Abstract
Possible links between periodontitis and various cardiometabolic and autoimmune diseases have been advocated on the basis of chronic inflammation or oxidative stress. However, the association between periodontitis and thyroid dysfunction is under-researched. Participants without previous thyroid disease or ongoing thyroid-related medication were included from a nationwide population-level survey. Participants were categorized into tertiles of thyroid stimulating hormone (TSH) levels (first tertile < 1.76 mIU/L; second tertile 1.76–2.83 mIU/L; third tertile > 2.83 mIU/L), and periodontal condition was assessed using the Community Periodontal Index. Of the total of 5468 participants, 1423 had periodontitis (26%). A significant difference in the weighted prevalence of periodontitis according to TSH tertiles was observed, with the highest prevalence in the first tertile (26.5%) and the lowest prevalence in the third tertile (20.9%, p = 0.003). Subjects in the first TSH tertile had higher odds for periodontitis than those in the third tertile (OR 1.36, 95% CI 1.10–1.68; p for trend = 0.005) after adjusting for covariates. This association was consistent across subgroups and within sensitivity analyses among subjects without specific factors affecting thyroid function or diseases reported to be related to periodontitis. The present study demonstrated that low TSH levels were associated with significantly higher odds for periodontitis.
Similar content being viewed by others
Introduction
The inflammatory process underpins the etiology of a wide variety of systemic diseases, ranging from metabolic to psychological disorders1. Chronic inflammation can lead to hypertension, dyslipidemia, cardiovascular disease (CVD), type 2 diabetes mellitus (DM), non-alcoholic fatty liver disease (NAFLD), chronic kidney disease (CKD), and neurodegenerative conditions. These conditions are the primary causes of morbidity and mortality worldwide, accounting for more than 50% of all deaths2,3.
Periodontitis is one of the most common chronic infectious diseases in humans, occurring as a result of dysregulation of the host immune response triggered by subgingival microorganisms4,5. It is highly prevalent worldwide with severe periodontitis affecting 7.4% of the global population, posing a significant economic burden and major public health challenge6,7. The potential connections between periodontal disease and systemic disorders have attracted considerable interest in recent decades. In fact, a large body of evidence supports the association between periodontitis and various diseases, including CVDs8,9,10, DM11,12, metabolic syndrome13, obesity14, NAFLD15, CKD16, osteoporosis17,18, rheumatoid arthritis19, Alzheimer's disease20, and Parkinson’s disease21. Inflammation (mediated in part by an increase in levels of the serum C-Reactive Protein and inflammatory cytokines tumor necrosis factor-α [TNF-α], interleukin-1 [IL-1], and IL-6), oxidative stress, and endothelial dysfunction have been proposed as the underlying mechanisms for the interrelationship between periodontitis and these diseases22,23,24,25,26,27.
The thyroid is a typical organ within which organ-specific autoimmune disease and chronic inflammation occur frequently. Thyroid hormones play an important role in oxidative stress and inflammation in humans28. However, most previous studies on the association between periodontitis and thyroid dysfunction consist of animal studies, case reports, and retrospective chart review analyses with a limited number of patients29. Therefore, this study aimed to evaluate the link between periodontitis and thyroid function using data from Korea National Health and Nutrition Examination Survey (KNHANES), which is a large population-level survey performed by Korean government that provides latest nationally representative data containing credible health data carried out by well-trained staffs30.
Results
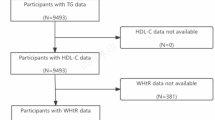
From a total of 6255 participants, representative of 13,033,409 Koreans, a final sample of 5468 subjects with data on thyroid function status and Community Periodontal Index (CPI) scores were eligible for analyses (Fig. 1). Participants were categorized based on thyroid stimulating hormone (TSH) tertiles. The ranges of serum TSH levels were < 1.76 mIU/L for the first tertile, 1.76–2.83 mIU/L for the second tertile, and > 2.83 mIU/L for the third tertile.
Baseline characteristics of the study subjects
Table 1 summarizes the baseline characteristics of the study participants according to TSH tertile. Participants presented with a median age of 41 years in all three TSH groups, and 47.6% of the participants were female. A higher proportion of female participants was observed in the highest tertile group than in the other groups (p < 0.001). Accordingly, fewer participants smoked and consumed alcohol in the third tertile than in the first or second tertiles (p < 0.001 for smoking and p = 0.001 for alcohol). No significant differences were observed with regard to fasting glucose, SBP, or total cholesterol among the three groups, though the estimated glomerular filtration rate (eGFR) was slightly higher in the first tertile group than in the other groups (p = 0.003). Logarithmically transformed urine iodine concentration and TPOAb positivity were both highest in the third tertile group compared to the other groups (p < 0.001 for both urine iodine and TPOAb).
