DNA hybridization clearly delineated the six Elizabethkingia species. Phylogenetic analysis confirmed that E. bruuniana, E. ursingii, and E. occulta were closer to E. miricola than to E. meningoseptica and E. anophelis. A total of 2,609 clusters of orthologous groups were identified among the six type strains of the Elizabethkingia genus. Metabolism-related clusters of orthologous groups accounted for the majority of gene families in KEGG analysis. New genes were identified that substantially increased the total repertoire of the pan genome after the addition of 86 Elizabethkingia genomes, which suggests that Elizabethkingia has shown adaptive evolution to environmental change. This study presents a comparative genomic analysis of Elizabethkingia, and the results of this study provide knowledge that facilitates a better understanding of this microorganism.
Similar content being viewed by others
Introduction
The species of the Elizabethkingia genus are usually found in natural environments, including water, soil, and plants, but they rarely cause diseases in humans1,2,3,4,5,6,7,8,9. The Elizabethkingia genus presently comprises six species. Among these, E. meningoseptica, initially named as Flavobacterium meningosepticum, was first identified by an American bacteriologist, Elizabeth O. King, in 195910. This species was categorized into a new genus (i.e., the Chryseobacterium genus) and was renamed as C. meningosepticum in 199411. With the application of 16 S ribosomal RNA (rRNA) in the taxonomy of microorganisms, C. meningosepticum and another novel species identified in 2003 (i.e., C. miricola) were assigned into a new genus (i.e., the Elizabethkingia genus) in 200512. The third species, E. anophelis, was initially discovered from the midgut microbiota of the mosquito Anopheles gambiae in the Gambia, Africa, in 201113. Lastly, three novel species, namely E. bruuniana, E. ursingii, and E. occulta, were proposed by Nicholson et al. in August 201714.
Recently, Elizabethkingia infections in humans have been increasingly reported and emerged as a critical public health issue. Life-threatening infections caused by E. anophelis have been described in Africa, Singapore, Hong Kong, the USA, and Taiwan2,3,4,5,6,7,8,9. The most severe outbreak of E. anophelis reported on to date occurred in Wisconsin and Illinois between November 1, 2015 and April 12, 20175,7,9.
With advances in molecular biology and biotechnology, whole-genome sequencing has become a popular technique in microbiology research. Whole-genome sequences of microbes can provide comprehensive information on virulence factors, pathogenesis, drug resistance, metabolism, host–pathogen interaction, host–environment reaction, and others. We previously published whole-genome sequences for two Elizabethkingia species: E. anophelis (strain EM361-97; GenBank accession number, LWDS00000000)15 and E. miricola (strain EM798-26; GenBank accession number, CP023746)16. E. miricola strain EM798-26 has been re-classified as E. bruuniana according to the whole-genome analysis17,18. However, few studies have investigated the comparative genomics of the six species in the Elizabethkingia genus. In this study, we used several methods to comprehensively analyze and compare the genomic features of all available whole-genome sequences of Elizabethkingia strains in GenBank.
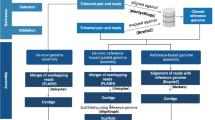
Materials and Methods
Ethics and experimental biosafety statements
This study was approved by the Institutional Review Board of E-Da Hospital (EMRP-106-105). The need for patient’s informed consent was waived by the Institutional Review Board of E-Da Hospital as the retrospective analysis of clinical data posed no more than minimal risk of harm to subjects and involved no procedures for which written consent was normally required outside of the research context. The experiments in this study were approved by the Institutional Biosafety Committee of E-Da Hospital. All experiments were performed in accordance with relevant guidelines and regulations.
Isolates for genome sequencing
Two clinical strains, namely E. anophelis EM361-97 and E. bruuniana EM798-26, were isolated from the blood of two cancer patients in Taiwan. The detailed information has been described previously15,16. In brief, the genomic DNA of these two strains was sequenced using an Illumina HiSeq. 2000 sequencing platform (Illumina, San Diego, CA, USA), and the DNA short reads were assembled using SOAP19. The sequences of the E. bruuniana strain EM798-26 were further analyzed using PacBio (Pacific Biosciences of California, Menlo Park, CA, USA) and optical mapping (Bionano Genomics, San Diego, CA, USA). Finally, 26 scaffolds and 27 contigs were generated for the E. anophelis strain EM361-9715, and the complete whole-genome sequence of the E. bruuniana strain EM798-26 was constructed16,17. The gene annotations of these two strains were performed using the Prokaryotic Genome Annotation Pipeline of the National Center for Biotechnology Information (NCBI)20.
