Abstract
Group cohesion relies on the ability of its members to process social signals. Songbirds provide a unique model to investigate links between group functioning and brain processing of social acoustic signals. In the present study, we performed both behavioral observations of social relationships within a group of starlings and individual electrophysiological recordings of HVC neuronal activity during the broadcast of either familiar or unfamiliar individual songs. This allowed us to evaluate and compare preferred partnerships and individual electrophysiological profiles. The electrophysiological results revealed asymmetric neuronal activity in the HVC and higher responsiveness to familiar than to unfamiliar songs. However, most importantly, we found a correlation between strength of cerebral asymmetry and social integration in the group: the more preferred partners a bird had, the more its HVC neuronal activity was lateralized. Laterality is likely to give advantages in terms of survival. Our results suggest that these include social skill advantages. Better knowledge of links between social integration and lateralization of social signal processing should help understand why and how lateralization has evolved.
Similar content being viewed by others
Introduction
Group cohesion relies in particular on the perception and appropriate processing of signals from conspecifics, i.e. on social cognition1,2. The ability to identify group membership3, to process social familiarity4 and to recognize individual group members5 appears to be widespread among animals. Songbirds in particular are very good models for studying links between “social and vocal brains”4. Since its discovery by Nottebohm6, their brains’ «song system» keep revealing more and more relationships between communication skills and social interactions7. However, understanding how social cohesion, communication skills and brain processes are interrelated requires us to bridge the gap between group functioning and brain processes.
Social relationships enhance attention8, which is important for memory formation and perceptual tuning9. The mere presence of a singing adult model in a group of young naive starlings is not sufficient for them to develop all typical adult song features. Individual bonding is essential to trigger appropriate selective attention, i.e. focusing on specific group members or social models4,10. Attention is a key element in perceptual lateralization11 and loss of attention can lead to total disappearance of the species-specific lateralized responses observed in brain processing of social signals12,13. Evolution of social attentional skills may have been part of social evolution and as such may have been associated with the evolution of perceptual lateralization12. Authors have proposed several different, sometimes contradictory, hypotheses concerning the advantages of sensory lateralization14,15. However, researchers generally accept that lateralized individuals possess higher skills for a variety of tasks, especially dual tasks requiring them, for example, to divide their attention between searching for food and detecting a predator16. Some types of lateralization develop with experience18,19. For example, hens’ type of mothering can influence chicks’ lateralization, probably through attentional processes involving social interactions with mother or between chicks20. Lateralization of zebra finches’ song processing in the secondary auditory area (NCM) correlates with the fidelity of their imitation of their model21. Recently, several reports have shown in addition the importance of the influence of the timing of memory formation on the direction and strength of the lateralization of song processing in this area2,22,23,24. Yet, we still do not know how the quality of interactions between individuals can influence social signal brain processing and its lateralization, especially concerning population level asymmetries. Since more gregarious starlings show increased levels of opioids25 and since more socially integrated (i.e. with more bonding partners) ravens are less stressed physiologically26, social bonding could well play a major role in guiding neurophysiological functioning.
European starlings are open-ended learners and their social bonding is associated with vocal sharing27. This is also the case for other social species of songbirds28. Outside the breeding season, individual adult starlings associate with preferred, often same-sex and same-age partners with which they share parts of their song repertoire. Changes in group composition lead to new associations and rapid changes in song repertoires so that newly formed partnerships are associated with new patterns of vocal sharing29. While females generally form same-sex pairs30, males form small same-sex groups whose individuals differ in their degree of «social integration» (i.e. number of preferred partners). This degree of «social integration» is correlated with the degree of vocal «conformity» (i.e. proportion of shared structures in the vocal repertoire)29. This suggests individual variations in the levels of social motivation, tolerance and/or social attention.
If lateralization of sensory processes reflects attentional and social skills, we would expect that the more socially «engaged» male starlings would show higher levels of lateralization in brain processing of social signals. To test this hypothesis, we determined first the social relationships within a group of non-breeding captive starlings and then we recorded the HVC (as a proper name) electrophysiological activity of some of the males while they were listening to either familiar or unfamiliar male starling songs. HVC neurons of starlings not only respond to the bird’s own song (as in other species31) but also to individual whistles of conspecifics13,32. These responses show a right hemisphere dominance when the birds are awake, but not when they are anesthetized33. This reinforces the idea that processing complex social signals requires attention34.
