Abstract
Investigations were conducted to examine the effects of amine type and initial concentration, free chlorine concentration, UV light intensity, pH and tert-butyl alcohol (TBA) on the formation of dichloronitromethane (DCNM) under UV/chlorine. Methylamine (MA), dimethylamine (DMA) and poly-dimethyl diallyl ammonium chloride (PolyDADMAC) were selected as the amine precursors of DCNM. And the reaction products of amines were explored through observing the contents of various nitrogen under UV/chlorine. Experimental results indicated that the higher of the intensity of UV light, the concentration of amines and free chlorine, the greater of the amount of DCNM formation; the amine substance with simple structure is more likely oxidized to form DCNM, so the potential of MA to form DCNM is the largest among three amines; the formation of DCNM decreased with increasing pH from 6.0 to 8.0; due to adding TBA into the reaction solution, halogen and hydroxyl radicals were restrained which resulted the DCNM formation decreased. In the reaction process, the formation of DCNM from amines increased at the beginning, then decreased and almost disappeared due to photodegradation. During the formation and photodegradation of DCNM, the dissolved organic nitrogen could be transformed into the ammonia-nitrogen (NH3-N) and nitrate-nitrogen (NO3−-N).
Similar content being viewed by others
Introduction
In the past 10 years, more and more attention has been paid to the safety of drinking water supply. Disinfection of public water sources is one of the important ways to protect people's health. It is significant to reduce the occurrence and spread of waterborne diseases in water1. And the impacts of many water-related infectious diseases have been greatly reduced through disinfection of drinking water2. Therefore, urban water supply system can provide quality drinking water to residents every day, and meet people's daily needs. However, chemical disinfection also poses a severe issue to us that forming new chemical substances which called disinfection by-products (DBPs). Chlorine is also commonly used in the water treatment process, which can maintain a certain residual chlorine of the effluent to keep the subsequent disinfection. In addition, chlorine also can inactivate waterborne pathogens so that it can reduce the public health risk3. But in the 1970s, previous research confirmed that chloroform would form during the chlorine disinfection process, which means that chlorination would bring a range of toxic DBPs4,5. Since then, DBPs attracted extensive attention from all walks of life. To date, more than 800 DBPs have been reported, and most of these were identified in the laboratory by simulated disinfection studies6. Besides, several epidemiological studies have shown that prolonged exposure to some DBPs will pose a risk of carcinogenicity. Some of these may lead to bladder cancer or colorectal cancer7. Even there are some potential health problems from DBPs for humans, such as developmental and reproductive complications8.
About DBPs, a lot of studies have been searched about carbonaceous DBPs (C-DBPs), especially trihalomethanes (THMs) and haloacetic acids (HAAs). Compared with THMs and HAAs, the content of nitrogenous DBPs (N-DBPs) in water always at a lower level, resulted in less research about N-DBPs in the initial study on DBPs4,9. For instance, in drinking water distribution systems (DWDSs), halonitromethanes (HNMs) level is in between 0.16 and 1.50 mg/L and generally higher in summer than in winter or spring10,11. In swimming pools, HNMs is in the range of 0.2 to 0.7 μg/L12. N-DBPs have lately received great attention for their higher genotoxicity and cytotoxicity than those of regulated C-DBPs13,14. Among N-DBPs, HNMs, haloacetonitriles (HANs), haloacetamides (HAcAms), and N-nitrosamines (NAs) are frequently detected in drinking water and wastewater, which brings serious health risks to human9. Toxicological research has uncovered that the cytotoxicity and genotoxicity of HAN and HNM are 1–2 orders of magnitude higher than their haloacetic acid analogs15. Zhang’s group found that HNMs can also cause oxidative damage of DNA in mice16. Thus, many regions have begun monitoring HNMs in drinking water to protect human health. As one typical class of HNMs, DCNM has the characteristics of low concentration levels (average 1.0 mg/L in DWDSs), low reported frequency and high toxicity (cytotoxicity value: 3.73 × 10–4; genotoxicity value: 4.21 × 10–4)17,18. At present, the precursors and the formation mechanism of HNMs are still at the stage of exploration. Bond et al. proposed that there was still great uncertainty about the characteristics of chemical group of HNMs precursors in drinking water (hydrophobicity or hydrophilic), and the key factor might be whether there were functional groups which can convert to nitro (e.g. amines or phenols)19. This study also claimed that dissolved organic nitrogen (DON) was the most relevant parameters to the formation of HNMs. And it has been reported that nitrite was potential source of nitrogen for the formation of chloropicrin20. Liew et al. research confirmed the increase of HANs formation in water with high content of NH3-N due to monochloramine formation from the chlorination of ammonia17. There is an important link between NH3-N and the formation of HANs.