Strains in this study
At the time of writing this paper, 86 whole-genome sequences of Elizabethkingia species, including 17 E. meningoseptica strains, 44 E. anophelis strains, 11 E. miricola strains, 8 E. bruuniana strains, 4 E. ursingii strains, and 2 E. occulta strains, were available in the NCBI genome sequence repository of GenBank (https://www.ncbi.nlm.nih.gov/genome/). Among these genomic sequences, 21 were complete genomes and 65 were shotgun sequences that presented as scaffolds or contigs. All these genome sequences were downloaded for comparison and analysis (Supplementary File 1).
In silico DNA–DNA hybridization
For genome-based species delineation, in silico DNA–DNA hybridization (DDH) was performed using the Genome-to-Genome Distance Calculator (GGDC) (http://ggdc.dsmz.de/home.php)21. The results of formula 2 were adopted according to the suggestion of the authors in a previous study21. A cutoff value of 70% was used as the delimitation criteria of microorganism species21. The heat map was generated using CIMminer (https://discover.nci.nih.gov/cimminer/).
Construction of whole-genome sequence-based phylogenetic tree
The whole-genome sequence-based phylogenetic tree was constructed using the Reference sequence Alignment based Phylogeny builder (REALPHY) (https://realphy.unibas.ch/fcgi/realphy)22. The whole-genome sequences of the 86 Elizabethkingia strains were submitted to the online pipeline of REALPHY in the FASTA format. The sequence alignments of phylogeny were performed using PhyML. The phylogenetic tree was edited using Dendroscope23.
Analysis of orthologous genes
To evaluate the evolution of ancestor genes in the different species, the online program OrthoVenn (http://www.bioinfogenome.net/OrthoVenn/) was used to evaluate the clusters of orthologous groups (COGs) in the six type strains of the Elizabethkingia genus24. The whole-genome sequences of the six type strains, namely E. meningoseptica KC1913T (=ATCC 13253T), E. anophelis R26T, E. miricola GTC 862T (=KCTC 12492T = W3-B1), E. bruuniana G0146T, E. ursingii G4122T, and E. occulta G4070T, were included for comparison of the COGs. The virulence factors were analysed using the Virulence Factor Database (VFDB)25. The antimicrobial resistance-associated genes and transposable element (transposon) genes were identified using the Rapid Annotations based on Subsystem Technology (RAST) Prokaryotic Genome Annotation Server (http://rast.nmpdr.org/)26.
Pan genome analysis
To perform pan genome analysis of Elizabethkingia species, the core (conserved), accessory (dispensable), and unique (strain-specific) genes were analyzed using a software package, namely the Bacterial Pan Genome Analysis Tool (BPGA)27. The pan genome and core genome phylogenies were generated using BPGA with default settings as per the manufacturer’s instructions.
Kyoto encyclopedia of genes and genomes (KEGG)
To understand the high-level functions of the Elizabethkingia strains, we used BPGA27 to access the KEGG database. The gene families of metabolism, genetic information processing, environmental information processing, cellular processes, organismal systems, human diseases, and drug development were analyzed.
Results and Discussion
Species delineation through in silico DDH
Figure 1 presents the results of in silico DDH for the 86 Elizabethkingia strains. The in silico DDH values between E. meningoseptica and the other five species were obviously lower than the values among the other species. The three novel species, namely E. bruuniana, E. ursingii, and E. occulta, were close to E. miricola in the dendrogram. The heat map built from the similarity matrix clearly displayed the delineation of the six species in the Elizabethkingia genus.
Dendrogram and heat map generated using in silico DNA–DNA hybridization (DDH) among 86 Elizabethkingia strains. The in silico DDH values between E. meningoseptica and the other five species were obviously lower than the values between the other species (light green). E. bruuniana, E. ursingii, and E. occulta were the most related to E. miricola in the dendrogram.
