Abstract
Despite improvements in systemic therapy options for renal cancer, it remains one of the most drug-resistant malignancies. Interestingly, reports have shown that kahweol and cafestol, natural diterpenes extracted from coffee beans, exhibit anti-cancer activity. However, the multiple potential pharmacological actions of both have yet to be fully understood. This study therefore investigated the effects of kahweol acetate and cafestol on human renal cancer ACHN and Caki-1 cells. Accordingly, the combination of kahweol acetate and cafestol administration synergistically inhibited cell proliferation and migration by inducing apoptosis and inhibiting epithelial–mesenchymal transition. Mechanistic dissection revealed that kahweol acetate and cafestol inhibited Akt and ERK phosphorylation. Moreover, kahweol acetate and cafestol downregulated the expression of not only C–C chemokine receptors 2, 5, and 6 but also programmed death-ligand 1, indicating their effects on the tumor microenvironment. Thus, kahweol acetate and cafestol may be novel therapeutic candidates for renal cancer considering that they exert multiple pharmacological effects.
Similar content being viewed by others
Introduction
Renal cancer represents the 6th and 10th most frequently diagnosed malignancy among men and women, accounting for 5% and 3% of all oncological diagnoses in the United States, respectively1. Increased tumor size and metastasis have been the major causes of renal cancer-related death2,3. Despite advancements in molecular-targeted therapies and immunotherapies, such as tyrosine kinase inhibitors targeting vascular endothelial growth factor (VEGF) and immune checkpoint inhibitors4,5,6,7,8, advanced renal cancer remains among the most drug-resistant malignancies. As such, novel therapeutic strategies that improve prognosis of patients with renal cancer are urgently needed.
Reports have suggested that natural products may serve as substitutes for anti-cancer agents with novel biochemical mechanisms that inhibit cell proliferation, modulate cell differentiation, and induce apoptosis9. We had previously reported, for the first time, that kahweol acetate and cafestol, natural diterpenes extracted from coffee beans, synergistically inhibit prostate cancer cell progression in vitro and in vivo10. Kahweol acetate and cafestol have a wide variety of bioactive properties, including anti-inflammation, anti-angiogenesis, and anti-tumorigenic properties and it is estimated that three or four cups of coffee reach bioactive serum concentration of kahweol and cafestol11. Therefore, the present study sought to examine the anti-cancer activity of kahweol acetate and cafestol in renal cancer cells.
Results
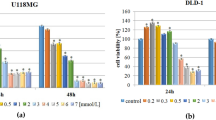
Suppression of human renal cancer cell proliferation following kahweol acetate and cafestol treatment
Considering our previous findings suggesting that kahweol acetate and cafestol had strong anti-proliferative effect in prostate cancer cells10, we determined whether kahweol acetate and cafestol could suppress cell proliferation of human renal cancer cells. Accordingly, kahweol acetate treatment significantly inhibited ACHN and Caki-1 proliferation after 24 and 48 h in a dose-dependent manner, respectively (Fig. 1a,b). Compared to control, ACHN and Caki-1 proliferation was around 40% and more than 90% lower 48 h after 30 and 100 µM of kahweol acetate treatment, respectively. Similarly, cafestol treatment significantly inhibited ACHN and Caki-1 cell proliferation after 24 and 48 h in a dose-dependent manner, respectively (Fig. 1c,d). Compared to control, ACHN and Caki-1 cells proliferation was 30–50% and more than 90% lower 48 h after 30 and 100 µM of cafestol treatment, respectively. To examine whether kahweol acetate and cafestol affect normal kidney cells, proliferation assay of proximal tubular cells from normal adult human kidney (HK-2) was performed. Since the combination of 30 µM kahweol acetate and 30 µM cafestol did not affect viability of HK-2 cells at all, 30 µM of kahweol acetate and cafestol was thought as non-cytotoxic concentrations of kahweol acetate and cafestol (Supplementary Figure S1). These results indicate that medium concentrations (10–30 µM) of kahweol acetate and cafestol exert an anti-proliferative effect on renal cancer cells without impairment of normal kidney cells.