Our subjects for the present study were a group of eight adult male starlings caught in the wild as adults 2 years before the experiment and kept since then in an outdoor aviary. Six months before the behavioral observations, they integrated a mixed group of 20 starlings (9 females and 3 other males) and they were observed during the non-breeding season which is favorable for determining social preferences (e.g. Henry et al.30). After the end of the behavioral observations, we recorded the neuronal activity of both left and right HVC during the playback of individual whistles of either familiar or unfamiliar male starlings (Fig. 1) using multielectrode systematic recordings35. We calculated the proportion of neuronal sites of both hemispheres responding to these individual whistles and used a hierarchical ascending classification based on an ANOVA to build individual neuronal profiles that integrate both neuronal preferences for familiar versus unfamiliar songs and asymmetries in brain processing.
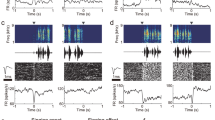
A set of stimuli including 10 Class-II individual male starling whistles. Left: six familiar whistles recorded in the experimental birds’ home aviary. These familiar whistles differed from one bird to another (birds’ own songs were not included). Right: four whistles recorded from unfamiliar distant birds. These unfamiliar whistles were the same for all birds.
Results
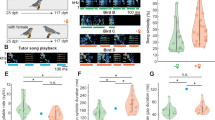
The birds spent most of their time in close proximity to another bird (only in 16.64 ± 8.53% of the scans was a subject at more than 50 cm from another bird). Aggression was rare (only threats: 1.36 aggression/hour/individual). The sociogram showed a pattern with strong dyadic spatial preferences and some birds that were less integrated (Fig. 2).
Spatial preferences and neuronal profiles of adult male starlings in our group of starlings (A2). Sociogram of proximity: Squares represent males and circles represent females. Filled colored squares represent males whose neuronal activity was recorded. Open blue squares and pink circles represent individuals that were housed in the aviary with the 8 experimental starlings. Solid black arrows link birds that were observed more often than expected by chance with another bird as their closest neighbor (χ2 test, large arrow: p ≤ .001, thin arrow, p ≤ .05). The different colors correspond to different clusters of neurophysiological profiles that are represented as radar plots. Each radius corresponds to the proportion of neuronal sites that responded to either the familiar or the unfamiliar stimuli in the left (left axes) and right (right axes) hemispheres.
We recorded the brain activity of 1,264 neuronal sites (632 sites/hemisphere; mean ± S.E.: 158 ± 17.28 sites/bird). Among these sites, 140 (11.1%) responded to at least one of the acoustic stimuli.
Significantly more neuronal sites were responsive in the right (mean ± S.E.: 10.87 ± 3.94) than in the left (mean ± S.E.: 6.75 ± 3.84) hemisphere (Wilcoxon test, n = 8, T = 1.5, p = 0.02). Each neuronal site responded to 2.70 ± 0.20 stimuli (mean ± SE). Most of them responded to only one or two stimuli (66.4%) whereas 33.5% responded to more than 2 stimuli.
Overall familiar stimuli elicited more responses than unfamiliar songs (Wilcoxon test, N = 7, T = 1, p = 0.05). However, one bird (A2M7) showed more responses to the unfamiliar songs.
We then evaluated the proportions and locations (right or left) of responsive neuronal sites in relation to type and familiarity of the stimulus. A cluster analysis revealed 4 distinct individual profiles (Fig. 3): cluster 1 (one single bird: M7) was characterized by a strong right hemisphere dominance and more responses to the songs of unfamiliar than familiar birds; cluster 2 (3 birds: M1, M2 and M3 ) was characterized by a strong right hemisphere preference for familiar songs; cluster 3 (one bird: M8) was characterized by a left hemisphere dominance for both familiar and unfamiliar stimuli; and cluster 4 (3 birds: M6, M4 and M5) was characterized by bilateral responses, hence weak lateralization. The neuronal responses were thus, except for one cluster, clearly lateralized with sometimes responsive neuronal sites in one hemisphere only.