UV disinfection is a commonly used disinfection method in water treatment, which can effectively inactivate various microorganisms in water. It has the advantages of fast sterilization, no harmful DBPs, no need for additional chemical drugs, and it does not introduce disinfectant resistance to bacteria21. However, in actual operation, there are some problems of UV disinfection such as low disinfection efficiency, no subsequent anti-virus ability, and unstable disinfection test results. So it is usually coupled with chlorine in water treatment12,22,23. As an emerging advanced oxidation process (AOP), UV/chlorine treatment can produce diverse reactive species which are beneficial to degrade a variety of contaminants24. Most studies believed that UV treatment can reduce the concentration of DBPs in drinking water25. But other studies showed that the formation of N-DBPs was related to the chlorination of natural organic matter (NOM), and UV treatment can degrade NOM into lower molecular weight products, which could react with chlorine or chloramine to promote the formation of DBPs5,26,27,28,29. For example, during the UV–chlorination process, THMs formed were higher than chlorine alone treatment, and the formation of HANs and HNMs also increased30,31. Lu’s group specified UV/chlorine pre-treatment of clofibric acid (CA) could promote the concentration of TCNM32. And in previous research, we did found that TCNM formation increased by UV–chloramination compared by chlorination alone33. It also has been reported UV–chloramination process formed the DBPs which are more cytotoxic than those by chloramination alone34.
Amines are widely distributed in water environments due to decomposition of protein in domestic sewage and extensive use of various amines as chemical raw materials35. During the treatment of sewage or drinking water, primary and secondary amines can react with disinfectants to form carcinogenic and mutagenic nitrosamines, which brings potential health risk. Until now, there are few reports on the effect of amines in water on the formation of DCNM under UV/chlorine disinfection. Therefore, in this study, three different classes of amines (MA, DMA and PolyDADMAC) were selected as the precursor of DCNM. The effects of amines concentration, free chlorine concentration, light intensity, pH and additional TBA on DCNM formation were explored with three different amine precursors. The DCNM formation potential of three amine precursors and the variation of different forms of nitrogen in the reaction system was analysed.
The aims of the present study were (1) to evaluate the effects of amine on the formation and photodegradation of DCNM under UV/chlorine with influencing factors of light intensity, free chlorine, pH and TBA and reaction time, etc., (2) to analyse the formation and photodegradation of DCNM from amine under UV/chorine, (3) to explore DON could be transformed into ammonia-nitrogen (NH3-N) and nitrate-nitrogen (NO3−-N) during the formation and photodegradation of DCNM, (4) to compare the formation and photodegradation of DCNM in three different amine precursors. In order to better show the phenomena of these experiments and the requirements of instrument detection, the concentrations of chlorine and amine used in these experiments were slightly larger than that in the actual water body. The results of the current study could be very useful for controlling HNMs formation and contributing to the development of new disinfection method in the drinking water and sewage treatment.
Materials and methods
Chemicals and reagents
Sources of chemicals and reagents were provided in the Supplementary Text S1.
Experimental equipment
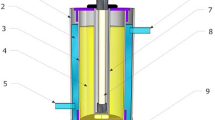
The experiments were carried out in a self-made quartz glass reaction equipment which has two layers (see Fig. 1). The outer layer was used for the condensing water circulation to maintain the constant temperature of reaction solution (22 ± 2 °C), and the reaction proceeded in the inner layer with the conventional low-pressure UV mercury lamps (5, 10, 15 W), which emits almost monochromatic light at 254 nm. A magnetic stirrer was arranged under the reactor to ensure a uniform mixture of the reaction solution. Before conducting the experiment, the equipment should be placed in a box so that the reaction could be carry out under a dark condition.