Prevalence of periodontitis according to TSH tertiles
We observed significant differences in the weighted prevalence of periodontitis according to TSH tertile (p = 0.003, Fig. 2A); 26.5% of participants in the first TSH tertile presented with periodontitis, whereas this prevalence decreased to 23.1% in the second tertile and 20.9% in the third tertile. Similar results were observed in the subgroup of male participants (p = 0.009, Fig. 2B). However, the association between periodontitis and TSH level was not statistically significant among female participants (p = 0.587, Fig. 2C).
Association between periodontitis and serum TSH levels
The impact of serum TSH levels on periodontitis was evaluated using multiple logistic regression analyses (Table 2). In the minimally adjusted model (adjusted for age and sex; model 1), the odds ratio (OR) for periodontitis was 1.34 (95% confidence interval [CI] 1.10–1.63) for the first tertile of TSH compared to the third tertile. The association remained statistically significant after adjusting for age, sex, BMI, smoking, alcohol, exercise, fasting glucose, SBP, total cholesterol, eGFR, AST, and ALT, with an OR of 1.39 (95% CI 1.14–1.71, model 2). Further adjustment for log-transformed urine iodine levels and TPOAb (model 3) showed significantly higher odds for periodontitis among subjects in the lowest TSH tertile group than among those in the third tertile (OR 1.36, 95% CI 1.10–1.68; p for trend = 0.005).
Subgroup analyses
Figure 3 shows forest plots of the ORs for the first tertile group compared to the third tertile group as well as according to prespecified subgroups defined at baseline. No differences were observed in subgroups stratified by age (< 65 years vs. ≥ 65 years), sex (male vs. female), BMI (< 23 kg/m2 vs. ≥ 23 kg/m2), smoking (no vs. yes), alcohol intake (no vs. yes), and regular exercise (no vs. yes), with no statistically significant p-values for multiplicative interaction.
Sensitivity analyses
Sensitivity analyses were performed among participants without TPOAb, excessive iodine intake (urine iodine > 300 μg/L according to World Health Organization (WHO) criteria31), DM, stroke, coronary artery disease, rheumatoid arthritis, or CKD (defined as eGFR < 60 mL/min). We conducted these sensitivity analyses to examine the robustness of our findings when excluding participants presenting with specific factors affecting thyroid function and/or diseases with well-established associations with periodontitis. These multiple logistic regression analyses using model 33 showed strong associations between TSH levels and periodontitis, with adjusted ORs of 1.33 (1.08–1.65) in participants without TPOAb (Supplementary Table 1), 1.37 (1.04–1.81) in participants with urine iodine ≤ 300 μg/L (Supplementary Table 2), 1.37 (1.10–1.70) in participants without DM (Supplementary Table 3), 1.26 (1.02–1.57) in participants without stroke (Supplementary Table 4), 1.25 (1.01–1.55) in participants without coronary artery disease (Supplementary Table 5), 1.25 (1.01–1.55) in participants without rheumatoid arthritis (Supplementary Table 6), and 1.35 (1.09–1.66) in participants without rheumatoid arthritis (Supplementary Table 7) when comparing participants in the first TSH tertile to those in the third tertile.
Discussion
This nationwide cohort study of 5468 participants showed that thyroid function was significantly associated with periodontitis. Specifically, lower serum TSH levels were associated with higher prevalence of periodontitis, after adjustment for various confounding factors. This association was consistent within subgroups stratified by age, sex, BMI, smoking status, alcohol consumption, or exercise. Moreover, higher ORs for periodontitis were observed in participants without TPOAb, excessive iodine intake, and diseases well known to be associated with thyroid disorders or periodontitis.