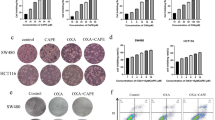
Anti-proliferative and migration effects of kahweol acetate and cafestol on renal cancer cells. (a–d) ACHN (a,c) and Caki-1 (b,d) cells were seeded in 12-well plates (5 × 104 cells/well) with RPMI containing 10% FBS. Each cell was treated with or without pre-determined concentrations of kahweol acetate and cafestol for 24 and 48 h. (e,f) Anti-proliferative effects of the combination of kahweol acetate and cafestol on ACHN (e) and Caki-1 (f) cells for 24 and 48 h. (g,h) Anti-migration effect of the combination of kahweol acetate and cafestol on ACHN (g) and Caki-1 (h) cells for 12 h. Bar = 500 µm. All experiments were performed in triplicate. Data are presented as means ± standard error of the mean. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. #Synergistic effects were observed.
Synergistic inhibition of human renal cancer cell proliferation and migration following combined kahweol acetate and cafestol treatment
Given that kahweol acetate and cafestol are both derived from coffee, these diterpenes can be ingested concurrently when drinking coffee11,12. In fact, we had also reported that the combination of these diterpenes synergistically affected prostate cancer cell viability10. The anti-cancer effects of combined kahweol acetate and cafestol treatment on human renal cancer cell proliferation and migration was evaluated by calculating the combination index (CI)13. Accordingly, the combination of 30 µM kahweol acetate and 30 µM cafestol promoted greater inhibition of ACHN and Caki-1 proliferation after 24 and 48 h compared to 30 µM kahweol acetate or 30 µM cafestol alone (CI < 1; synergism) (Fig. 1e,f). Even low-dose combination treatment (i.e., 10 µM kahweol acetate and 10 µM cafestol for 24 and 48 h) promoted inhibition of cell proliferation comparable to 30 µM of each diterpene treatment alone (CI < 1). Given studies showing that diterpenes had multifunctional anti-cancer effects11,12,14, cell migration assays were also conducted to verify the effects of kahweol acetate and cafestol on ACHN and Caki-1 cells. Similar to results of proliferation assays, the combination of 30 µM kahweol acetate and 30 µM cafestol promoted significantly greater inhibition of cell migration compared to 30 µM kahweol acetate or 30 µM cafestol alone (CI < 1). In addition, we performed wound healing assay to further confirm above results from transwell migration assay. The combination of 30 µM kahweol acetate and 30 µM cafestol promoted significantly greater inhibition of cell migration compared to 30 µM kahweol acetate or 30 µM cafestol alone (Supplementary Figure S2). Importantly, the combination of 30 µM of diterpenes showed comparable effects to 100 µM of each diterpene alone (Fig. 1g,h).
Apoptosis and downregulation of anti-apoptotic proteins in human renal cancer cells following kahweol acetate and cafestol treatment
TdT-mediated dUTP-biotin nick end labeling (TUNEL) assays of ACHN and Caki-1 cells were performed to assess whether kahweol acetate and cafestol could induce apoptosis. Consistent with the results of proliferation assays, kahweol acetate and cafestol strongly induced apoptosis, even with 30 µM of each diterpene alone (Fig. 2a,b). Next, western blot analyses were performed to determine changes in key proteins involved in cancer cell proliferation, including apoptosis-related proteins. Accordingly, the combination of 30 µM kahweol acetate and 30 µM cafestol hampered STAT3 activation (Fig. 2c,d). Moreover, the combination of 30 µM kahweol acetate and 30 µM cafestol promoted significantly lower expression of Bcl-2 and Bcl-xL, downstream of STAT3, compared to untreated cells (Fig. 2e,f). In addition, Bcl-2-associated X protein (Bax) increased by kahweol acetate and cafestol (Supplementary Figure S3). We further checked caspase-related apoptosis proteins to examine whether apoptosis induced by kahweol acetate and cafestol was caspase-dependent or not, there were no fragmentation of caspase-3 and PARP observed regardless of the concentration of kahweol acetate and cafestol (Supplementary Figure S4). The combination of 30 µM diterpenes inhibited the aforementioned apoptosis-related proteins to an extent comparable to high concentrations (100 µM) of each diterpene alone.