Experimental methods and analyses
According to the experimental requirements, the initial reaction solution with different concentration was prepared for adding into the reaction device. The UV lamp was immersed in the reaction solution, while the height of the tube holder was adjusted to control the lamp to a suitable depth. After the reaction started, 5.0 mL of the reaction solution was transferred to a brown sample vial with cap, at regular intervals. 2 mL methyl tert-butyl ether (MTBE) was used to liquid–liquid extraction (LLE) before analysis. Then, taking 1 mL MTBE to a gas chromatography (GC) vial from the extracted solution and then analysed the samples using GC-ECD system. The initial operating temperature of GC was 50 °C. After the instrument was operated at the initial temperature for 5 min, the temperature was raised to 140 °C at 10 °C/min, and then raised to 280 °C at 20 °C/min. Among them, the temperature of the inlet of instrument was 235 °C and the ECD was 280 °C. Nitrogen was the carrier gas of GC, and the flow rate was 1.0 mL/min. In addition, in this experiment, alkaline potassium persulfate digestion UV-spectrophotometry method was used for determination of total nitrogen (TN); NH3-N was determined by Nessler’s reagent spectrophotometry; NO3−-N and nitrite-nitrogen (NO2−-N) were determined by ultraviolet spectrophotometry. All experiments were performed at least twice, and error bars represent one standard deviation of the average values.
Results and discussion
Effects of amine type and initial concentration
In order to investigate the DCNM formation potential of amine precursors, the solutions of MA, DMA and PolyDADMAC containing the organic nitrogen of 0.5, 1.0 and 1.5 mmol/L were prepared for the experiments, respectively. And the reaction solution was subjected to 15 W UV irradiation under the condition of 60 mg/L free chlorine at pH 7. From Fig. 2a, when MA acted as precursor of DCNM, the concentration of DCNM formation increased quickly at the beginning, and reached the maximum at 4 min, then the declined tendency of DCNM formation was observed after that. As shown in Fig. 2b,c, when DMA and PolyDADMAC acted as precursor of DCNM, DCNM formed reached the maximum at 6 min, and then decreased with the increase of reaction time. When the reaction solution contained 0.5 mmol/L organic nitrogen, in the presence of MA, DMA, PolyDADMAC, the maximum amount of DCNM formation was 77.83, 61.98 and 33.92 μg/L respectively, and then decreased to 21.95, 6.50 and 5.02 μg/L at 10 min. When the organic nitrogen concentration of MA, DMA, PolyDADMAC was 1.0 mmol/L, the maximum production of DCNM was 109.10, 81.48 and 38.48 μg/L, respectively. When the organic nitrogen concentration of MA, DMA, PolyDADMAC increased to 1.5 mmol/L, the maximum production of DCNM was 136.36, 110.02 and 46.90 μg/L. Compared to the reaction with 1.0 mmol/L organic nitrogen, the maximum amount of DCNM formed increased 25.0%, 35.0% and 21.9% respectively. Thus it was demonstrated that the increase of precursor concentration can promote the formation of DCNM. At the same time, the formation of DCNM from simple structure amines was higher. During UV/chlorine process, UV irradiation can provide energy for the bond-breaking and ring cleavage of PolyDADMAC to destroy its structure36,37. Therefore, PolyDADMAC would be degraded into simple structure amines firstly, and then continue to react with free chlorine to form DCNM. Polymers such as PolyDADMAC required more energy and oxidants to degrade into low-molecular products than amines with simple structure, which caused the concentration of DCNM formation by the three amines is MA > DMA > PolyMAMDAC, under the same experimental conditions.
Effect of free chlorine concentration
Free chlorine plays a vital role in the process of DCNM formation under UV/chlorine. The reaction solutions containing the organic nitrogen of 1.0 mmol/L (MA, DMA and PolyDADMAC, respectively) were prepared for the experiments, respectively. The effect of free chlorine concentration on forming DCNM was investigated by adding 40.0, 60.0 and 80.0 mg/L free chlorine into the reaction solutions, respectively. As shown in Fig. 3, when the concentration of free chlorine was 40 mg/L, the maximum amount of DCNM formation was 87.28, 43.78 and 33.59 μg/L in the reaction solutions of MA, DMA and PolyDADMAC, respectively. When the concentration of free chlorine was 60.0 mg/L, the maximum production of DCNM was 109.1, 81.48 and 38.48 μg/L from MA, DMA, PolyDADMAC respectively, which increased 25.0%, 86.1%, 14.6% in comparison with 40 mg/L free chlorine. When the concentration of free chlorine reached 80.0 mg/L, the amount of DCNM formation continued to up to 121.24, 96.02 and 42.16 μg/L respectively, which increased 38.0%, 119.3%, 25% in comparison with 40 mg/L free chlorine. It also can be found that the formation of DCNM from DMA was the most sensitive to the change of chlorine dose, followed by MA, PolyDADMAC. Therefore, the experiment results showed that the concentration of DCNM formation was positively correlated to the concentration of free chlorine in the presence of amine. It was different from the result of chloramphenicol (CAP) as a precursor which indicated the formation of DCNM from CAP decreased with the increase of chlorine dose. As chlorine increased, the rate of degradation of CAP would decrease, resulting in the decrease of free radicals produced by CAP degradation31. However, for amines, the high concentration of free chlorine could accelerate the breaking of bond and oxidation process, which might contribute to subsequent chlorination of the intermediates. So, the concentration of DCNM formed by amines increased with the increase of chlorine.