Most importantly, we evidenced a correlation between “social engagement” (number of unilateral or bilateral associated partners) and the degree of lateralization of neuronal responses to the social acoustic signals (absolute laterality index: |L − R|/L + R; Spearman test of correlation, r = 0.83 p = 0.01) (Fig. 4).
The five birds (M1, M2, M3, M7 andM8) that showed neuronal responses in only one hemisphere (either the right or the left one) were involved in more preferred partnerships than the three birds that showed clear bilateral responses (M5, M6, M4) (Mann Whitney U test, n1 = 5, n2 = 3, U = 0, p = 0.018).
Discussion
Our neurophysiological data show important inter-individual variations of lateralization of auditory responses. The degree of lateralization, whatever its direction, was correlated with social engagement: the closer the birds were socially associated, the more their brain processing of individual social information was lateralized. Auditory-responsive neurons in the HVC showed a bias for processing the songs of familiar rather than those of unfamiliar conspecifics and those of the most «socially engaged» subjects were clearer lateralization, with a bias in favor of the right hemisphere.
Overall, more HVC auditory-responsive neuronal sites responded to familiar songs, especially in the right hemisphere. This is in accordance with the diverse hypotheses concerning the role of this hemisphere, for example in processing individual information36 or attention37. Playback experiments showed that female starlings paid more attention to songs of familiar birds38, or became more aroused39, maybe because they were surprised to hear a familiar song in a non-familiar setting. Recent findings suggest that young mammals position themselves with their left body side towards their mother, especially in affiliative contexts, reinforcing the idea of a right hemisphere dominance for social processing40. Anyway, this confirms that familiarity is a major aspect of social cognition. When typical adult starlings hear a conspecific they modulate the responses of their auditory-responsive neurons according to whether the song is familiar or unfamiliar, indicating a cross-modal representation of familiar conspecifics in the brain41.
Lateralization of the processing of social signals is common in songbirds42, as in all vertebrates14 but its potential relationships with social integration and cohesion have not been investigated. Here we show that the more socially engaged animals presented the strongest lateralization of HVC responses to individual conspecific’s songs. The advantages of lateralization have been largely discussed, often in terms of predator avoidance (at the level of the population) or conflict resolution between the two hemispheres (at the individual level17,43). Recent studies related lateralization to social systems: social bee species show a lateralization pattern for diverse functions that is not found in asocial species44. Populational biases of the direction of lateralization have been related to sociality, hypothesizing that social coordination may require alignment45,46,47. However, asocial mason bees present the same lateralized pattern during aggressive interactions as social bees48. Even basic social processes may thus have triggered the evolution of lateralization45.
The finding here that more lateralized individuals have more preferred social partners fuels the discussion. Interestingly, the absolute degree of lateralization rather than its direction appeared important here, although overall the right hemisphere tended to present more neuronal responses. Selection for strength, but not direction, of lateralization is possible15; this means that strength may reflect evolutionary processes better. Since social bonding is associated with well-being25, being more lateralized may advantage an individual. Possibly the ability to create and maintain social bonds could be related to increased social attention, a cognitive ability that allows better vocal copying and may enhance social bonding49,50. Attention has been proposed as a possible important process in the evolution of lateralization11,12. Anyway, relationships between lateralization of social signal processing and social skills deserve further consideration thus opening future lines of research.
Methods
Birds
our subjects were eight adult male starlings (A2). They had been caught in the wild as adults in Normandy (France) more than 2 years before the experiment. We observed them in October while they were housed in a mixed group of 20 starlings (11 females and 1 other male) that had been together for 6 months in an outdoor aviary (2 m × 4 m × 2.5 m) with many branches, shelter and open spaces where birds could forage for insects in the soil or the air. The aviary was located in a wooded area where wild starlings could be seen and heard. Water and food were provided ad libitum (commercial pellets and apples). We ringed all birds with a unique combination of colors on both legs.
Behavioral observations
Social preferences were evaluated following Hausberger et al.’s29 method. This method is based on recording the nearest neighbor (within a 50 cm radius). Social proximity is a very reliable indicator of social preferences51,52. We noted who was the closest neighbor using instantaneous scan sampling53 with one scan every 2 min. Observation sessions lasted 40 min and we obtained 323 scans per individual. For every bird, we evaluated at what frequency (proportion of scans) each other bird was its nearest neighbor. We decided whether this association occurred more or less often than expected by chance using chi-square analyses. This allowed us to include asymmetrical relationships. We then built sociograms based on spatial preferences.