Kahweol acetate and cafestol treatment induced apoptosis by downregulating anti-apoptotic proteins in renal cancer cells. (a,b) TdT-mediated dUTP-biotin nick end labeling (TUNEL) assay (a) and statistics (b) showing apoptosis of ACHN or Caki-1 cells under kahweol acetate and cafestol treatment. ACHN and Caki-1 cells (3 × 104 cells) were exposed to predetermined concentrations of kahweol acetate and cafestol for 12 h on sterile slide coverslips, after which TUNEL assays were performed. All experiments were performed in triplicate. Data are presented as means ± standard error of the mean (SEM). Bar = 50 µm. (c–f) Protein levels of apoptosis-associated genes, p/tSTAT3 in ACHN (c) and Caki-1 (d) cells, Bcl-2/Bcl-xL in ACHN (e) and Caki-1 (f) cells, treated for 24 h were measured using Western blot analysis and quantitatively analyzed using densitometry with ImageJ software as shown by bar graphs. Equal amounts of cell lysates (10 µg) were subjected to electrophoresis. Data are presented means ± SEM (n = 4). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Downregulation of epithelial–mesenchymal transition-related proteins following kahweol acetate and cafestol treatment
Western blot analyses of epithelial–mesenchymal transition (EMT)-related proteins were performed to trace the mechanism whereby cell migration was inhibited. Accordingly, the combination of 30 µM kahweol acetate and 30 µM cafestol, as well as high concentrations (100 µM) of each diterpene alone, clearly reduced Snail expression in ACHN and Caki-1 cells (Fig. 3a,b). The combination of 30 µM kahweol acetate and 30 µM cafestol, as well as high concentrations (100 µM) of each diterpene alone, also clearly reduced expression of Twist, which had been reported to interact with Snail15, in ACHN and Caki-1 cells (Fig. 3c,d). Among other EMT-related proteins we examined, although E-cadherin and Slug did not express at all regardless of the concentration of kahweol acetate and cafestol, N-cadherin and β-catenin showed the tendency to decrease by kahweol acetate and cafestol in ACHN and Caki-1 cells (Supplementary Figure S5). The aforementioned results indicated that kahweol acetate and cafestol decreased migration ability of human renal cancer cells through the inhibition of EMT.
Kahweol acetate and cafestol treatment promoted downregulation of epithelial–mesenchymal transition (EMT)-related proteins in renal cancer cells. (a–d), Protein levels of EMT-associated genes, Snail in ACHN (a) and Caki-1 (b) cells, Twist in ACHN (c) and Caki-1 (d) cells, treated for 24 h were measured using Western blot analysis and quantitatively analyzed using densitometry with ImageJ software as shown by bar graphs. Soluble cell lysates (30 µg in Snail, 10 µg in Twist) were subjected to electrophoresis. Data were presented as means ± standard error of the mean (n = 4). *p < 0.05; **p < 0.01.
Downregulation of Akt and ERK signaling following kahweol acetate and cafestol treatment
Studies have reported Akt and ERK as factors strongly associated with the acceleration of renal cancer cell aggressiveness in terms of both growth and metastasis16,17. Akt and ERK, which promote EMT, are activated by STAT3 through downstream signaling18,19. Accordingly, the combination of 30 µM kahweol acetate and 30 µM cafestol rapidly inhibited Akt (Fig. 4a, b) and ERK phosphorylation (Fig. 4c,d) in ACHN and Caki-1 cells.
The combination of 30 µM kahweol acetate and 30 µM cafestol inhibited Akt and ERK signaling in renal cancer cells. (a–d) Phosphorylation of Akt in ACHN (a) and Caki-1 (b) cells, phosphorylation of ERK in ACHN (c) and Caki-1 (d) cells, at the indicated times after combination treatment were assessed using Western blot analysis and quantitatively analyzed using densitometry with ImageJ software as shown by bar graphs. Equal amounts of cell lysates (10 µg) were subjected to electrophoresis. Data are presented as means ± standard error of the mean (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Inhibition of immune signaling proteins after kahweol acetate and cafestol treatment
Cancer progression can be regulated by not only internal cancer cell signals but also external factors. Some immune cells that infiltrate into cancer tissues promote cancer progression by secreting chemokines that often activate cancer cells20,21. We thus investigated the influence of kahweol acetate and cafestol on C–C chemokine receptors (CCR2, 5, and 6) of representative chemokines activating renal cancer cells22,23,24,25,26. Accordingly, the combination of 30 µM kahweol acetate and 30 µM cafestol or 100 µM kahweol acetate administration reduced the expression of CCR2, CCR5, and CCR6 in both ACHN and Caki-1 cells (Fig. 5a,b). Moreover, with the emergence of immune checkpoint inhibitors as the prominent treatment method for renal cancer nowadays, the role of programmed death-ligand 1 (PD-L1) has also become a significant concern7. Accordingly, the combination of 30 µM kahweol acetate and 30 µM cafestol or 100 µM kahweol acetate administration reduced PD-L1 expression in both ACHN and Caki-1 cells (Fig. 5c,d). The aforementioned results indicated that kahweol acetate and cafestol may also affect the tumor microenvironment by inhibiting the immunological tolerance of renal cancer cells.
