Abstract
The study estimates the parameters of the photosynthesis–irradiance relationship (PN/I) of a sedge-grass marsh (Czech Republic, Europe), represented as an active “green” surface—a hypothetical “big-leaf”. Photosynthetic parameters of the “big-leaf” are based on in situ measurements of the leaf PN/I curves of the dominant plant species. The non-rectangular hyperbola was selected as the best model for fitting the PN/I relationships. The plant species had different parameters of this relationship. The highest light-saturated rate of photosynthesis (Asat) was recorded for Glyceria maxima and Acorus calamus followed by Carex acuta and Phalaris arundinacea. The lowest Asat was recorded for Calamagrostis canescens. The parameters of the PN/I relationship were calculated also for different growth periods. The highest Asat was calculated for the spring period followed by the summer and autumn periods. The effect of the species composition of the local plant community on the photosynthetic parameters of the “big-leaf” was addressed by introducing both real (recorded) and hypothetical species compositions corresponding to “wet” and “dry” hydrological conditions. We can conclude that the species composition (or diversity) is essential for reaching a high Asat of the “big-leaf ”representing the sedge-grass marsh in different growth periods.
Similar content being viewed by others
Introduction
Carbon cycling and its exchange between primary producers (vegetation) and the atmosphere is a central process directly connected with processes of global climate change1,2. Individual processes of carbon exchange and sequestration by the vegetation are studied using different approaches and methods from the theoretical and technical points of view3,4. A frequently used approach is the simplification of the whole canopy of primary producers to a hypothetical “big-leaf”, representing the active “green” surface of the ecosystem in the meaning of the inclusion of the leaf and canopy models5,6. The “big-leaf” concept is also used in post-processing of eddy covariance data7 and is associated with the photosynthesis–irradiance relationship (PN/I). The eddy covariance technique measures the net ecosystem exchange (NEE) of CO2 as the balance between CO2 released by ecosystem respiration (Re) and CO2 fixation by photosynthesis. The fundamental photosynthesis–irradiance relationship (PN/I) is used for separation of underlying processes such as Re. The PN/I curves are fitted to daytime NEE measurements, and respiration is estimated from the intercept of the ordinate7. This approach allows assessing photosynthetic parameters of a whole plant community as those of a “big-leaf”, without any knowledge of individual contributions of dominant plant species forming this community. The species richness and diversity are important for long-term ecosystem stability due to the higher probability of species-rich communities to survive unfavourable environmental conditions inducing physiological stress8,9,10,11. Important in this case is functional diversity of the plants in a community, which affects the functions and services of a given ecosystem12. Plants have various adaptations determining how they manage different stressors or survive under physiologically unsuitable conditions13,14. Knowledge of the photosynthetic parameters of individual dominant plant species based on in situ measurements makes it possible to determine the functional contributions of these species to the photosynthesis of the whole ecosystem characterised as a green “big-leaf” surface. Variation of the “big-leaf” photosynthesis under varied environmental conditions in relation to species composition (diversity) and functional diversity enables us to describe and understand the respective roles of individual species in the photosynthesis of a whole plant community.
The aims of our study are as follows: (1) individual estimation of the parameters of the photosynthesis–irradiance relationship (PN/I curve) of five dominant plant species of the sedge-grass marsh community, based on in situ measurements. (2) Based on these estimates, to upscale the parameters obtained for individual species to the “big-leaf” concept of the sedge-grass marsh ecosystem for each growth period (spring, summer and autumn) and also for the whole growing season.
We expected that the wetland plant species studied will differ in values of their photosynthetic parameters, i.e., apparent quantum use efficiency (α), light-saturated rate of photosynthesis (Asat), rate of dark respiration (Rd) and compensation point (Icomp). These parameters will change during the individual periods of growth, reflecting changing in situ conditions (e.g., position of water table). Contributions of photosynthesis of individual plant species will differ in different growth periods. These differences show the involvement of individual plant species in carbon cycling in the sedge-grass marsh ecosystem.
Material and methods
Plant nomenclature
Plant nomenclature: Kaplan, Z., ed (2019)15.