More than half of all-cause mortality worldwide is attributable to inflammation-related diseases such as CVDs, DM, NAFLD, CKD, and autoimmune and neurodegenerative disorders1,2. Recent studies have suggested that periodontitis may be a risk factor for these wide range of systemic diseases8,9,10,11,12,15,16,19. Periodontitis is a periodontal disorder characterized by both dysbiosis of the oral microbiota and dysregulated inflammatory interactions32. Imbalances between proinflammatory and anti-inflammatory cytokines have deleterious effects on tissue destruction in chronic periodontitis33,34. These inflammatory processes, along with host immune response, provide the basis for the possible associations between periodontitis and the aforementioned noncommunicable diseases27,35. Moreover, studies have observed that inflammatory reactions in periodontitis are related to increased local and systemic oxidative stress and impaired antioxidant capacity, thereby affecting systemic health23. Indeed, it is globally recognized that dental care and oral health are key factors for maintaining health and protecting against various systemic diseases36. These circumstances highlight the need to evaluate disorders that affect periodontal health. However, the association between thyroid function and periodontitis has not been adequately established, and the present study is yet the largest study examining this association using a nationally representative dataset.
Previous studies regarding the possible link between thyroid function and periodontitis mostly focused on the impact of hypothyroidism in a limited number of patients37. Rahangdale et al. reported that clinical parameters associated with periodontal status differed among hypothyroid patients on thyroxine therapy (n = 52) and those in the control group (n = 50)37. However, this study was confined to a small group of thyroxine-treated patients with hypothyroidism and did not adhere to an established definition of periodontitis, such as the CPI. Feitosa et al. showed a positive association between hypothyroidism and bone loss in a rat model of ligature-induced periodontitis38. In fact, a recent review assessing the relationship between hypothyroidism and periodontitis concluded that, to date, very few high-quality studies have been conducted to determine this association, although the review suggested a modest association between these diseases based on current epidemiological and laboratory-based reports29. Our population-based study used TSH levels as an indicator of thyroid function, thereby covering the whole range of thyroid function (including euthyroid and hyperthyroidism). We observed that low TSH levels were rather associated with a higher prevalence of periodontitis, as defined by the CPI criteria.
There is a complex immunopathology underlying the etiology of periodontitis, which is now recognized as a chronic inflammatory disease39. Previous studies have investigated the pathogenesis of periodontitis and its association with the inflammatory cytokines IL-1β, IL-4, IL-6, IL-10, interferon-γ (IFN-γ), and TNF-α39. Interestingly, various cytokines, including IL-1β, IL-6, IL-10, IFN-γ, and TNF-α, are found in the thyroid follicular cells that augment the inflammatory response40. The importance of cytokines and chemokines in the pathogenesis of autoimmune thyroid disorders has been emphasized in recent studies. Recruited T helper 1 (Th1) lymphocytes stimulate IFN-γ and TNF-α production from the thyroid cells, which enhances the secretion of CXCL10, an IFN-γ-inducible Th1 chemokine41. This mechanism launches an amplification feedback loop and sustains the autoimmune process41. The active phase of hyperthyroidism is associated with increased circulating levels of these chemokines42. Moreover, thyroid dysfunction and thyroid autoantibodies have been commonly reported in patients with systemic autoimmune diseases such as rheumatoid arthritis, highlighting the possibility of common pathogenic mechanisms within various autoimmune diseases43.
Oxidative stress is a pivotal mechanism underlying the process of inflammation, and a vicious cycle exists between oxidative stress and chronic inflammation28. Increased oxidative stress is a characteristic of hyperthyroidism44 and hypermetabolic states in hyperthyroidism can cause oxidative tissue injury45. Thyroid hormones have been shown to regulate the oxidative system by increasing reactive oxidative species and lowering antioxidant availability28,46. It can directly induce DNA damage or enhance nitric oxide synthase gene expression to overproduce nitric oxide45,47. Antioxidative status may also be regulated through an increase in the turnover of mitochondrial proteins and mitoptosis48. These mechanisms share epidemiological associations with periodontitis—furthermore with systemic inflammatory diseases—and may explain the observed impact of TSH levels on periodontitis in this study. Although bacteria induce periodontitis, previous research indicates that inflammation and oxidative stress may be the main causes of tissue damage progression in periodontitis49.
The association between vitamin D levels and autoimmune thyroid disease has been widely studied in recent years50. Previous evidence supports the influence of vitamin D deficiency on the increased risk of developing thyroid diseases and/or increased antibody titers50,51. Interestingly, a systematic review and meta-analysis demonstrated that circulating vitamin D levels were significantly lower in patients with chronic periodontitis than in healthy controls52. These results suggest a possible pathophysiological role of vitamin D in linking thyroid dysfunction and periodontitis. Unfortunately, the present study could not analyze or adjust for vitamin D levels due to the lack of data in KNHANES VI.