Kahweol acetate and cafestol treatment promoted the downregulation of immune signaling molecules in renal cancer cells. (a–d) Protein levels of C–C chemokine receptors (CCRs; CCR2/5/6) in ACHN (a) and Caki-1 (b) cells, and programmed death-ligand 1 (PD-L1) in ACHN (c) and Caki-1 (d) cells treated for 24 h were measured using Western blot analysis and quantitatively analyzed using densitometry with ImageJ software as shown by bar graphs. The soluble cell lysates (10 µg in CCR2/5 and PD-L1, 20 µg in CCR6) were subjected to electrophoresis. Data were presented as means ± standard error of the mean (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Discussion
Recently, several studies have shown that kahweol or cafestol can regulate tumor cell activity and apoptosis-related proteins through multiple targets, individually contributing to the inhibition of renal cancer cell proliferation27,28,29,30. The present study examined the anti-cancer properties of coffee kahweol acetate and cafestol and their synergistic effects on two renal cancer cells, following our previous study focusing on prostate cancer cells10. Accordingly, our results showed that kahweol acetate and cafestol inhibited the proliferation and migration of both ACHN and Caki-1 cells, with their synergistic effects apparent at relatively low concentrations. Moreover, the combination of kahweol acetate and cafestol induced downregulation in not only anti-apoptotic proteins (Bcl-2 and Bcl-xL) but also EMT-related proteins. Inhibition of STAT3, Akt, and ERK signaling pathway, which play pivotal roles in tumor cell proliferation, invasion, and migration, had also been observed. Among apoptosis-related proteins, although the fragmentation of caspase-3 and PARP was not observed, Bax was increased by kahweol acetate and cafestol. Recently, Bax is reported to induce caspase-independent apoptosis31. The diterpenes may induce apoptosis through the caspase-independent apoptotic pathway: however, the role and the mechanism of this pathway in renal cell cancer should be further investigated. The aforementioned results therefore suggest that kahweol acetate and cafestol, which have been identified as potential anti-cancer agents, can directly suppress renal cancer cell activities.
Immunotherapy, such as IFN-α and IL-2, had been the mainstream renal cancer therapy until the 1990s given the immunogenic property of renal cancer32. Recently, however, immune checkpoint inhibitors have become the prominent treatment method for renal cancer, with immune tolerance again becoming the main concern in the treatment of renal cancer7. Our previous studies had revealed that chemokines and their receptors were strong mediators of prostate and renal cancer progression with their effects on the tumor microenvironment33,34,35,36,37. Macrophage-like cells prepared from human monocytic leukemia cell line THP-1 promoted renal cancer cell migration, while CCL20, a specific ligand of CCR6, was more involved in macrophage-induced renal cancer cell migration than other cytokines37. Furthermore, AKT activation was involved in the promotion of renal cancer cell migration through the CCL20–CCR6 axis37. Some studies have already reported ERK activation is also induced by CCL20–CCR6 axis in cancer cells38,39. Therefore, it is interesting to note that kahweol acetate and cafestol treatment reduced CCR2, CCR5, and CCR6. The CCL2–CCR2 axis was reported to promote both cancer cell proliferation and migration by paracrine and by autocrine40. The CCL5–CCR5 axis also can arrange an immune-suppressive microenvironment, especially, BRCA1-associated protein 1-mutant clear cell renal cancer41. Moreover, studies have shown that PD-L1 expression was a negative prognostic factor and was associated with more advanced clinical features in patients with renal cancer42,43. Accordingly, our results showed that kahweol acetate and cafestol treatment also significantly inhibited PD-L1. T lymphocytes express PD-1 which is the receptor of PD-L1 and regulates the activity of T lymphocytes44. Therefore, inhibition of PD-L1 by kahweol acetate and cafestol treatment may increase the activity of T lymphocytes attacking renal cancer cells. An exploratory clinical study showed coffee consumption did not affect the number of T lymphocytes45. In addition, kahweol acetate and cafestol did not damage DNA of human peripheral lymphocytes46. Hence, although we did not clarify the direct effect of kahweol acetate and cafestol on T lymphocytes in this study, the diterpenes may not have significant effects on T lymphocytes. The aforementioned results thus indicate that kahweol acetate and cafestol can indirectly suppress renal cancer cell activities through by controlling the immunological tolerance of renal cancer cells. As shown in Fig. 6, kahweol acetate and cafestol exhibit multifunctional direct and indirect anti-tumor effects in renal cancer cells. Moreover, previous studies have suggested kahweol and cafestol could exhibit anti-angiogenic properties mainly by downregulating VEGF receptor-2, a primary mediator of the pro-angiogenic effect of VEGF14, with VEGF receptor inhibitor plus kahweol showing a synergistic effect in inducing renal cancer cell apoptosis30. Hence, the combination of kahweol acetate and cafestol may enhance the anti-cancer effects of conventional therapeutic agents, including tyrosine kinase inhibitors.