We recorded agonistic interactions ad libitum because they are rare and short events.
Only one experimenter made all the behavioral observations from outside the aviary (1 m away).
Electrophysiological recordings
In December, after the behavioral observations, we recorded the electrophysiological activity of the birds’ HVC during the playback of acoustic social signals (i.e. song elements) while they were awake and restrained. This avoided potential biases related to anesthesia, such as a loss of lateralization or selectivity in neuronal responses12,33,34.
Each bird was exposed to a set of stimuli of 10 Class-II individual male starling whistles (Fig. 1). These whistles characterize individual birds or closely associated birds27. We recorded the familiar stimuli in the birds’ home aviary. The 6 familiar whistle types differed between birds (a bird’s own songs were not included). Four stimuli came from unfamiliar distant birds, i.e. birds that the experimental animals could not have heard (e.g. recorded in New Zealand). All the recordings were made with a Marantz PMD670 digital recorder and a Sennheiser MKH416 directional microphone.
We arranged the stimuli randomly in a single sequence lasting 19.74 ± 0.02 s. The mean duration of the stimuli was 641.55 ± 27.65 ms. As HVC neurons can respond up to 350 ms after the end of a stimulus54, we avoided any interference between two successive responses by using a mean interval between stimuli of 722.66 ± 10.74 ms with a minimum of 514 ms.
Using the approach described by George et al.35, we recorded neuronal activity systematically throughout the HVC of each hemisphere while broadcasting the acoustic stimuli to starlings that were awake and restrained. We used a four-microelectrodes array (two electrodes in each hemisphere) made of tungsten wires insulated by epoxylite (FHC). Electrodes were 1.2 mm apart in the sagittal plane and 5 mm apart in the coronal plane. Electrodes’ impedance was in the range of 5–6 MΩ.
We positioned the electrodes very precisely (± 2.5 µm) using stereotaxic coordinates (first extrapolated from the canary atlas of Stokes et al.55 and then confirmed by the 3D atlas of the starling brain made by De Groof et al.56). This approach is sufficiently precise (validated by earlier histological studies29,33), respects the 3R rules (reducing number of animals) and keeps the animals alive.
We made 12 penetrations throughout the HVC every 230 µm in a rostrocaudal row. As we lowered the four electrodes simultaneously, it required one to three recording sessions (only one session per day) to perform all the recordings. We therefore collected data in 1–3 days. We recorded electrophysiological activity systematically every 100 µm, dorso-ventrally along the path of the penetration of an electrode without any preselection of the recording sites with acoustic test stimuli. Between the recording sessions the birds were kept in individual cages placed in the same room where the birds could hear one another and interacted vocally. Food and water were provided ad libitum.
We played the acoustic stimuli in an anechoic soundproof chamber through a loudspeaker located 20 cm in front of the bird’s head. The peak sound pressure at the bird’s ears was 85 dB SPL for all stimuli which corresponded to 65 dB RMS for all stimuli. The whole sequence of stimuli was repeated 10 times at each recording site. The neuronal activity recorded by each electrode was visualized with raster plots in which each dot represented a spike. Spikes were detected with a voltage threshold trigger. At each recording site, before playing the stimuli, the experimenter manually adjusted the amplitude discrimination to limit recordings to the neurons exhibiting the biggest spikes, with a custom-made time- and level-window discriminator35. The spontaneous activity of each recording site was recorded during 1.5 s before the first acoustic stimulus of each sequence.
Neuronal responsiveness was assessed by comparing spontaneous activity level (number of action potentials) with activity during stimulation using binomial tests. Only responsive sites were analyzed further. We calculated the proportion of sites responding to each stimulus and to each type of stimulus. We then used the mean values calculated for individual birds for statistical comparisons.
Statistical analyses (see also Cousillas et al.57, George et al.32)
We used non-parametric statistics to analyze behavioral data, i.e. Chi-square tests to assess whether birds were more often than expected by chance close to some neighbors.
We compared neuronal responses between hemispheres and between stimuli using Wilcoxon tests for paired data. We compared responses to baseline with Chi-square/binomial tests.