The subgroup analyses in the present study clearly demonstrated that associations between TSH levels and periodontitis remained significant regardless of age, sex, BMI, smoking status, alcohol consumption, or exercise (Fig. 3). In addition, results were consistent when sensitivity analyses were performed in subjects without TPOAb or high urine iodine levels (> 300 μg/L). Given the strong and well-established association between periodontitis and rheumatoid arthritis (a classic autoimmune disease in humans19), TPOAb, which is the most sensitive marker for detecting autoimmune thyroid disease53, could plausibly be related to periodontitis. However, contrary to our expectation, TPOAb had no impact on periodontitis within the current study. This may indicate that thyroid hormone per se has an effect on periodontal inflammation through the above-mentioned mechanisms, although the low TPOAb positivity (4.9%) may have limited the likelihood of reaching statistical significance. In subjects without DM, stroke, coronary artery disease, rheumatoid arthritis, or CKD, the impact of TSH on periodontitis remained significant, supporting the robust association observed between thyroid function and periodontitis.
There are some limitations to address for this study. First, the major limitation lies on the cross-sectional design, and the observed association does not necessarily represent a causal relationship. Longitudinal studies evaluating the temporal association between periodontitis and thyroid function are necessary. Moreover, it would be useful to evaluate if periodontal treatment reduces the risk of thyroid dysfunction. Second, although we adjusted for various possible confounding variables identified through a thorough literature review, there could be residual bias that we were unable to eliminate. Third, the present study included only Korean subjects which prohibit the generalization of the results to the population across different ethnicities. As ethnicity and geographic locations have significant impact on thyroid disease54, the results from our study need to be confirmed in other ethnicities. Lastly, periodontitis was defined by CPI criteria only. Despite its longstanding widespread use for screening periodontitis, the CPI requires well-trained dentists, long examination time, and special equipment to perform55. Further studies using more recent screening tools with lower bias including half-reduced Centers of Disease Control and Prevention and American Academy of Periodontology case definition or the new 2018 periodontitis case definition by the European Federation of Periodontology and American Association of Periodontology would be valuable56,57. Despite these limitations, a substantial strength of this study is the availability of nationally representative data from a large epidemiological study with standardized high-quality clinical and laboratory procedures and a wide range of information about potential confounding factors. Furthermore, the extensive subgroup and sensitivity analyses conducted within this study support the consistency of the present findings.
In conclusion, TSH levels were significantly associated with periodontitis, independent of various risk factors for periodontitis. Given that numerous systemic diseases have been linked to periodontal disease, our findings may provide important guidance for clinicians to evaluate periodontal disease in patients with thyroid dysfunction.
Methods
Study design and population
Data were obtained from the KNHANES, a nationwide cross-sectional survey conducted by the Korea Centers for Disease Control and Prevention to provide national health, diet, and nutrition data representing the civilian, non-institutionalized Korean population. This study was conducted with data from version VI of the survey, conducted from 2013 to 2015. The research subjects were selected using two-stage stratified cluster sampling of the population as well as housing census data. Data were collected via household interviews and standardized physical examinations. The institutional review board of the Korea Centers for Disease Control and Prevention approved the protocol of the KNHANES (IRB approval number: 2013-07CON-03-4C, 2013-12EXP-03-5C, and 2015-01-02-6C). The study was performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from each participant before the survey, and secondary anonymized data were used in the analyses. The anonymized KNHANES database is publicly available at http://knhanes.cdc.go.kr/knhanes/eng.
This study initially selected participants with data on thyroid function status and CPI scores (n = 6255, Fig. 1). Participants with previous thyroid disease, those receiving thyroid-related medications were excluded from the analyses (n = 723). Participants with an inappropriate TSH response (free T4 > 1.76 ng/mL and TSH ≥ 0.62 mIU/L) or secondary hypothyroidism (free T4 < 0.89 ng/mL and TSH ≤ 6.8 mIU/L) were excluded as well (n = 64), as these findings are very unusual and generally require repeated thyroid function testing. A total of 5468 participants were included in the final study. The study protocol was approved by the Institutional Review Board of Korea University.