With the development of next-generation sequencing, numerous whole-genome sequences are available in the repositories of GenBank, and whole-genome sequencing can be used as a new method for species discrimination. For genome-based species delineation, in silico DDH is considered as an accurate substitution for traditional DDH21,28,29,30. Our study used GGDH, an in silico method for genome-to-genome comparison, to discriminate Elizabethkingia species. Similar to the results of the previous study performed by Nicholson et al.14, our study clearly supports that in silico DDH can noticeably distinguish Elizabethkingia species.
Phylogenetic evolution based on whole genomes
Figure 2 illustrates the phylogenetic tree based on the whole-genome sequences of the 86 Elizabethkingia strains, which was constructed using REALPHY. The six species of the Elizabethkingia genus were evidently separate from each other. Similar to the dendrogram generated based on in silico DDH, E. bruuniana, E. ursingii, and E. occulta were located close to E. miricola and were away from E. anophelis and E. meningoseptica. E. anophelis were divided into several sublineages.
Phylogeny constructed using whole-genome sequences is known to be more congruent than that built using a single gene or a small fraction of the genome, such as 16S rRNA or rpoB genes31. Through traditional DDH, Elizabethkingia species were previously classified as genomospecies. Based on the phylogenetic study of whole-genome sequences, E. miricola was found to be the most similar to genomospecies 2, E. anophelis was the closest to genomospecies 1, genomospecies 3 was near E. bruuniana, and genomospecies 4 was proposed as E. ursingii14. Moreover, the phylogenetic tree of E. anophelis strains showed several clearly demarcated sublineages in our study. The analysis of genomic features in the Wisconsin outbreak of E. anophelis also revealed different phylogenetic subclusters with distinctive temporal and geographic dynamics5. In the present study, we constructed the phylogenetic tree of the 86 Elizabethkingia strains using whole-genome sequences; the phylogenetic tree clearly demonstrated the phylogenetic relationship among these strains. The phylogeny generated using whole genomes provides not only species delineation but also comprehensive insights into comparative analyses of phylogenetic evolution across different species.
Functional COGs
COGs, also known as orthologs, are a group of genes in different species that have descended from a common gene in the same ancestor32. These genes usually retain the original function during the evolution of microorganisms, and they determine the relationships between the genome structure, gene function, and taxonomic classification32. Subsequently, it is crucial to recognize COGs and predict their functions, particularly in emerging pathogens with newly sequenced genomes32.
Figure 3 presents the COGs of the six Elizabethkingia type species. The total number of genes in the six species ranged from 3,066 to 3,629. The E. bruuniana strain G0146 possessed the highest number of genes, and the E. meningoseptica strain KC1913 had the lowest number of genes. A total of 2,609 shared COGs were identified among the six Elizabethkingia type species (Fig. 3; Supplementary File 2). The E. miricola strain GTC 862 had the lowest number of unique gene families (n = 2), and the E. anophelis strain R26 had the highest number of unique gene families (n = 25). The difference in the number of unique gene families may reflect the phenotypical traits that are specific to the group of bacteria33.
In the present study, the genome annotation of the six Elizabethkingia type species using the RAST Server revealed abundant putative genes associated with transposable elements. These transposons included hypothetical conjugative transposons BF0131, putative conjugative transposon mobilization protein BF0132, putative mobilization protein BF0133, and conjugative transposon protein TraA, TraB, TraE, TraF, TraG, TraJ, TraK, TraM, TraN, TraO, and TraQ. A recent study investigated putative integrative and conjugative elements (ICEs) in 13 complete genomes and 23 draft genomes of E. anophelis strains34. Among the 36 E. anophelis strains, ICEs were identified in 31 strains. ICEs are an important group of mobile genetic elements, which could transfer between bacteria horizontally via conjugation34. However, further studies are necessary to investigate if horizontal gene transfer occurs between different strains of Elizabethkingia.