Site description
The sedge-grass marsh is a part of a large wetland complex (450 ha) called “The Wet Meadows” located near the town of Třeboň, South Bohemia, Czech Republic (Central Europe) at the centre of the Třeboň Basin Biosphere Reserve of the UNESCO Man and the Biosphere Programme16. The sedge-grass marsh (426 m a.s.l., 49° 01′ 29″ N, 14° 46′ 13″ E) is situated in the inundation area of a large human-made lake (Rožmberk fishpond, water-surface area about 5 km2). Its water level is controlled by a system of ditches, which interconnect the system of human-made shallow lakes (fishponds) in the whole region, and is thus fairly stable throughout the year except flood or fishpond-drawdown periods. The traditional use of the Wet Meadows consisted of their regular mowing. The study site represents the wettest part of this wetland, which used to be mown once a year until the 1950s. The resulting vegetation was formed mainly by tall sedges (Carex acuta [syn. C. gracilis], C. vesicaria) and wetland grasses (mostly Calamagrostis canescens)17,18,19,20,21. This community is classified as the association Caricetum gracilis Almquist 192922,23. During the last 60 years, after cessation of mowing, a distinct structure of hummocks and hollows has developed. The hummocks are formed mainly by tussocks of C. acuta. Accompanying species usually colonize hummock edges and extend down into the hollows only during dry years. C. canescens spreads on tops of the hummocks in dry years and thus forms an important part of the C. acuta–dominated plant community24. The other species include Glyceria maxima and Acorus calamus, which occur either together with C. acuta or form monodominant stands. Phalaris arundinacea used to colonize only the banks of drainage ditches and small patches of sandy substrate within continuous stands of tall sedges25. Surprisingly it has spread over a large area of the sedge-grass marsh after the extreme and long lasting summer flood of 200226.
Light-curve measurements
The measurements of PN/I curves were conducted during the growing season of 2013, from mid-April to early October at weekly intervals except for early June, when the wetland was flooded. The measurements were designed so as to minimise variation among shoots of a particular species, among leaves of different age on a shoot, and random effects of the environmental conditions. A group of adjacent shoots (of G. maxima, P. arundinacea and A. calamus) or individual tussocks (of C. acuta and C. canescens) were labelled and then repeatedly used for measurements taken during the whole growing season. On a particular date, two shoots were chosen within a labelled plant and their young but fully developed leaves (2nd–4th youngest leaves) were used for the measurement. Four leaves of C. acuta and C. canescens, respectively, or two leaves of G. maxima, P. arundinacea and A. calamus, respectively, were enclosed in the leaf chamber so as to cover its whole area. The measured leaf segments were situated at approximately 3/4 of the distance from the leaf-blade base to its apex. All the leaves originated from the top layer of the canopy and were therefore adapted to full sunlight. One photosynthetic light curve was measured for each species on each sampling date.
The rate of net photosynthesis was measured using an open gas-exchange system (Li-Cor 6400, Li-Cor, Inc., Lincoln, NE, USA) equipped with a modulable light source (6400-02B LED). The rapid light response curves were obtained according to the methodology provided in the LICOR-6400 manual. Gas exchange measurements started with a high PAR flux density (2000 µmol (photon) m–2 s–1) and successively dropped to 1500, 1000, 600, 400, 200, 100, 30 and 0 μmol (photon) m–2 s–1. The time period between the successive measurements followed the standard protocol (Manual of Li-6400, Book 1, 4–24). i.e., it was at least 2 min followed by a minimum of 15 s of stability of the output signal. This interval was extended to 4 min after the irradiance was set to 0 μmol (photon) m–2 s–1, i.e. prior to the measurement of dark respiration. The area of the leaf chamber was 6 cm2.
Concentration of CO2 was set at 400 µmol of mol (air)–1 inside the leaf chamber. The flux of the air entering the leaf chamber was set at 300–400 µmol s−1 and was measured under natural light conditions at the beginning of each measurement. In order to minimize its fluctuations, the block temperature was set according to the observed ambient temperature in different periods spring (May), summer (June to August) and autumn (September to October). The gas exchange measurements were restricted to the time period from 07:00 to 11:00 h of Central European time in order to minimize the effects of the afternoon depression of photosynthesis28.
Accessory meteorological and leaf area measurements
Meteorological measurements were executed at an ecosystem CZECHWET (CZ-wet) station of ICOS (http://www.europe-fluxdata.eu/icos/home), Fluxnet (http://sites.fluxdata.org/CZ-wet/) and CzeCOS (http://www.czecos.cz/en.html) flux networks. The instruments and sensors are placed on a pontoon which floats during floods (Fig. 1). Photosynthetically active radiation (PAR, sensor EMS Brno, Czech Republic), air temperature (Pt 100, EMS Brno, Czech Republic), water table (LPM-307, BD sensors, Czech Republic) and precipitation (rain gauge Model 376, Met One Instruments, USA) were measured at intervals of 30 s and 30-min averages were saved. Leaf area index (LAI, Fig. 2) was measured using the leaf area meter Li-3100 (Li-Cor, Inc., Lincoln, NE, USA) on 9 occasions during the growing season.
Location of the “Wet Meadows” study site in the Czech Republic (Europe) and picture of the sedge-grass marsh with scientific infrastructure of ICOS (http://www.europe-fluxdata.eu/icos/home) and Fluxnet (http://sites.fluxdata.org/CZ-wet/) flux networks. The map was drawn using the R packages27 “rworldmap” version 1.3-6 (https://cran.r-project.org/web/packages/rworldmap/).