Effect of UV light intensity
Low pressure UV could degrade amines into lower molecular weight products, which react with chlorine to promote the formation of DBPs. And the photolysis of free chlorine using conventional UV mercury lamps could produce reactive oxygen species (e.g., ozone and HO·) and reactive chlorine species (RCS), which will promote DBPs formation38,39,40. To investigate the influence of UV light intensity on the formation and photodegradation of DCNM from MA, DMA and PolyDADMAC, the reaction solution (MA, DMA and PolyDADMAC) containing 1.0 mmol/L and free chlorine 60 mg/L at pH 7.0 and 22 °C under 5, 10, and 15 W irradiation, respectively. In addition, each precursor group was set up with a control experiment under dark condition. As shown in Fig. 4, the amount of DCNM formation from DMA fluctuated between 1.98 and 3.95 μg/L under dark condition, while the amount of DCNM formation from MA and PolyDADMAC was about 11.71–16.81 μg/L and 11.44–15.59 μg/L, respectively. When MA, DMA and PolyDADMAC were used as precursors of DCNM, the formation of DCNM increased rapidly under UV irradiation, and reached the maximum at 4 min, 6 min, 6 min respectively. Under 5 W UV irradiation, in the presence of MA, DMA and PolyDADMAC in the reaction solution respectively, the maximum concentration of DCNM was 81.20, 57.69 and 29.87 μg/L. The maximum concentration of DCNM was 98.42, 72.23, 35.85 μg/L respectively under 10 W UV irradiation. Under 15 W UV irradiation, the maximum formation of DCNM from MA, DMA and PolyDADMAC was 109.10, 81.48 and 38.48 μg/L respectively. In comparison with 10 W UV irradiation, DCNM formation increased 10.9%, 12.8% and 7.3% under 15 W UV light intensity. According to the experimental result, DMA was the most affected by the UV light intensity among three types of amine precursors. When light intensity increased from 5 to 15 W, the increase percentage of DCNM formation from DMA was the largest (41.2%), followed by MA (34.4%), PolyDADMAC (28.8%). These phenomena were similar with the results reported by Wang's group, which described the formation of TCNM increased with the increase of UV irradiation intensity41. UV light irradiation played an important role in the formation of DCNM, in addition, the higher the light intensity of UV was, the faster the formation and photodegradation of DCNM was. The reason might be the increase of light intensity promoted the oxidation of amines and was helpful for DCNM formation, while the photodegradation of DCNM increased with the increase of light intensity because of its nitrile (–CN) and nitro (–NO2) functional groups with appropriate optical absorption properties.
To explore the reasons why the concentration of DCNM formation decreased gradually after the maximum concentration in Figs. 2, 3 and 4, the effect of low-pressure UV irradiation on the photodegradation of DCNM was investigated. The experiments were carried out in 100 µg/L DCNM solution at pH = 7.0 under different light intensity (5, 10 and 15 W). The results showed that the photodegradation efficiency of DCNM increased with increasing light intensity. As shown in Fig. 5, the degradation percentage of DCNM was 8.33% after 20 min without UV. It demonstrated that DCNM in solution could be volatilized or hydrolyzed under dark condition. After 20 min, the degradation percentage of DCNM by 5 W UV irradiation was 79.98%, while that by 10 W and 15 W UV irradiation was 89.67% and 92.57%, respectively. The DCNM degradation data of all four conditions were processed by the pseudo-first-order kinetics model (Eq. (1)), and the insert figure in Fig. 5 shows that four linear fitting curves (with R2 > 0.98) were obtained. The kobs,T values of UV treatment (5, 10, 15 W) was 0.0833, 0.1127, 0.1297 min−1 respectively, which was 19–29 folds that observed from the dark condition (0.0045 min−1). The kobs,T was related to UV light intensity and increased with the increase of light intensity. It can found that UV can effectively promote the DCNM degradation and high UV light intensity providing more energy to promote photodegradation of DCNM.
where kobs,T is the pseudo-first-order rate constants (min−1).