Patients infected with Elizabethkingia have shown a mortality rate of 34% to 60%. The immune status of patients and virulence factors of microorganisms may be associated with the high case-fatality rate of Elizabethkingia infections. Previous studies have shown that patients with Elizabethkingia infections frequently had chronic illnesses. For example, 85% of patients with E. anophelis infections had comorbidities, such as malignancy (45%), cardiovascular diseases (37%), and diabetes mellitus (25%)6. In the present study, many homologs of offensive, defensive, nonspecific, and virulence-associated regulatory factors were identified in the COGs of Elizabethkingia isolates (Supplementary File 2). The potential virulence factor homologs and their associated genes of the six Elizabethkingia species predicted using the VFDB are shown in Table 1. These virulence factors included heat shock protein, phospholipase, capsular polysaccharide, catalase, peroxidase, and others. The distribution of virulence factors was similar among E. meningoseptica KC1913T, E. anophelis R26T, and E. bruuniana G0146T. Some virulence factors, such as polar flagella (flmH), exopolysaccharide (galE, pgi, acpXL, hemL, ureB, ureG), Mg2+ transport (mgtB), and type III secretion system effectors (hopJ1) were identified only in E. miricola GTC 862T, E. ursingii G4122T, and E. occulta G4070T. It is interesting that there are no flagella in Elizabethkingia species. However, the putative flmH was identified in E. miricola, E. ursingii, and E. occulta
. Further studies are necessary to investigate the function and source of this putative gene.
Functional analysis of the COGs in the 86 Elizabethkingia genomes revealed that the majority of core genomes were associated with metabolism, and the unique gene families were mostly related to “information storage and processing” (Fig. 4A). In the COG analysis, “information storage and processing” includes RNA processing and modification, chromosome dynamics, translation, transcription, replication, recombination, and repair35. The function of COGs with “information storage and processing” might be associated with intracellular survival36. However, the exact reason for the large presence of genes with function of “information storage and processing” in unique gene families is not clear.
According to the functional prediction of genomes, R (general function prediction only) accounted for the largest part of COGs, followed by K (transcription) and L (replication, recombination, and repair). Gene families associated with D (cell cycle control, cell division, and chromosome partitioning) occupied the least part (Fig. 4B). Regarding the constituents of each functional gene family, core genomes consisted of 64.7% of J (translation, ribosomal structure, and biogenesis), and accessory genomes accounted for the largest part of M (cell wall, membrane, envelope, and biogenesis) (45.7%). The unique genes accounted for 56.3% and 53.7% of L (replication, recombination, and repair) and V (defense mechanisms), respectively.
KEGG
In the KEGG analysis, genes associated with metabolism accounted for the largest part (Fig. 5A). Of these genes, most were associated with carbohydrate metabolism, followed by amino acid metabolism, cofactor and vitamin metabolism, and energy metabolism (Fig. 5B). For genes associated with carbohydrate metabolism, core genes accounted for 30.4%, accessory genes for 35.7%, and unique genes for 33.9%.
Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of the 86 Elizabethkingia strains. (A) The majority of core, accessory, and unique genes were associated with metabolism. (B) Functional annotations showed that gene families associated with carbohydrate metabolism, amino acid metabolism, cofactor and vitamin metabolism, and energy metabolism accounted for the largest part in these 86 Elizabethkingia strains.
The antimicrobial resistance-associated proteins in the six Elizabethkingia type species are shown in Table 2. Multidrug resistance efflux pumps, β-lactamases, proteins associated with resistance to vancomycin, and quinolone-resistance determining regions (DNA gyrase and topoisomerase IV) were extensively identified in these species. Several antimicrobial resistance-associated proteins, such as multidrug resistance efflux pumps, multiple antibiotic resistance MAR locus, and multidrug resistance, tripartite systems, were identified in some species. No antimicrobial resistance-associated genes were identified on ICEs in the genome annotation using the RAST Server.
Previous studies have shown that Elizabethkingia isolates were frequently resistant to many antimicrobial agents, including most β-lactams, β-lactams/β-lactamase inhibitors, aminoglycosides, macrolides, tetracycline, vancomycin, and carbapenems. But they showed variable susceptibility to piperacillin, piperacillin-tazobactam, fluoroquinolones, minocycline, tigecycline, and trimethoprim-sulfamethoxazole2,3,4,5,6,37. Genes associated with drug resistance in the Elizabethkingia genus have been reported. For example, Opota et al. reported that blaGOB-13 and blaB-9 carbapenemase-encoding genes were identified in a carbapenemase-producing clinical isolate, Elizabethkingia miricola EM_CHUV38. Previous reports indicated that E. meningoseptica and E. anophelis were resistant to several classes of antimicrobials2,3,4,5,6. A recent study revealed that E. bruuniana has a similar antimicrobial susceptibility pattern to other Elizabethkingia species18. The present study also showed that the antimicrobial resistance-associated genes of the three new Elizabethkingia species are similar to those of the other three species.