In addition we built neurophysiological individual profiles: these profiles were built on the basis of the percentage of neuronal responses recorded in each hemisphere for each type of stimulus (familiar/unfamiliar). A hierarchical cluster analysis (HCA), based upon a Correspondence Factorial Analysis followed by a hierarchical cluster analysis was conducted to characterize individual bird’s electrophysiological profiles (Fig. 3). Birds displaying the same distribution of responses were identified and their data combined to represent profile clusters. Clusters, based on multivariate analyses, have proved useful in studies of personality and family psychology58 or individual behavioral profiles50. Each profile was represented by a radar plot and each ray of the plot represented the proportion of neuronal responses in one hemisphere for a given stimulus. Tests to identify the predominant responses on each radar were χ2 tests. We used Mann Whitney U tests to compare groups (i.e. clusters).
We calculated correlations between behavioral and neurophysiological data using a Spearman correlation test.
Ethical approval
These studies comply with the French laws related to animal experimentation and the European directive 86/609/CEE and 2010/63/UE and were approved by the Rennes local Animal Care Committee (CREEA).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Seebacher, F. & Krause, J. Physiological mechanisms underlying animal social behaviour. Philos. Trans. R. Soc. B Biol. Sci. 382, 20160231 (2017).
Olson, E. M., Maeda, R. K. & Gobes, S. M. H. Mirrored patterns of lateralized neuronal activation reflect old and new memories in the avian auditory cortex. Neuroscience 330, 395–402 (2016).
Lemasson, A., Boutin, A., Boivin, S., Blois-Heulin, C. & Hausberger, M. Horse (Equus caballus) whinnies: a source of social information. Anim. Cogn. 12, 693–704 (2009).
Kuhl PK Human speech and birdsong: communication and the social.brain. PNAS 100: 9645–9646 (2003).
Boeckle, M. & Bugnyar, T. Long-term memory for affiliates in ravens. Curr. Biol. 22, 801–806 (2012).
Nottebohm, F. Ontogeny of bird song. Science 167, 950–956 (1970).
Woolley, S. M. N. Early experience shapes vocal neural coding and perception in songbirds. Dev. Psychobiol. 54, 612–631 (2012).
Posner, M. I. & Rothbart, M. K. Attention, self-regulation and consciousness. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 353, 1915–1927 (1998).
Bolhuis, J. J., Van Mil, D. P. & Houx, B. B. Song learning with audiovisual compound stimuli in zebra finches. Anim. Behav. 58, 1285–1292 (1999).
Bertin, A., Hausberger, M., Henry, L. & Richard-Yris, M.-A. Adult and peer influences on starling song development. Dev. Psychobiol. 49, 362–384 (2007).
Andrew, R. J. & Watkins, J. A. S. Evidence for cerebral lateralization from senses other than vision. In Comparative Vertebrate Lateralization (eds Rogers, L. J. & Andrew, R.) 365–382 (Cambridge University Press, Cambridge , 2002).
Hausberger, M. et al. A crucial role of attention in lateralisation of sound processing?. Symmetry 11, 48 (2019).
George, I., Cousillas, H., Richard, J.-P. & Hausberger, M. State-dependent hemispheric specialization in the songbird brain. J. Comput. Neurol. 488, 48–60 (2005).
Vallortigara, G. & Rogers, L. J. A function for the bicameral mind. Cortex 124, 274–285 (2020).
Vallortigara, G. The evolutionary psychology of left and right: costs and benefits of lateralization. Dev. Psychobiol. 48, 418–427 (2006).
Rogers, L. J., Zucca, P. & Vallortigara, G. Advantages of having a lateralized brain. Proc. R. Soc. 271, 420–422 (2004).
Vallortigara, G. & Rogers, L. J. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, (2005).
Bolhuis, J. J., Hetebrij, E., Boer-Visser, A. M. D., Groot, J. H. D. & Zijlstra, G. G. O. Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. Eur. J. Neurosci. 13, 2165–2170 (2001).
Bolhuis, J. J., Zijlstra, G. G. O., den Boer-Visser, A. M. & Van der Zee, E. A. Localized neuronal activation in the zebra finch brain is related to the strength of song learning. Proc. Natl. Acad. Sci. 97, 2282–2285 (2000).