Schematic illustration of the anticancer mechanisms of kahweol acetate and cafestol. Kahweol acetate and cafestol inhibited cell proliferation and migration by inducing apoptosis and inhibiting epithelial–mesenchymal transition (orange and yellow colored molecules, respectively). Kahweol acetate and cafestol also inhibited CCR2/5/6, may potentially target the chemokine axis, and may affect the tumor immune environment by downregulating PD-L1 (blue colored molecules). Moreover, kahweol acetate and cafestol also inhibited the phosphorylation of Akt and ERK, which play central roles in tumor progression (gray colored molecules).
The current study revealed that kahweol acetate and cafestol may serve as not only therapeutic agents but also promising natural ingredients exhibiting anti-tumor effects against renal cancer cells. Although kahweol and cafestol are diterpenes included in unfiltered coffees, such as French press, espresso, and boiled coffees, concentrations of both diterpenes vary depending on the quality/blend and process of coffee preparation47. Studies have shown that 17.2 and 19.7 mg of kahweol and cafestol levels were present per cup (150 mL) of Arabica or Robusta coffee brewed using French press, respectively12. Assuming that an adult has a blood volume of 5000 mL and considering that approximately 70% of the consumed kahweol and cafestol can be absorbed in small intestine11, kahweol and cafestol concentrations may reach 30 µM each with three or four cups of coffee. Hence, achieving a concentration of 30 µM for both kahweol and cafestol, at which both exerted anti-cancer effects against renal cancer cells in our experiment, seems to be practicable.
In conclusion, the current study suggested that kahweol acetate and cafestol synergistically contributed to the inhibition of not only renal cancer proliferation and migration, by inducing apoptosis and inhibiting EMT, but also tumor microenvironment-related pathways, such as CCRs and PD-L1. Therefore, the combined use of kahweol acetate and cafestol may be a potentially effective therapeutic modality against renal cancer.
Materials and methods
Reagents and antibodies
Kahweol acetate (sc-228383A) and cafestol (sc-204663) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). The following antibodies were used during western blot analyses: mouse anti-Bcl-2 (15071S), rabbit anti-Bcl-xL (2764S), rabbit anti-STAT3 (8719S), rabbit anti-phospho-STAT3 (9131S), rabbit anti-Akt (9272S), rabbit anti-phospho-Akt (Ser473; 9271S), rabbit anti-ERK (9102S), rabbit anti-phospho-ERK (Thr202/Tyr204; 9101S), rabbit anti-PD-L1 (13684S), and horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (7074S) antibodies from Cell Signaling Technology (Danvers, MA, USA); mouse anti-Snail (ab117866), mouse anti-Twist (ab175430), rabbit anti-CCR2 (ab155321), rabbit anti-CCR5 (ab32048), and rabbit anti-CCR6 (ab227036) from Abcam (Cambridge, MA, USA); mouse anti-GAPDH (NB300-221) from Novus Biologicals (Littleton, CO, USA); and HRP-conjugated anti-mouse IgG (1706516) antibody from Bio-Rad Laboratories (Hercules, CA, USA). Antibody information used in western blot analyses for Supplementary Figures is shown in Supplementary Figure S6.