Effect of pH
The pH of natural water or water plant effluents generally ranged from 6.0 to 8.0. To study the formation and photodegradation of DCNM at different pH values, the experiments were carried out in reactive solutions containing 60 mg/L free chlorine and 1.0 mmol/L organic nitrogen (MA, DMA and PolyDADMAC, respectively) under 15 W UV irradiation at pH 6.0, 7.0, and 8.0. From Fig. 6a, at pH 6.0, the maximum concentration of DCNM formation reached 120.04 μg/L in the presence of MA. When DMA and PolyDADMAC worked as the precursors, the maximum concentration of DCNM was 94.43 and 45.62 μg/L respectively (from Fig. 6b,c). At pH 7.0, the maximum concentration of DCNM formation from MA, DMA or PolyDADMAC decreased to 109.10 81.48 and 38.48 μg/L respectively. At pH 8.0, the maximum formation of DCNM from MA, DMA or PolyDADMAC decreased to 100.97, 73.01 and 36.45 μg/L respectively, which decreased 15.9%, 22.7%, 20.1% compared to the condition of pH 6.0. And DMA was the most affected by pH among three types of amine precursors. The experimental results revealed that the formation of DCNM decreased with increasing pH from 6.0 to 8.0. This is similar to the research results of Dong et al. that the acidic condition was more favorable for the generation of chloronitromethane (CNM) during UV/chlorine disinfection process31. The concentration of hydroxyl radical (HO·) and chlorine free radicals (Cl·) showed a decreased trend with the increase of pH, which might be the reason of the decrease of DCNM formation42.
Effect of TBA
TBA was selected as free radical quenching reagent to restrain HO· and Cl· under UV/chlorine. The experiments were conducted in the reaction solutions which contained 1.0 mmol/L organic nitrogen (MA, DMA and PolyDADMAC, respectively) and free chlorine (60 mg/L) at pH = 7, and 15 W UV irradiation. To ensure halogen and hydroxyl radicals completely suppressed in the reaction process, 5.0 g/L TBA was added in the reaction solutions. The experimental results were shown in Fig. 7, it illustrated that TBA had an important effect on the formation and photodegradation of DCNM. Under dark condition, the maximum concentration of DCNM formation from MA, DMA and PolyDADMAC was to 16.81, 3.95 and 15.59 μg/L, respectively. And with 15 W UV irradiation, in the reaction solution without TBA, the maximum formation of DCNM was to 109.1, 81.48 and 38.48 μg/L respectively. With 15 W UV irradiation and adding excess TBA, the maximum concentration of DCNM formation from MA, DMA and PolyDADMAC was 92.92, 65.59 and 31.37 μg/L, which decreased 14.8%, 19.5% and 18.5% compared to the absence of TBA. In the reaction process, the oxidation processes of precursors were hindered due to halogen and hydroxyl radicals being restrained, which resulted in the decrease of DCNM formation. Due to photodegradation, the concentration of DCNM dropped to 31.27, 15.10 and 6.55 μg/L at 10 min, respectively. However, compared to the absence of TBA, the final concentration of DCNM from DMA and PolyDADMAC was larger. This might be due to the presence of radicals scavenging compounds (TBA), which led to the weakening of the contribution of hydroxyl radicals to DCNM degradation. Therefore, it can be concluded that TBA could change the distribution of halogen and hydroxyl radicals in the solution might change, then affecting the formation and photodegradation of DCNM from amines.
Changes of various forms of nitrogen from MA, DMA and PolyDADMAC under UV/chlorine
To study the final products of the reaction further, a correlation between the formation and photodegradation of DCNM and the various forms of nitrogen under UV/chlorine was investigated. The experiments were carried out in the reaction solution containing 1.0 mmol/L organic nitrogen (MA, DMA and PolyDADMAC, respectively) and 60.0 mg/L free chlorine at pH 7.0 under 15 W UV irradiation and dark condition. As shown in Fig. 8, it showed that the TN had a declining trend with the increase of reaction time. With 15 W UV irradiation, the concentration of TN from MA, DMA and PolyDADMAC decreased from 13.78, 13.29 and 13.87 to 12.61, 11.93 and 12.26 mg/L respectively, and decreased by 1.17, 1.36, 1.61 mg/L in 20 min. And under dark condition, the concentration of TN also decreased by 1.33–1.63 mg/L. The loss of TN might be due to the formation of volatile nitrogen-containing organic compounds and N2 in the reaction process. At the beginning, NH3-N did not present in the reaction solution. And then, at 20 min, the concentration of NH3-N from MA, DMA and PolyDADMAC increased to 1.86, 2.44, 2.00 mg/L respectively, which was slightly higher than that in dark conditions (1.25, 1.26, 1.60 mg/L). The NO3−-N concentration from MA, DMA and PolyDADMAC increased initially from 0 to 0.56, 0.22 and 0.38 mg/L in 2 min and then decreased to 0.36, 0.12 and 0.20 mg/L at 20 min respectively. However, NO3−-N was not detected under dark condition. Due to the condition of free chlorine, the NO2−-N in the reaction solution was considered to be at a negligible level. Therefore, DON in the reaction solution was calculated via Eq. (2).