Laboratory test
Thyroid function tests were conducted in one-third of all KNHANES VI participants, (approximately 2400 persons aged ≥ 10 years annually). The sum of 3 years of testing represents the entire population of Korea. Serum TSH was measured using an electrochemiluminescence immunoassay (E-TSH kit, Roche Diagnostics, Mannheim, Germany), and the TSH reference interval was determined to be between the 2.5th and 97.5th percentile of serum TSH levels measured in the reference population (0.62–6.86 mIU/L)58. TSH levels were logarithmically transformed to normalize the distribution. In this study, we used the TSH levels to represent the thyroid function as TSH is the most sensitive marker of thyroid function, influenced by minute changes in free T4 concentrations59. Serum free T4 was also measured through an electrochemiluminescence immunoassay (E-Free T4 kit, Roche Diagnostics, Mannheim, Germany), with a reference range of 0.89–1.76 ng/mL. Anti-thyroid peroxidase antibody (TPOAb) levels were measured using an E-Anti-TPO kit (Cobas e 801; Roche Diagnostics) with a reference range of 0–34 IU/mL. Urine iodine concentrations were measured using an inductively coupled plasma mass spectrometer (ICP-MS; Perkin Elmer ICP-MS, Waltham, MA, USA) with an iodine standard (Inorganic Venture, Christiansburg, VA, USA).
Definition of periodontitis
An oral health examination was performed within the KNHANES by calibrated dentists. Participants’ periodontal condition was assessed using the CPI, which was developed by the WHO60. Periodontitis was defined as a CPI code ≥ 3, indicating that at least one site had a > 3.5-mm pocket in the index tooth (11, 16, 17, 26, 27, 31, 36, 37, 46, and/or 47 according to the Federation Dentaire Internationale system).
Statistical analysis
R version 3.4.0 software, the R libraries survey (RODBC), and Cairo were used for data analysis (R Foundation for Statistical Computing, Vienna, Austria; available at http://www.R-project.org). All statistics were calculated using sample weights assigned to the sample participants within the KNHANES. The sample weights were constructed to attain unbiased estimates representing the entire Korean population, with consideration of the stratified multistage probability sampling design of each survey year. Continuous variables were presented as medians with interquartile ranges (IQRs) and categorical variables were presented as numbers with percentages. The distributions of continuous and categorical variables were compared using the Kruskal–Wallis and chi-square tests, respectively. Associations between periodontitis and TSH levels were analyzed using multivariable logistic regression models adjusted for potential confounding factors, including age, sex, body mass index (BMI), smoking, alcohol intake, exercise, fasting glucose levels, systolic blood pressure (SBP), total cholesterol, eGFR (calculated by the MDRD equation), AST, ALT, log urine iodine, and TPOAb. Sensitivity analyses were performed by assessing associations between periodontitis and serum TSH levels among participants without specific factors affecting thyroid function, as well as among participants without diseases reported to be related to periodontitis within previous studies. Associations were considered statistically significant at a p value of < 0.05.
Data availability
The anonymized KNHANES database is publicly available at http://knhanes.cdc.go.kr/knhanes/eng.
References
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832. https://doi.org/10.1038/s41591-019-0675-0 (2019).
Roth, G. A. et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788. https://doi.org/10.1016/s0140-6736(18)32203-7 (2018).
World Health, O. Assessing national capacity for the prevention and control of noncommunicable diseases: Report of the 2019 global survey. ix, 101 p. (World Health Organization, 2020).
Pihlstrom, B. L., Michalowicz, B. S. & Johnson, N. W. Periodontal diseases. Lancet 366, 1809–1820. https://doi.org/10.1016/s0140-6736(05)67728-8 (2005).
Cekici, A., Kantarci, A., Hasturk, H. & Van Dyke, T. E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000(64), 57–80. https://doi.org/10.1111/prd.12002 (2014).
Botelho, J. et al. Economic burden of periodontitis in the United States and Europe: An updated estimation. J. Periodontol. https://doi.org/10.1002/jper.21-0111 (2021).
Kassebaum, N. J. et al. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 96, 380–387. https://doi.org/10.1177/0022034517693566 (2017).
Beck, J. D. et al. Periodontal disease and coronary heart disease: A reappraisal of the exposure. Circulation 112, 19–24. https://doi.org/10.1161/circulationaha.104.511998 (2005).
Hansen, G. M., Egeberg, A., Holmstrup, P. & Hansen, P. R. Relation of periodontitis to risk of cardiovascular and all-cause mortality (from a Danish Nationwide Cohort Study). Am. J. Cardiol. 118, 489–493. https://doi.org/10.1016/j.amjcard.2016.05.036 (2016).
Grau, A. J. et al. Periodontal disease as a risk factor for ischemic stroke. Stroke 35, 496–501. https://doi.org/10.1161/01.Str.0000110789.20526.9d (2004).