Cell culture
ACHN and Caki-1 human renal cancer cells, and HK-2 human normal kidney proximal tubular cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Human renal cancer cell line cells and human kidney cell line cells were maintained in a growth medium containing RPMI-1640 (R8758; Sigma-Aldrich, St. Louis, MO, USA) and DMEM (D5796; Sigma-Aldrich), respectively, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in a humidified incubator at 37 °C with 5% CO2.
Cell proliferation assay
ACHN and Caki-1 cells were seeded in 12-well plates (5 × 104 cells/well) with RPMI containing 10% FBS. Each was cell treated with or without predetermined concentrations of kahweol acetate and cafestol for 24 and 48 h. After the cells were harvested, cell numbers were counted using a hemocytometer.
Cell migration assay
ACHN and Caki-1 cells (1 × 104 cells/well) were seeded onto the upper chamber of transwell plates (cell culture inserts with 8.0-μm pore sizes for 24-well plates) with RPMI containing 0.1% FBS, while the lower compartment was filled with predetermined concentrations of kahweol acetate and cafestol in RPMI containing 10% FBS. Cells were then incubated for 12 h at 37 °C in a humidified incubator containing 5% CO2. Thereafter, the cells on the filter of cell culture inserts were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min. The cells on top of the filter were carefully removed with a cotton swab, while those on the back of the filter were stained with 0.1% crystal violet for 20 min. The stained filter was then microscopically photographed, after which migrated cells in two random fields were counted.
Western blot analyses
Cell lysates were prepared using RIPA lysis buffer (FUJIFILM Wako Pure Chemical Corporation, Japan) containing 1% protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma-Aldrich). Soluble lysates (10–30 µg) were mixed with a lithium dodecyl sulfate sample buffer and sample reducing agent, both obtained from Thermo Fisher Scientific (Waltham, MA, USA), and separated through sodium dodecyl sulfate polyacrylamide gel electrophoresis. Separated proteins were then transferred to nitrocellulose membranes. The membranes were blocked with 1% gelatin and 0.05% Tween in Tris-buffered saline for 1 h at room temperature and then incubated overnight at 4 °C with primary antibody according to the manufacturer’s instructions. After washing, the membranes were incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibody for 1 h at room temperature. Protein bands were detected using the Super Signal West Femto maximum sensitivity substrate (Thermo Fisher Scientific). Full length images of cropped blots presented in Figs. 2, 3, 4, and 5 are shown in Supplementary Figure S7.
Apoptosis assay
Apoptosis was detected using TUNEL assay with the DeadEndTM Fluorometric TUNEL System (Promega, Madison, WI, USA) as per the manufacturer's instructions. Briefly, ACHN and Caki-1 cells (3 × 104 cells) were exposed to predetermined concentrations of kahweol acetate and cafestol for 12 h on sterile slide coverslips. The cells were then washed twice with PBS, fixed with 4% methanol-free paraformaldehyde for 25 min, washed twice with PBS, and permeabilized with 0.2% Triton X-100 for 20 min. After two more washes, each glass slide was covered with equilibration buffer for 10 min. Thereafter, the buffer was aspirated, and the glass slides were incubated with rTdT buffer at 37 °C for 1 h under shading. Chromosomal DNA was stained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) for 15 min, after which the stained cells mounted on slides were examined using an OLYMPUS cellSens Standard (OLYMPUS, Japan).
Combination index
Synergistic effect was evaluated using combination index (CI) analysis widely used for evaluating drug interactions in combination cancer chemotherapy. The CI is calculated using the following formula: CI = (D)1/(Dx)1 + (D)2/(Dx)2. In the denominator, (Dx)1 represents the concentration at which Drug 1 “alone” inhibits a system x%, while (Dx)2 represents the concentration at which Drug 2 “alone” inhibits a system x%. In the numerators, (D)1 and (D)2 represent the concentrations for Drug 1 and Drug 2 that (D)1 + (D)2 “in combination” inhibit a system x%. The CI-isobologram equation allows for the quantitative determination of drug interactions, where CI < 1, = 1, and > 1 indicate synergism, additive effect, and antagonism, respectively13.
Statistical analyses
Protein expression was quantitatively analyzed through densitometry using ImageJ software (Bethesda, MD, USA). Graphs are presented as means ± standard error of mean (SEM). Statistical analysis was performed using GraphPad Prism version 6.07 (GraphPad Software, San Diego, CA, USA). Statistically significant differences were determined using one-way ANOVA followed by Tukey’s post hoc analysis, with p values < 0.05 indicating statistical significance.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019).