It is noteworthy that vancomycin resistance gene (vanW) was found in these six Elizabethkingia type species. vanW is included in the vanB gene cluster. The exact function of vanW is still unknown. However, mutations in vanW has been identified in microorganisms with VanB-type glycopeptide resistance39. Vancomycin has been anecdotally reported to successfully treat patients with E. meningoseptica meningitis40. However, several recent studies revealed that most Elizabethkingia species exhibited a high minimum inhibitory concentration of vancomycin41,42. Therefore, the use of vancomycin is not suggested for patients with Elizabethkingia infections41,42.
Core and pan genome analysis
In 2005, Tettelin et al. proposed pan genome as the whole-genomic repertoire of a microorganism43. Pan genome analysis can be used to discriminate the diversity of genomes and explore the core, accessory, and unique genes44. To understand the pan genome of Elizabethkingia, the whole-genome sequences of the 86 strains were examined. The distribution of gene families and the number of new genes are shown in Fig. 6A,B, respectively. The core, accessory, and unique genes are presented as a flower plot in Fig. 6C. In the 86 Elizabethkingia strains, 1,154 core (conserved) genes were recognized. In each strain, the number of accessory genes ranged from 996 to 2,738, and the number of unique genes ranged from 0 to 215. With the addition of new genome sequences, the genes of the pan genome increased from 2,110 to 9,794, and core genes decreased from 3,002 to 824 (Fig. 6D).
Pan genome analysis of 86 Elizabethkingia strains. (A) Distribution of gene families among the 86 Elizabethkingia strains. (B) Distribution of new genes among the 86 Elizabethkingia strains. (C) Flower plot showing the numbers of core orthologous genes (in the center) and strain-specific orthologous genes (in the petals) in each Elizabethkingia strain. (D) Core-pan plot of the 86 Elizabethkingia strains. The orange and purple lines indicate the numbers of pan genomes and core genomes, respectively. The cyan lines represent the change in gene families in pan genomes when genomes were added sequentially. This curve of pan genome denotes that the pan genome is still open after adding 86 genomes. The pink lines indicate the change in gene families in core genomes when genomes were added sequentially.
Conclusions
This study presents a comparative genomic analysis of 86 Elizabethkingia strains with whole-genome sequences available in GenBank. Because Elizabethkingia infections have emerged as a critical public health issue worldwide, knowledge on the clinical, molecular, and genetic characteristics is of paramount importance. The results of this study provide information to understand the population genomics, phylogenetic distinctness, evolutionary features, and genetic functions of this emerging and life-threatening pathogen.
Data availability
The names of organisms, strains, biosample numbers, bioproject numbers, assembly numbers, isolated origins, and release dates of bacteria used in this study are shown in Supplementary File 1. All data are available in the NCBI genome sequence repository.
References
Loch, T. P. & Faisal, M. Emerging flavobacterial infections in fish: A review. J. Adv. Res. 6, 283–300 (2015).
Breurec, S. et al. Genomic epidemiology and global diversity of the emerging bacterial pathogen Elizabethkingia anophelis. Sci. Rep. 6, 30379 (2016).
Frank, T. et al. First case of Elizabethkingia anophelis meningitis in the Central African Republic. Lancet 381, 1876 (2013).
Lau, S. K. P. et al. Elizabethkingia anophelis bacteremia is associated with clinically significant infections and high mortality. Sci. Rep. 6, 26045 (2016).
Perrin, A. et al. Evolutionary dynamics and genomic features of the Elizabethkingia anophelis 2015 to 2016 Wisconsin outbreak strain. Nat. Commun. 8, 15483 (2017).
Lin, J. N., Lai, C. H., Yang, C. H., Huang, Y. H. & Lin, H. H. Clinical manifestations, molecular characteristics, antimicrobial susceptibility patterns and contributions of target gene mutation to fluoroquinolone resistance in Elizabethkingia anophelis. J. Antimicrob. Chemother. 73, 2497–2502 (2018).
CDC. Recent Outbreaks, Elizabethkingia. Available at: https://www.cdc.gov/elizabethkingia/outbreaks/. (Accessed: 26th December 2016).