Preshaw, P. M. et al. Periodontitis and diabetes: A two-way relationship. Diabetologia 55, 21–31. https://doi.org/10.1007/s00125-011-2342-y (2012).
Negrato, C. A., Tarzia, O., Jovanovič, L. & Chinellato, L. E. M. Periodontal disease and diabetes mellitus. J. Appl. Oral Sci. Rev. FOB 21, 1–12. https://doi.org/10.1590/1678-7757201302106 (2013).
Kim, J. S. et al. Association between periodontitis and metabolic syndrome in a Korean nationally representative sample of adults aged 35–79 years. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph16162930 (2019).
Martinez-Herrera, M., Silvestre-Rangil, J. & Silvestre, F. J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal 22, e708–e715. https://doi.org/10.4317/medoral.21786 (2017).
Akinkugbe, A. A. et al. Periodontitis and non-alcoholic fatty liver disease, a population-based cohort investigation in the study of health in Pomerania. J. Clin. Periodontol. 44, 1077–1087. https://doi.org/10.1111/jcpe.12800 (2017).
Chambrone, L. et al. Periodontitis and chronic kidney disease: A systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J. Clin. Periodontol. 40, 443–456. https://doi.org/10.1111/jcpe.12067 (2013).
Koduganti, R. R., Gorthi, C., Reddy, P. V. & Sandeep, N. Osteoporosis: “A risk factor for periodontitis”. J. Indian Soc. Periodontol. 13, 90–96. https://doi.org/10.4103/0972-124X.55841 (2009).
Esfahanian, V., Shamami, M. S. & Shamami, M. S. Relationship between osteoporosis and periodontal disease: Review of the literature. J. Dent. (Tehran) 9, 256–264 (2012).
Bingham, C. O. 3rd. & Moni, M. Periodontal disease and rheumatoid arthritis: The evidence accumulates for complex pathobiologic interactions. Curr. Opin. Rheumatol. 25, 345–353. https://doi.org/10.1097/BOR.0b013e32835fb8ec (2013).
Abbayya, K., Puthanakar, N. Y., Naduwinmani, S. & Chidambar, Y. S. Association between periodontitis and Alzheimer’s disease. N. Am. J. Med. Sci 7, 241–246. https://doi.org/10.4103/1947-2714.159325 (2015).
Kaur, T., Uppoor, A. & Naik, D. Parkinson’s disease and periodontitis: The missing link? A review. Gerodontology 33, 434–438. https://doi.org/10.1111/ger.12188 (2016).
Cardoso, E. M., Reis, C. & Manzanares-Céspedes, M. C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 130, 98–104. https://doi.org/10.1080/00325481.2018.1396876 (2018).
Wang, Y., Andrukhov, O. & Rausch-Fan, X. Oxidative stress and antioxidant system in periodontitis. Front. Physiol. 8, 910. https://doi.org/10.3389/fphys.2017.00910 (2017).
Ambati, M. et al. Evaluation of oxidative stress in chronic periodontitis patients following systemic antioxidant supplementation: A clinical and biochemical study. J. Nat. Sci. Biol. Med. 8, 99–103. https://doi.org/10.4103/0976-9668.198366 (2017).
Amar, S. et al. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler. Thromb. Vasc. Biol. 23, 1245–1249. https://doi.org/10.1161/01.Atv.0000078603.90302.4a (2003).
Holtfreter, B. et al. Periodontitis is associated with endothelial dysfunction in a general population: A cross-sectional study. PLoS ONE 8, e84603–e84603. https://doi.org/10.1371/journal.pone.0084603 (2013).
Machado, V. et al. Serum C-reactive protein and periodontitis: A systematic review and meta-analysis. Front. Immunol. https://doi.org/10.3389/fimmu.2021.706432 (2021).
Mancini, A. et al. Thyroid hormones, oxidative stress, and inflammation. Mediat. Inflamm. 6757154–6757154, 2016. https://doi.org/10.1155/2016/6757154 (2016).
Aldulaijan, H. A., Cohen, R. E., Stellrecht, E. M., Levine, M. J. & Yerke, L. M. Relationship between hypothyroidism and periodontitis: A scoping review. Clin. Exp. Dent. Res. 6, 147–157. https://doi.org/10.1002/cre2.247 (2020).
Kweon, S. et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 43, 69–77. https://doi.org/10.1093/ije/dyt228 (2014).