Galuret, S., Lumineau, S., Pouzol, D. & George, I. Mothering influences domestic chick’s laterality. Anim. Behav. 159, 69–79 (2020).
Moorman, S. et al. Human-like brain hemispheric dominance in birdsong learning. Proc. Natl. Acad. Sci. 109, 12782–12787 (2012).
Bell, B. A., Phan, M. L. & Vicario, D. S. Neural responses in songbird forebrain reflect learning rates, acquired salience, and stimulus novelty after auditory discrimination training. J. Neurophysiol. 113, 1480–1492 (2015).
Yang, L. M. & Vicario, D. S. Exposure to a novel stimulus environment alters patterns of lateralization in avian auditory cortex. Neuroscience 285, 107–118 (2015).
Tsoi, S. C. et al. Hemispheric asymmetry in new neurons in adulthood is associated with vocal learning and auditory memory. PLoS ONE 9, e108929 (2014).
Riters, L. V., Kelm-Nelson, C. A. & Spool, J. A. Why Do Birds Flock? A Role for Opioids in the Reinforcement of Gregarious Social Interactions. Front. Physiol. 10, (2019).
Stocker, M. et al. Loner or socializer? Ravens’ adrenocortical response to individual separation depends on social integration. Horm. Behav. 78, 194–199 (2016).
Hausberger, M. Social influences on song acquisition and sharing in the European starling (Sturnus vulgaris). In Social Influences on Vocal Development (eds Snowdon, C. T. & Hausberger, M.) 128–156 (Cambridge University Press, Cambridge , 1997).
Brown, E. D. & Farabaugh, S. M. What birds with complex social relationships can tell us about vocal learning: Vocal sharing in avian groups. In Social Influences on Vocal Development (eds Snowdon, C. T. & Hausberger, M.) 98–127 (Cambridge University Press, Cambridge , 1997).
Hausberger, M., Richard-Yris, M.-A., Henry, L., Lepage, L. & Schmidt, I. Song sharing reflects the social organization in a captive group of European starlings (Sturnus vulgaris). J. Comput. Psychol. 109, 222–241 (1995).
Henry, L., Bourguet, C., Coulon, M., Aubry, C. & Hausberger, M. Sharing mates and nest boxes is associated with female “friendship” in European starlings, Sturnus vulgaris. J. Comput. Psychol. 127, 1–13 (2013).
Margoliash, D. Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J. Neurosci. 6, 1643–1661 (1986).
George, I., Cousillas, H., Richard, J.-P. & Hausberger, M. New insights into the auditory processing of communicative signals in the HVC of awake songbirds. Neuroscience 136, 1–14 (2005).
George, I., Cousillas, H., Richard, J.-P. & Hausberger, M. Auditory responses in the HVC of anesthetized starlings. Brain Res. 1047, 245–260 (2005).
Karino, G. et al. Anesthesia and brain sensory processing: impact on neuronal responses in a female songbird. Sci. Rep. 6, 39143 (2016).
George, I., Cousillas, H., Richard, J.-P. & Hausberger, M. A new extensive approach to single unit responses using multisite recording electrodes: application to the songbird brain. J. Neurosci. Methods 125, 65–71 (2003).
Vallortigara, G. & Andrew, R. J. Differential involvement of right and left hemisphere in individual recognition in the domestic chick. Behav. Processes 33, 41–57 (1994).
Basile, M. et al. Socially dependent auditory laterality in domestic horses (Equus caballus). Anim. Cogn. 12, 611–619 (2009).
Hausberger, M., Forasté-Mathelier, M., Richard-Yris, M.-A. & Nygren, C. Differential response of female starlings to shared and nonshared song types. Etología 5, 31–38 (1997).
Rogers, L. J. & Andrew, R. Comparative Vertebrate Lateralization (Cambridge University Press, Cambridge, 2002).
Karenina, K., Giljov, A., Ingram, J., Rowntree, V. J. & Malashichev, Y. Lateralization of mother–infant interactions in a diverse range of mammal species. Nat. Ecol. Evol. 1, 1–4 (2017).
George, I., Richard, J.-P., Cousillas, H. & Hausberger, M. No need to talk, i know you: familiarity influences early multisensory integration in a Songbird’s brain. Front. Behav. Neurosci. 4, (2011).