Bhindi, B. et al. Are we using the best tumor size cut-points for renal cell carcinoma staging?. Urology 109, 121–126 (2017).
Klatte, T., Rossi, S. H. & Stewart, G. D. Prognostic factors and prognostic models for renal cell carcinoma: a literature review. World J. Urol. 36, 1943–1952 (2018).
Choueiri, T. K. & Motzer, R. J. Systemic therapy for metastatic renal-cell carcinoma. N. Engl. J. Med. 376, 354–366 (2017).
Capitanio, U. & Montorsi, F. Renal cancer. Lancet 387, 894–906 (2016).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Rini, B. I. et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 393, 2404–2415 (2019).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803 (2020).
Iwamoto, H. et al. Coffee diterpenes kahweol acetate and cafestol synergistically inhibit the proliferation and migration of prostate cancer cells. Prostate 79, 468–479 (2019).
Ren, Y., Wang, C., Xu, J. & Wang, S. Cafestol and kahweol: a review on their bioactivities and pharmacological properties. Int. J. Mol. Sci. 20, 4238 (2019).
Moeenfard, M. & Alves, A. New trends in coffee diterpenes research from technological to health aspects. Food Res. Int. 134, 109207 (2020).
Chou, T. C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 58, 621–681 (2006).
Moeenfard, M. et al. Anti-angiogenic properties of cafestol and kahweol palmitate diterpene esters. J. Cell Biochem. 117, 2748–2756 (2016).
Rembold, M. et al. A conserved role for Snail as a potentiator of active transcription. Genes Dev. 28, 167–181 (2014).
Guo, H. et al. The PI3K/AKT pathway and renal cell carcinoma. J. Genet. Genom. 42, 343–353 (2015).
Campbell, L., Nuttall, R., Griffiths, D. & Gumbleton, M. Activated extracellular signal-regulated kinase is an independent prognostic factor in clinically confined renal cell carcinoma. Cancer 115, 3457–3467 (2009).
Li, B. & Huang, C. Regulation of EMT by STAT3 in gastrointestinal cancer (review). Int. J. Oncol. 50, 753–767 (2017).
Yu, H. & Jove, R. The STATs of cancer: new molecular targets come of age. Nat. Rev. Cancer 4, 97–105 (2004).
Beatty, G. L. & Gladney, W. L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 21, 687–692 (2015).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Wang, Z. et al. CCL2/CCR2 axis is associated with postoperative survival and recurrence of patients with non-metastatic clear-cell renal cell carcinoma. Oncotarget 7, 51525–51534 (2016).
Guan, X., Liu, Z., Zhang, J. & Jin, X. Myeloid-derived suppressor cell accumulation in renal cell carcinoma is correlated with CCL2, IL-17 and IL-18 expression in blood and tumors. Adv. Clin. Exp. Med. 27, 947–953 (2018).
Schuler, Y. et al. Osteoblast-secreted factors enhance the expression of dysadherin and CCL2-dependent migration of renal carcinoma cells. Int. J. Cancer 130, 288–299 (2012).
Arakaki, R. et al. CCL2 as a potential therapeutic target for clear cell renal cell carcinoma. Cancer Med. 5, 2920–2933 (2016).
Wu, Y., Li, Y., Matsushima, K., Baba, T. & Mukaida, N. CCL3–CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. J. Immunol. 181, 6384–6393 (2008).
Woo, S. M. et al. Cafestol overcomes ABT-737 resistance in Mcl-1-overexpressed renal carcinoma Caki cells through downregulation of Mcl-1 expression and upregulation of Bim expression. Cell Death Dis. 5, e1514 (2014).
Choi, M. J. et al. Cafestol, a coffee-specific diterpene, induces apoptosis in renal carcinoma Caki cells through down-regulation of anti-apoptotic proteins and Akt phosphorylation. Chem. Biol. Interact. 190, 102–108 (2011).
Um, H. J. et al. The coffee diterpene kahweol sensitizes TRAIL-induced apoptosis in renal carcinoma Caki cells through down-regulation of Bcl-2 and c-FLIP. Chem. Biol. Interact. 186, 36–42 (2010).
Min, K. J., Um, H. J., Kim, J. I. & Kwon, T. K. The coffee diterpene kahweol enhances sensitivity to sorafenib in human renal carcinoma Caki cells through down-regulation of Mcl-1 and c-FLIP expression. Oncotarget 8, 83195–83206 (2017).