Seasonal courses of (A) daily mean air temperature (red line) and its daily fluctuations (grey field), (B) daily precipitation (grey vertical bars) and positions of water table (blue line) with (C) standard light conditions characterized as daily sums of incident photosynthetically active radiation (PAR) at the sedge-grass marsh.
Photosynthesis–irradiance model and parameters evaluation
In general, photosynthesis or net photosynthesis (PN) increases with increasing PAR irradiance (I)29. Many different mathematical models can be used for exact description of this relationship (see Supplementary Information for details). Non-rectangular hyperbola was selected as the best model of the PN/I relationship in our study29,30 on the basis of previous precise analyses of other available models used for describing the PN/I relationship (see Supplementary Information for details).
Individual parameters of the photosynthetic models were estimated as follows: apparent maximum quantum yield (α), light compensation point (Icomp) and the light-saturated rate of net photosynthesis (Asat) were modelled using the function nls2 (https://cran.r-project.org/web/packages/nls2/) in the R statistical software version 3.6.127. This function determines the non-linear (weighted) least-square estimates of the parameters of a non-linear model31. Dark respiration (Rd) was estimated as the intersection of the modelled curve with the Y axis. The models were parametrized separately for each species for each measurement day and for each growth period. The parameter of convexity (θ) in the non-rectangular hyperbola model was fitted within a fixed interval from 0 to 1 according to Ögren32.
Obtained parameters of the PN/I relationship were first tested for normality by the Shappiro–Wilk normality test33. As they did not have a normal distribution, the non-parametric Kruskal–Wallis test33 was used for subsequent evaluation of differences in parameters of photosynthetic light-curve between examined plant species. Post-hoc testing was performed using the Dunn test with Bonferroni correction for multiple comparisons by means of the Kruskal–Wallis test. The relationship between the parameters of environmental conditions was analysed by correlation analysis (Spearman’s nonparametric correlation coefficient34).
The big-leaf photosynthetic parameters
As the most suitable parameter for upscale in situ measurements at leaf level to an ecosystem level the sedge-grass “big-leaf” was used leaf area index (LAI) of studied plant species. Average parameters of the PN/I relationships calculated for individual species were weighted by corresponding portions of LAI for different growth periods as well as the whole growing season. The parameters of PN/I “big-leaf” relationships was obtained as the sum of individual weighted parameters of the plant species. The final course of the PN/I relationship of the sedge-grass “big-leaf” was presented by substituting obtained parameters into the equation of a non-rectangular hyperbola (model 4, Supplementary Information Table S1).
Results
Environmental conditions during the growing season
The environmental conditions varied characteristically during the year in accordance with the site location in the temperate zone (Fig. 2). In spring, the daily average amount of incident photosynthetically active radiation (PAR) was 5.4 MJ m−2 d−1 (maximum PPFD 1645 µmol m−2 s−1). The highest daily average amount of incident PAR was recorded in summer 8.7 MJ m−2 d−1 (maximum PPFD 2063 µmol m−2 s−1). In the autumn the daily average amount of incident PAR was the lowest only 4.1 MJ m−2 d−1 (maximum PPFD 1558 µmol m−2 s−1). A similar pattern was found for the daily mean temperature, which was 10.4 °C, 16.6 °C, and 10.7 °C in spring, summer and autumn, respectively.
Precipitation differed among individual periods and months (Fig. 2B). The highest monthly sums of precipitation were recorded in May (109 mm) and June (212 mm). Very low precipitation (less than 1 mm in total) was recorded in July. The low precipitation period persisted also in August (58 mm, which is about half of the long-term average).
Water level fluctuated within periods and also within individual months (Fig. 2B). It remained near the soil surface in spring. A flood (with maximum water level at 1.75 m) occurred in June after several days of extremely high precipitation. As a result of no precipitation in July, the water level sank to 0.44 m below the soil surface and remained there until mid- September. The water level fluctuations were significantly correlated with the precipitation in the spring (Rspearman = 0.31, p < 0.05) and summer (Rspearman = 0.32, p < 0.01) periods, but not in the autumn period.
Vegetation composition and leaf area index
Vegetation of a sedge-grass marsh was without significant changes appearing in changing abundances and species composition during the studied growth season. The relative cover of the dominant species was following: Phalaris arundinacea 35%, Carex acuta 28.6%, Glyceria maxima 16.5%, Calamagrostis canescens 18.7% and Acorus calamus 1.2%. Different state of plant developing was determined by changes of leaf area index (LAI, Fig. 3) among periods of the growing season. Different LAI among periods and plant species relate to slightly different plant phenology.