World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers, 3rd ed. https://apps.who.int/iris/handle/10665/43781 (2007).
Yucel-Lindberg, T. & Båge, T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 15, e7. https://doi.org/10.1017/erm.2013.8 (2013).
Liu, Y. C., Lerner, U. H. & Teng, Y. T. Cytokine responses against periodontal infection: Protective and destructive roles. Periodontology 2000(52), 163–206. https://doi.org/10.1111/j.1600-0757.2009.00321.x (2010).
Garlet, G. P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 89, 1349–1363. https://doi.org/10.1177/0022034510376402 (2010).
Hajishengallis, G. & Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 21, 426–440. https://doi.org/10.1038/s41577-020-00488-6 (2021).
Rahangdale, S. & Galgali, S. Periodontal status of hypothyroid patients on thyroxine replacement therapy: A comparative cross-sectional study. J. Indian Soc. Periodontol. 22, 535–540. https://doi.org/10.4103/jisp.jisp_316_18 (2018).
Rahangdale, S. I. & Galgali, S. R. Periodontal status of hypothyroid patients on thyroxine replacement therapy: A comparative cross-sectional study. J. Indian Soc. Periodontol. 22, 535–540. https://doi.org/10.4103/jisp.jisp_316_18 (2018).
Feitosa, D. S. et al. The influence of thyroid hormones on periodontitis-related bone loss and tooth-supporting alveolar bone: A histological study in rats. J. Periodontal. Res. 44, 472–478. https://doi.org/10.1111/j.1600-0765.2008.01144.x (2009).
Medara, N. et al. A review of T helper 17 cell-related cytokines in serum and saliva in periodontitis. Cytokine 138, 155340. https://doi.org/10.1016/j.cyto.2020.155340 (2021).
Khan, F. A., Al-Jameil, N., Khan, M. F., Al-Rashid, M. & Tabassum, H. Thyroid dysfunction: An autoimmune aspect. Int. J. Clin. Exp. Med. 8, 6677–6681 (2015).
Antonelli, A., Ferrari, S. M., Corrado, A., Di Domenicantonio, A. & Fallahi, P. Autoimmune thyroid disorders. Autoimmun. Rev. 14, 174–180. https://doi.org/10.1016/j.autrev.2014.10.016 (2015).
Ferrari, S. M. et al. Chemokines in hyperthyroidism. J. Clin. Transl. Endocrinol. 16, 100196. https://doi.org/10.1016/j.jcte.2019.100196 (2019).
Conigliaro, P. et al. Autoimmune thyroid disorders and rheumatoid arthritis: A bidirectional interplay. Autoimmun. Rev. 19, 102529. https://doi.org/10.1016/j.autrev.2020.102529 (2020).
Marcocci, C., Leo, M. & Altea, M. A. Oxidative stress in graves’ disease. Eur. Thyroid J. 1, 80–87. https://doi.org/10.1159/000337976 (2012).
Venditti, P. & Di Meo, S. Thyroid hormone-induced oxidative stress. Cell Mol. Life Sci. 63, 414–434. https://doi.org/10.1007/s00018-005-5457-9 (2006).
Resch, U., Helsel, G., Tatzber, F. & Sinzinger, H. Antioxidant status in thyroid dysfunction. Clin. Chem. Lab. Med. 40, 1132–1134. https://doi.org/10.1515/cclm.2002.198 (2002).
Dobrzyńska, M. M., Baumgartner, A. & Anderson, D. Antioxidants modulate thyroid hormone- and noradrenaline-induced DNA damage in human sperm. Mutagenesis 19, 325–330. https://doi.org/10.1093/mutage/geh037 (2004).
Venditti, P., Di Stefano, L. & Di Meo, S. Vitamin E management of oxidative damage-linked dysfunctions of hyperthyroid tissues. Cell Mol. Life Sci. 70, 3125–3144. https://doi.org/10.1007/s00018-012-1217-9 (2013).
Sulijaya, B., Takahashi, N. & Yamazaki, K. Host modulation therapy using anti-inflammatory and antioxidant agents in periodontitis: A review to a clinical translation. Arch. Oral Biol. 105, 72–80. https://doi.org/10.1016/j.archoralbio.2019.07.002 (2019).
Vieira, I. H., Rodrigues, D. & Paiva, I. Vitamin D and autoimmune thyroid disease-cause, consequence, or a vicious cycle? Nutrients https://doi.org/10.3390/nu12092791 (2020).