Lindenboim, L. et al. Apoptotic stress induces Bax-dependent, caspase-independent redistribution of LINC complex nesprins. Cell Death Discov. 6, 90 (2020).
Takezawa, Y. et al. Treatment outcome of low-dose interleukin-2 therapy in patients with metastatic renal cell carcinoma. Anticancer Res. 36, 4961–4964 (2016).
Izumi, K. et al. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Mol. Med. 5, 1383–1401 (2013).
Maolake, A. et al. Tumor necrosis factor-alpha induces prostate cancer cell migration in lymphatic metastasis through CCR7 upregulation. Cancer Sci. 109, 1524–1531 (2018).
Natsagdorj, A. et al. CCL2 induces resistance to the antiproliferative effect of cabazitaxel in prostate cancer cells. Cancer Sci. 110, 279–288 (2018).
Urata, S. et al. C–C motif ligand 5 promotes migration of prostate cancer cells in the prostate cancer bone metastasis microenvironment. Cancer Sci. 109, 724–731 (2018).
Kadomoto, S. et al. Tumor-associated macrophages induce migration of renal cell carcinoma cells via activation of the CCL20-CCR6 axis. Cancers (Basel) 12, 89 (2019).
Chin, C. C. et al. Interleukin-17 induces CC chemokine receptor 6 expression and cell migration in colorectal cancer cells. J. Cell Physiol. 230, 1430–1437 (2015).
Zhang, X. P. et al. Role of CCL20/CCR6 and the ERK signaling pathway in lung adenocarcinoma. Oncol. Lett. 14, 8183–8189 (2017).
Kuper, C., Beck, F. X. & Neuhofer, W. Autocrine MCP-1/CCR2 signaling stimulates proliferation and migration of renal carcinoma cells. Oncol. Lett. 12, 2201–2209 (2016).
Zhou, Q. et al. CCR5 blockade inflames antitumor immunity in BAP1-mutant clear cell renal cell carcinoma. J. Immunother. Cancer 8, e000228 (2020).
Wang, Z. et al. Prognostic and clinicopathological significance of PD-L1 in patients with renal cell carcinoma: a meta-analysis based on 1863 individuals. Clin. Exp. Med. 18, 165–175 (2018).
Kammerer-Jacquet, S. F. et al. Targeting the PD-1/PD-L1 pathway in renal cell carcinoma. Int. J. Mol. Sci. 20, 1692 (2019).
Xu, W., Atkins, M. B. & McDermott, D. F. Checkpoint inhibitor immunotherapy in kidney cancer. Nat. Rev. Urolo. 17, 137–150 (2020).
Melamed, I., Kark, J. & Spirer, Z. Coffee and the immune system. Int. J. Immunopharmacol. 12, 129–134 (1990).
Bichler, J. et al. Coffee consumption protects human lymphocytes against oxidative and 3-amino-1-methyl-5H-pyrido[4,3-b]indole acetate (Trp-P-2) induced DNA-damage: results of an experimental study with human volunteers. Food Chem. Toxicol. 45, 1428–1436 (2007).
van Cruchten, S. T. et al. The role of epoxidation and electrophile-responsive element-regulated gene transcription in the potentially beneficial and harmful effects of the coffee components cafestol and kahweol. J. Nutr. Biochem. 21, 757–763 (2010).
Acknowledgements
This work was supported by JSPS KAKENHI (Grant Nos. 19K09685 to K. Izumi and 20K09574 to Y. Kadono).
Author information
Authors and Affiliations
Contributions
K.I. had full access to all study data and takes responsibility for the integrity of the data and accuracy of data analysis. Study concept and design: K.I. Acquisition, analysis, or interpretation of data: T.M. Drafting of the manuscript: T.M. Critical revision of the manuscript for important intellectual content: K.H., H.K., T.S., T.N., S.K., R.N., H.I., H.Y., K.S., Y.K., H.N., Y.S., K.NG., N.S, Y.I, and T.W. Statistical analysis: T.M. Study supervision: A.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makino, T., Izumi, K., Hiratsuka, K. et al. Anti-proliferative and anti-migratory properties of coffee diterpenes kahweol acetate and cafestol in human renal cancer cells. Sci Rep 11, 675 (2021). https://doi.org/10.1038/s41598-020-80302-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-020-80302-4