George, I. Hemispheric asymmetry of songbirds. In Hemispheric Asymmetry of Songbirds (eds Hugdahl, K. & Westerhausen, R.) 102–131 (The MIT Press, London , 2010).
Lippolis, G., Bisazza, A., Rogers, L. J. & Vallortigara, G. Lateralisation of predator avoidance responses in three species of toads. Lateral. Asymmet. Body Brain Cogn. 7, 163–183 (2002).
Frasnelli, E. et al. The bee as a model to investigate brain and behavioural asymmetries. Insects 5, 120–138 (2014).
Ghirlanda, S. & Vallortigara, G. The evolution of brain lateralization: a game theoretical analysis of population structure. Proc. R. Soc. 271, 853–857 (2004).
Vallortigara, G. & Versace, E. Laterality at the neural, cognitive, and behavioral levels. In Handbook of Comparative Psychology: Vol. 1. Basic Concepts, Methods, Neural Substrate, and Behavior (ed. Call, J.) 557–577 (American Psychological Association (APA), Washington, DC, 2017).
Ghirlanda, S., Frasnelli, E. & Vallortigara, G. Intraspecific competition and coordination in the evolution of lateralization. Philos. Trans. R. Soc. Lond. 364, 861–866 (2009).
Rogers, L. J., Frasnelli, E. & Versace, E. Lateralized antennal control of aggression and sex differences in red mason bees, Osmia bicornis. Sci. Rep. 6, 29411 (2016).
Poirier, C. et al. Direct social contacts override auditory information in the song-learning process in starlings (Sturnus vulgaris). J. Comput. Psychol. 118, 179–193 (2004).
Grandgeorge, M. et al. Social rivalry triggers visual attention in children with autism spectrum disorders. Sci. Rep. 7, (2017).
Freeberg, T. M. Spatial associations provide a context for social learning of courtship patterns in brown-headed cowbirds (Molothrus ater). J. Comput. Psychol. 113, 327–332 (1999).
Mason, W. A. Ontogeny of social systems. Recent Adv. Primatol. 1, 5–14 (1978).
Altmann, J. Observational study of behavior: sampling methods. Behaviour 49, 227–266 (1974).
Margoliash, D. & Fortune, E. S. Temporal and harmonic combination-sensitive neurons in the zebra finch’s HVc. J. Neurosci. 12, 4309–4326 (1992).
Stokes, T. M., Leonard, C. M. & Nottebohm, F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J. Comput. Neurol. 156, 338–384 (1974).
De Groof, G. et al. A three-dimensional digital atlas of the starling brain. Brain Struct. Funct. 221, 1899–1909 (2016).
Cousillas, H. et al. Experience-dependent neuronal specialization and functional organization in the central auditory area of a songbird. Eur. J. Neurosci. 19, 3343–3352 (2004).
Henry, D. B., Tolan, P. H. & Gorman-Smith, D. Cluster analysis in family psychology research. J. Fam. Psychol. 19, 121–132 (2005).
Acknowledgements
We thank C. Aubry for taking care of the animals, I. Robles for her help in electrophysiological recordings and A. L’Azou for his help with bibliography. We are thankfull to Dr. A. Cloarec and A. L’Azou for improving the English Funding was provided by the French CNRS, Rennes 1 University and a Grant from the French ANR (Interimit, PI: Prof. A. Streri).
Author information
Authors and Affiliations
Contributions
H.C. and M.H. supervised the study; S.M., H.C., I.G. performed and analyzed the electrophysiological recordings; S.M., L.H. performed and analyzed the behavioral observations and M.H., H.C., L.H., I.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cousillas, H., Henry, L., George, I. et al. Lateralization of social signal brain processing correlates with the degree of social integration in a songbird. Sci Rep 10, 14093 (2020). https://doi.org/10.1038/s41598-020-70946-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-020-70946-7
This article is cited by
-
Effects of experience and familiarity on visual attention and behaviours of bottlenose dolphins (Tursiops truncatus) in presence of humans
Scientific Reports (2024)
-
Effect of visual lateralization on the spatial position of individuals within a school of oval squid (Sepioteuthis lessoniana)
Journal of Comparative Physiology A (2024)
-
Evolution and function of neurocognitive systems in non-human animals
Scientific Reports (2021)