Teo, J. et al. First case of E anophelis outbreak in an intensive-care unit. Lancet 382, 855–856 (2013).
Navon, L. et al. Notes from the field: Investigation of Elizabethkingia anophelis cluster - Illinois, 2014-2016. MMWR 65, 1380–1381 (2016).
King, E. O. Studies on a group of previously unclassified bacteria associated with meningitis in infants. Am. J. Clin. Pathol. 31, 241–247 (1959).
Vandamme, P., Bernardet, J. F., Segers, P., Kersters, K. & Holmes, B. Notes: New perspectives in the classification of the Flavobacteria: Description of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int. J. Syst. Evol. Microbiol. 44, 827–831 (1994).
Kim, K. K., Kim, M. K., Lim, J. H., Park, H. Y. & Lee, S. T. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int. J. Syst. Evol. Microbiol. 55, 1287–1293 (2005).
Kämpfer, P. et al. Elizabethkingia anophelis sp. nov., isolated from the midgut of the mosquito Anopheles gambiae. Int. J. Syst. Evol. Microbiol. 61, 2670–2675 (2011).
Nicholson, A. C. et al. Revisiting the taxonomy of the genus Elizabethkingia using whole-genome sequencing, optical mapping, and MALDI-TOF, along with proposal of three novel Elizabethkingia species: Elizabethkingia bruuniana sp. nov., Elizabethkingia ursingii sp. nov., and Elizabethkingia occulta sp. nov. Antonie Van Leeuwenhoek 111, 55–72 (2018).
Lin, J. N., Yang, C. H., Lai, C. H., Huang, Y. H. & Lin, H. H. Draft genome sequence of Elizabethkingia anophelis strain EM361-97 isolated from the blood of a cancer patient. Genome Announc. 4 (2016).
Lin, J. N., Lai, C. H., Yang, C. H., Huang, Y. H. & Lin, H. H. Complete genome sequence of Elizabethkingia miricola strain EM798-26 isolated from the blood of a cancer patient. Genome Announc. 6 (2018).
Lin, J. N., Lai, C. H., Yang, C. H., Huang, Y. H. & Lin, H. H. Genomic features, comparative genomics, and antimicrobial susceptibility patterns of Elizabethkingia bruuniana. Sci Rep. 9, 2267 (2019).
Lin, J. N., Lai, C. H., Yang, C. H., Huang, Y. H. & Lin, H. H. Elizabethkingia bruuniana infections in humans, Taiwan, 2005-2017. Emerg Infect Dis. 25, 1412–1414 (2019).
Li, R., Li, Y., Kristiansen, K. & Wang, J. SOAP: short oligonucleotide alignment program. Bioinforma. 24, 713–714 (2008).
Tatusova, T. et al. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 44, 6614–6624 (2016).
Meier-Kolthoff, J. P., Auch, A. F., Klenk, H.-P. & Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14, 60 (2013).
Bertels, F., Silander, O. K., Pachkov, M., Rainey, P. B. & van Nimwegen, E. Automated reconstruction of whole-genome phylogenies from short-sequence reads. Mol. Biol. Evol. 31, 1077–1088 (2014).
Huson, D. H. & Scornavacca, C. Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Syst. Biol. 61, 1061–1067 (2012).
Wang, Y., Coleman-Derr, D., Chen, G. & Gu, Y. Q. OrthoVenn: a web server for genome wide comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 43, W78–84 (2015).
Liu, B., Zheng, D. D., Jin, Q., Chen, L. H. & Yang, J. VFDB 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 47, D687–D692 (2019).
Overbeek, R. et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 42, D206–214 (2014).
Chaudhari, N. M., Gupta, V. K. & Dutta, C. BPGA- an ultra-fast pan-genome analysis pipeline. Sci. Rep. 6, 24373 (2016).
Wayne, L. G. International Committee on Systematic Bacteriology announcement of the report of the ad hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Zentralbl Bakteriol Mikrobiol Hyg A. 268, 433–434 (1988).
Janda, J. M. & Abbott, S. L. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J. Clin. Microbiol. 45, 2761–2764 (2007).
Goris, J. et al. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 57, 81–91 (2007).