According to calculated results, DON in the reaction solution had a large decrease during the process of UV/chlorine disinfection. With 15 W UV irradiation, the concentration of DON from MA, DMA and PolyDADMAC decreased from 13.78, 13.29, 13.87 to 9.40, 9.37, 10.06 mg/L respectively, decreased by 4.39, 3.92, 3.81 mg/L in 20 min, which showed that amines and DON could be decomposed into inorganic matter by oxidizing reaction. Under dark condition, DON from MA, DMA and PolyDADMAC also showed a decreased trend, which declined 2.58, 2.59, 3.23 mg/L in 20 min. Through comparison of DON between UV irradiation and dark condition, it was found that more DON participated in the UV/chlorine process. From the results, it can be seen that the DON in the reaction solution would eventually be converted into NH3-N and NO3−-N during the UV/chlorine disinfection process. And NO3−-N would react further with other dissolved organic matter or produce reactive nitrogen species (RNS) by UV photolysis, which led the concentration of NO3−-N in the reaction solution to rise firstly and then fall.
Conclusions
The experimental results showed that MA, DMA and PolyDADMAC were important precursors for DCNM formation. The combined UV/chlorine condition could promote the formation and photodegradation of DCNM from MA, DMA and PolyDADMAC in contrast to free chlorination alone. The increase of amine concentration, free chlorine concentration and UV light intensity could effectively increase the formation of DCNM. The production of DCNM decreased with increasing pH value. The halogen and hydroxyl radicals play an important role on the formation of DCNM from MA, DMA and PolyDADMAC. Among three types of amines, the potential of MA to form DCNM was the largest. According to the analysis of various nitrogen during UV/chlorine disinfection, it showed that amines were decomposed into the inorganic matter of NH3-N and NO3−-N. The study could be very useful to reduce the formation of HNMs through controlling disinfection conditions and to improve the disinfection methods for drinking water and sewage.
References
Gaffga, N. H., Tauxe, R. V. & Mintz, E. D. Cholera: A new homeland in Africa?. Am. J. Trop. Med. Hyg. 77, 705–713 (2007).
Unicef, WHO. Progress on Sanitation and Drinking Water. 2015 Update and MDG Assessment (2015).
Grellier, J., Rushton, L., Briggs, D. J. & Nieuwenhuijsen, M. J. Assessing the human health impacts of exposure to disinfection by-products—A critical review of concepts and methods. Environ. Int. 78, 61–81 (2015).
Shah, A. J. & Gilani, A. H. Aqueous-methanolic extract of sweet flag (Acorus calamus) possesses cardiac depressant and endothelial-derived hyperpolarizing factor-mediated coronary vasodilator effects. J. Nat. Med.-Tokyo 66, 119–126 (2012).
Yang, M. & Zhang, X. Current trends in the analysis and identification of emerging disinfection byproducts. Trends. Environ. Anal. 10, 24–34 (2016).
Culin, J. & Mustac, B. Environmental risks associated with ballast water management systems that create disinfection by-products (DBPs). Ocean Coast. Manag. 105, 100–105 (2015).
Chowdhury, S., Champagne, P. & McLellan, P. J. Uncertainty characterization approaches for risk assessment of DBPs in drinking water: A review. J. Environ. Manag. 90, 1680–1691 (2009).
Nieuwenhuijsen, M. J., Dadvand, P., Grellier, J., Martinez, D. & Vrijheid, M. Environmental risk factors of pregnancy outcomes: A summary of recent meta-analyses of epidemiological studies. Environ. Health-Glob. 12, 1–10 (2013).
Krasner, S. W. et al. Occurrence of a new generation of disinfection byproducts. Environ. Sci. Technol. 40, 7175 (2006).