Nettore, I. C., Albano, L., Ungaro, P., Colao, A. & Macchia, P. E. Sunshine vitamin and thyroid. Rev. Endocr. Metab. Disord. 18, 347–354. https://doi.org/10.1007/s11154-017-9406-3 (2017).
Machado, V., Lobo, S., Proenca, L., Mendes, J. J. & Botelho, J. Vitamin D and periodontitis: A systematic review and meta-analysis. Nutrients https://doi.org/10.3390/nu12082177 (2020).
Mariotti, S., Caturegli, P., Piccolo, P., Barbesino, G. & Pinchera, A. Antithyroid peroxidase autoantibodies in thyroid diseases. J. Clin. Endocrinol. Metab. 71, 661–669. https://doi.org/10.1210/jcem-71-3-661 (1990).
McLeod, D. S. A., Caturegli, P., Cooper, D. S., Matos, P. G. & Hutfless, S. Variation in rates of autoimmune thyroid disease by race/ethnicity in US military personnel. JAMA 311, 1563–1565. https://doi.org/10.1001/jama.2013.285606 (2014).
Leroy, R., Eaton, K. A. & Savage, A. Methodological issues in epidemiological studies of periodontitis: How can it be improved?. BMC Oral Health 10, 8. https://doi.org/10.1186/1472-6831-10-8 (2010).
Tran, D. T. et al. Assessment of partial-mouth periodontal examination protocols for periodontitis surveillance. J. Clin. Periodontol. 41, 846–852. https://doi.org/10.1111/jcpe.12285 (2014).
Botelho, J., Machado, V., Proença, L. & Mendes, J. J. The 2018 periodontitis case definition improves accuracy performance of full-mouth partial diagnostic protocols. Sci. Rep. 10, 7093. https://doi.org/10.1038/s41598-020-63700-6 (2020).
Kim, W. G. et al. Thyroid stimulating hormone reference range and prevalence of thyroid dysfunction in the Korean Population: Korea National Health and Nutrition Examination Survey 2013 to 2015. Endocrinol. Metab. (Seoul) 32, 106–114. https://doi.org/10.3803/EnM.2017.32.1.106 (2017).
Sheehan, M. T. Biochemical testing of the thyroid: TSH is the best and oftentimes, only test needed—A review for primary care. Clin. Med. Res. 14, 83–92. https://doi.org/10.3121/cmr.2016.1309 (2016).
World Health Organization. Oral health surveys : basic methods, 4th ed. https://apps.who.int/iris/handle/10665/41905 (1997).
Funding
This work was supported by Korea University Guro Hospital (Korea Research-Driven Hospital) and grant funded by Korea University Medicine (K2115701). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Conceptualization: E.S., K.M.C. Data curation: E.S., M.J.P., J.A.K., E.R., H.J.Y., S.H.B., K.M.C. Formal Analysis: E.S., J.H.Y., N.H.K1, K.M.C. Investigation: E.S., M.J.P., J.A.K., E.R., J.H.Y., N.H.K.1, H.J.Y., J.A.S., S.G.K., N.H.K.2, S.H.B., and K.M.C. Methodology: E.S., K.M.C. Project administration: K.M.C. Resources: E.S., M.J.P., J.A.K., E.R., J.H.Y., N.H.K.1, H.J.Y., J.A.S., S.G.K., N.H.K.2, S.H.B., and K.M.C. Supervision: H.J.Y., J.A.S., S.G.K., N.H.K.2, S.H.B., and K.M.C. Visualization: E.S., J.A.K., E.R., J.H.Y., N.H.K.1. Writing—original draft: E.S., K.M.C. Writing—review and editing: E.S., M.J.P., J.A.K., E.R., J.H.Y., N.H.K.1, H.J.Y., J.A.S., S.G.K., N.H.K.2, S.H.B., and K.M.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Song, E., Park, M.J., Kim, J.A. et al. Implication of thyroid function in periodontitis: a nationwide population-based study. Sci Rep 11, 22127 (2021). https://doi.org/10.1038/s41598-021-01682-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01682-9
This article is cited by
-
The effects of thyroid function on periodontal status: a systematic review and meta-analysis
BMC Oral Health (2025)
-
Bidirectional Relationship Between Periodontal Disease and Thyroid Diseases
Current Oral Health Reports (2025)
-
Exploring the Relationship Between Thyroid Disorders and Periodontitis
Indian Journal of Otolaryngology and Head & Neck Surgery (2025)