Rokas, A., Williams, B. L., King, N. & Carroll, S. B. Genome-scale approaches to resolving incongruence in molecular phylogenies. Nature 425, 798–804 (2003).
Tekaia, F. Inferring orthologs: Open questions and perspectives. Genomics Insights 9, 17–28 (2016).
Kahlke, T., Goesmann, A., Hjerde, E., Willassen, N. P. & Haugen, P. Unique core genomes of the bacterial family vibrionaceae: insights into niche adaptation and speciation. BMC Genomics 13, 179 (2012).
Xu, J., Pei, D., Nicholson, A., Lan, Y. & Xia, Q. In silico identification of three types of integrative and conjugative elements in Elizabethkingia anophelis strains isolated from around the world. mSphere 4 (2019).
Cheleuitte-Nieves, C. et al. Genotypic differences between strains of the opportunistic pathogen Corynebacterium bovis isolated from humans, cows, and rodents. PLoS One 13, e0209231 (2018).
Yang, X. et al. Ortholog-based screening and identification of genes related to intracellular survival. Gene 651, 134–142 (2018).
Lin, P. Y. et al. Clinical and microbiological analysis of bloodstream infections caused by Chryseobacterium meningosepticum in nonneonatal patients. J. Clin. Microbiol. 42, 3353–3355 (2004).
Opota, O. et al. Genome of the carbapenemase-producing clinical isolate Elizabethkingia miricola EM_CHUV and comparative genomics with Elizabethkingia meningoseptica and Elizabethkingia anophelis: evidence for intrinsic multidrug resistance trait of emerging pathogens. Int. J. Antimicrob. Agents 49, 93–97 (2017).
Santona, A. et al. Novel type of VanB2 teicoplanin-resistant hospital-associated Enterococcus faecium. Int. J. Antimicrob. Agents 44, 156–159 (2014).
Tai, I. C. et al. Outbreak of Elizabethkingia meningoseptica sepsis with meningitis in a well-baby nursery. J. Hosp. Infect. 96, 168–171 (2017).
Lin, J. N., Lai, C. H., Yang, C. H. & Huang, Y. H. Comparison of clinical manifestations, antimicrobial susceptibility patterns, and mutations of fluoroquinolone target genes between Elizabethkingia meningoseptica and Elizabethkingia anophelis isolated in Taiwan. J. Clin. Med. 7 (2018).
Han, M. S. et al. Relative prevalence and antimicrobial susceptibility of clinical isolates of Elizabethkingia species based on 16S rRNA gene sequencing. J. Clin. Microbiol. 55, 274–280 (2017).
Tettelin, H. et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial ‘pan-genome’. Proc. Natl. Acad. Sci. USA 102, 13950–13955 (2005).
Vernikos, G., Medini, D., Riley, D. R. & Tettelin, H. Ten years of pan-genome analyses. Curr. Opin. Microbiol. 23, 148–154 (2015).
Acknowledgements
This work was supported by grants EDAHP106007 and EDPJ108068 from E-Da Hospital and MOST 106-2314-B-214-009-MY2 and 108-2314-B-214-004 from the Ministry of Science and Technology, Taiwan.
Author information
Authors and Affiliations
Contributions
C.-Y.L. and J.-N.L. conceived and designed the study. C.-H.Y., C.-H.L., Y.H.H. and J.-N.L. analyzed and discussed the results. C.-Y.L., C.-H.Y. and J.-N.L. wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, CY., Yang, CH., Lai, CH. et al. Comparative Genomics of 86 Whole-Genome Sequences in the Six Species of the Elizabethkingia Genus Reveals Intraspecific and Interspecific Divergence. Sci Rep 9, 19167 (2019). https://doi.org/10.1038/s41598-019-55795-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-019-55795-3
This article is cited by
-
Genomic and Pan-Genomic Characterization of Elizabethkingia meningoseptica Reveals Resistance Gene Profiles, Phylogenetic Clusters, and Functional Landscape
Current Microbiology (2025)
-
Comprehensive genome analysis of Burkholderia contaminans SK875, a quorum-sensing strain isolated from the swine
AMB Express (2023)
-
Draft genome sequence of Staphylococcus aureus sequence type 5 SA01 isolated from bloodstream infection and comparative analysis with reference strains
Functional & Integrative Genomics (2023)