Mian, H. R., Hu, G., Hewage, K., Rodriguez, M. J. & Sadiq, R. Prioritization of unregulated disinfection by-products in drinking water distribution systems for human health risk mitigation: A critical review. Water. Res. 147, 112–131 (2018).
Zhou, X. et al. Factors influencing DBPs occurrence in tap water of Jinhua Region in Zhejiang Province, China. Ecotox. Environ. Saf. 171, 813–822 (2019).
Carter, R. A. A., Allard, S., Croue, J.-P. & Joll, C. A. Occurrence of disinfection by-products in swimming pools and the estimated resulting cytotoxicity. Sci. Total. Environ. 664, 851–864 (2019).
Plewa, M. J., Wagner, E. D., Muellner, M. G., Hsu, K. M. & Richardson, S. D. Comparative mammalian cell toxicity of N-DBPs and C-DBPs. In Occurrence, Formation, Health Effects and Control of Disinfection By-Products in Drinking Water. Symposium Series No. 995 (eds Karanfil, T. et al.) 36 (American Chemical Society, Washington, DC, 2008).
Plewa, M. J. & Wagner, E. D. Charting a new path to resolve the adverse health effects of DBPs. In Recent Advances in Disinfection By-Products. Symposium Series No. 1190 (eds Karanfil, T. et al.) 3–32 (American Chemical Society, Washington, DC, 2015).
Plewa, M. J. et al. Halonitromethane drinking water disinfection byproducts: Chemical characterization and mammalian cell cytotoxicity and genotoxicity. Environ. Sci. Technol. 38, 62–68 (2004).
Zhang, L. et al. Comparison of DNA damage in human-derived hepatoma line (HepG2) exposed to the fifteen drinking water disinfection byproducts using the single cell gel electrophoresis assay. Mutat. Res-Gen. Toxicol. Environ. 741, 89–94 (2012).
Liew, D., Linge, K. L. & Joll, C. A. Formation of nitrogenous disinfection by-products in 10 chlorinated and chloraminated drinking water supply systems. Environ. Monit. Assess. 188, 518 (2016).
US National Library of Medicine. Toxnet Database. US National Library of Medicine (US). https://toxnet.nlm.nih.gov/ (2017).
Bond, T., Templeton, M. R. & Graham, N. Precursors of nitrogenous disinfection by-products in drinking water––A critical review and analysis. J. Hazard Mater. 235–236, 1–16 (2012).
Choi, J. & Richardson, S. D. Formation studies of halonitromethanes in drinking water. AWWA In Water Quality Technology Conference, Denver, CO, USA (2004).
Mori, M. et al. Development of a new water sterilization device with a 365 nm UV-LED. Med. Biol. Eng. Comput. 45, 1237–2124 (2007).
Sun, W., Liu, W., Cui, L. & Liu, L. Impact of AOC and chlorine residual on regrowth of microbes in a model distribution system receiving UV-treated potable water. J. Water Supply Res. Technol.-AQUA 61, 372–380 (2012).
Ye, Z., Liu, W., Sun, W., Nie, X. & Ao, X. Role of ammonia on haloacetonitriles and halonitromethanes formation during ultraviolet irradiation followed by chlorination/chloramination. Chem. Eng. J. 337, 275–281 (2018).
Xiang, Y., Fang, J. & Shang, C. Kinetics and pathways of ibuprofen degradation by the UV/chlorine advanced oxidation process. Water. Res. 90, 301–308 (2016).
Magnuson, M. L. et al. Effect of UV irradiation on organic matter extracted from treated Ohio river water studied through the use of electrospray mass spectrometry. Environ. Sci. Technol. 36, 5252–5260 (2002).
Han, J. & Zhang, X. Evaluating the comparative toxicity of DBP mixtures from different disinfection scenarios: A new approach by combining freeze-drying or rotoevaporation with a marine polychaete bioassay. Environ. Sci. Technol. 18, 10552 (2018).
Roccaro, P., Vagliasindi, F. G. A. & Korshin, G. V. Quantifying the formation of nitrogen-containing disinfection by-products in chlorinated water using absorbance and fluorescence indexes. Environ. Sci. Technol. 63, 40–44 (2011).
Shah, A. D., Dotson, A. D., Linden, K. G. & Mitch, W. A. Impact of UV disinfection combined with chlorination/chloramination on the formation of halonitromethanes and haloacetonitriles in drinking water. Environ. Sci. Technol. 45, 3657–3664 (2011).
Zhang, M. et al. Effects of ion species on the disinfection byproduct formation in artificial and real water. Chemosphere 217, 706–714 (2019).
Guo, Z., Lin, Y. & Xu, B. Factors affecting THM, HAN and HNM formation during UV–chlor(am)ination of drinking water. Chem. Eng. J. 306, 1180–1188 (2016).
Dong, H., Qiang, Z., Hu, J. & Qu, J. Degradation of chloramphenicol by UV/chlorine treatment: Kinetics, mechanism and enhanced formation of halonitromethanes. Water Res. 121, 178–185 (2017).
Lu, X. et al. Investigation of clofibric acid removal by UV/persulfate and UV/chlorine processes: Kinetics and formation of disinfection byproducts during subsequent chlor(am)ination. Chem. Eng. J. 331, 364–371 (2018).
Deng, L., Wen, L. J., Dai, W. J. & Singh, R. P. Impact of tryptophan on the formation of TCNM in the process of UV/chlorine disinfection. Environ. Sci. Pollut. Res. 25, 23227–23235 (2018).
Lyon, B. A. et al. Integrated chemical and toxicological investigation of UV–chlorine/chloramine drinking water treatment. Environ. Sci. Technol. 48, 6743–6753 (2014).
Poste, A. E., Grung, M. & Wright, R. F. Amines and amine-related compounds in surface waters: A review of sources. Sci. Total Environ. 481, 274–279 (2014).
Lau, T. K., Chu, W. & Graham, N. The degradation of endocrine disruptor di-n-butyl phthalate by UV irradiation: A photolysis and product study. Chemosphere 60, 1045–1053 (2005).
An, D. et al. Lower molecular weight fractions of PolyDADMAC coagulants disproportionately contribute to N-nitrosodimethylamine formation during water treatment. Water Res. 150, 466–472 (2019).
Fang, J., Fu, Y. & Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 48, 1859–1868 (2014).
Remucal, C. & Manley, D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment. Environ. Sci. Water Res. Technol. 2, 565–579 (2016).
Sun, P., Lee, W. N., Zhang, R. & Huang, C. H. Degradation of DEET and caffeine under UV/chlorine and simulated sunlight/chlorine conditions. Environ. Sci. Technol. 50, 13265–13273 (2016).
Wang, A. Q., Xu, B., Zhang, T. Y., Chen, Y. Y. & Gao, N. Y. Effect of UV irradiation and UV/chlorine processes on trichloronitromethane formation during chlorination of ronidazole. Clean-Soil Air Water 45, 1600163 (2017).
Hua, Z. et al. PPCP degradation and DBP formation in the solar/free chlorine system: Effects of pH and dissolved oxygen. Water Res. 150, 77–85 (2019).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21677032), the National Key R&D Program of China (Grant No. 2017YFC0 504505) and the Fundamental Research Funds for the Central Universities. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Author information
Authors and Affiliations
Contributions
L.D. conceived and designed the study, analysed the data, and wrote the manuscript. X.L. and J.S. carried out the experiment of the effect of amine type, free chlorine and UV light intensity, analysed the data, and edited the manuscript. L.D. and B.X. carried out the experiment of the effect of pH and TBA. L.D. analysed the data of the changes of various forms of nitrogen. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deng, L., Liao, X., Shen, J. et al. Effects of amines on the formation and photodegradation of DCNM under UV/chlorine disinfection. Sci Rep 10, 12602 (2020). https://doi.org/10.1038/s41598-020-69426-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-020-69426-9
This article is cited by
-
Halonitromethanes Formation From Aspartic Acid in the Presence of Cu2+ During UV254/chloramine Treatment: Experimental and Computational Studies
Water, Air, & Soil Pollution (2025)
-
Microbiological Shelf-Life Extension and Quality Retention of a Novel Vegetable Product Through an Optimized Preservation Treatment Combining Citric Acid, Oregano Essential Oil, and UV-C Radiation
Food and Bioprocess Technology (2025)
-
Formation of halonitromethanes from benzylamine during UV/chlorination: Impact factors, toxicity alteration, and pathways
Environmental Science and Pollution Research (2024)
-
Impact factors and pathways of halonitromethanes formation from aspartic acid during LED-UV265/chlorine disinfection
Frontiers of Environmental Science & Engineering (2024)
-
Development and modeling of a novel type of photoreactors with exterior ultraviolet (UV) reflector for water treatment applications
Scientific Reports (2023)