Leaf area index (LAI) of individual dominant plant species of the sedge-grass marsh during different growth periods: spring, summer and autumn. Average LAI values are presented with standard errors of mean (SE). Numbers above the SE-indicating abscissae are percentages of total LAI formed by each of the five dominant plant species in each growth period.
LAI increased gradually from the spring (1.95 m2 m−2) to the summer period, when total LAI reached its maximum of about 9.7 m2 m−2. During the autumn, LAI decreased to about 5.5 m2 m−2. LAI values significantly differed between the three growth periods (χ2 = 20.6, p < 0.05). The shares of individual plant species in total LAI were also different across the growth periods. The highest proportion of total LAI was formed by Glyceria maxima: 43% in spring, 29% in summer and 27% in the autumn. During the summer period, LAI values of individual species were quite balanced within the range of 17% to 19% of total LAI, except Glyceria maxima (29%). The lowest proportions of total LAI were those of Carex acuta and Acorus calamus plants in the spring and autumn. In the autumn period, Phalaris arundinacea and Glyceria maxima formed the highest proportions of total LAI (Fig. 3). For the whole growing season, the relative LAI proportions of the five dominant species were as follows: P. arundinacea 23.0%, C. acuta 15.3%, A. calamus 11.6%, C. canescens 20.1% and G. maxima 30.0%.
Based on relative proportions of LAI, two hypothetical species composition variants of the plant community were created. They were composed of Phalaris arundinacea, Carex acuta, Glyceria maxima, Calamagrostis canescens and Acorus calamus (Fig. 4). Two habitat variants corresponding to two hypothetical simple hydrological situations: “wet” and “dry”, were set separately for each growth period, i.e., spring, summer and autumn. The species composition variants (percentage proportions of each species’ LAI Fig. 4) were also set for the “wet” and “dry” hydrological situations. The hypothetical “wet situation is determined by the position of the water level closely below or above the soil surface (up to + 0.4 m), while the “dry” hypothetical situation is determined by the water level situated markedly below the soil surface (maximally sinking to − 0.4 m). At the hypothetical “wet” situation, we assume Carex acuta to form the highest LAI proportion during the summer period, with Glyceria maxima and Phalaris arundinacea forming it during the spring and autumn periods, respectively. Calamagrostis canescens is not represented in the hypothetical plant community at the “wet” situation. On the other hand, in the “dry” situation, we assume the highest LAI proportion being formed by C. canescens and P. arundinacea. Other plant species were represented at minute to negligible hypothetical LAI proportions.
Photosynthesis–irradiance weighted relationship (PN/I) of a hypothetical “big-leaf” for the hypothetical “wet” and “dry” hydrological situations and corresponding hypothetical plant species compositions. The PN/I relationships were modelled by non-rectangular hyperbolas for individual growth periods and also for the whole growing season. The proportional bar plot shows the plant species composition (proportions of LAI) in two hypothetical hydrological situations for individual growth periods.
P N /I relationship and individual curve parameters
The relationship between photosynthesis and PAR irradiance modelled by a non-rectangular hyperbola (Fig. 5) differs among the plant species studied. They can be divided into three groups according to the Asat curve parameter (Table S5) which determine a position of PN/I curve relative to other plant species. The position of curves of the plants, with the highest Asat, included A. calamus and G. maxima, their Asat reaching on average 16.61 µmol m−2 s−1 and 18.36 µmol m−2 s−1, respectively were above others. The group comprised P. arundinacea and C. acuta with average Asat values 9.41 µmol m−2 s−1 and 10.83 µmol m−2 s−1, respectively had PN/I curves lower. The last species C. canescens, with the lowest Asat (Asat = 7.02 µmol m−2 s−1 in average) was the lowest position of PN/I curve from the plant species studied. The differences among species Asat were statistically significant at p < 0.05 probability level.
The highest proportion of the variability of PN/I relationship explained by the model was calculated for A. calamus and G. maxima (R2 = 0.97 and 0.91). Explained variability of C. acuta and C. canescens, were lower (R2 = 0.91 and 0.79). The lowest variability explained by the PN/I model was calculated for P. arundinacea (R2 = 0.63). The weighted PN/I curve of the “big-leaf” explained about 84% of variability of the PN/I relationship (Fig. 5).
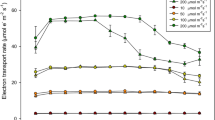
Light-saturated photosynthesis (Asat)
The maximum rates of photosynthesis varied considerably among the selected plant species (Fig. 6). The highest values of Asat were found in G. maxima and A. calamus during May and June (about 26 µmol m−2 s−1). Their Asat decreased by about 25% from July to the middle of August. And finally, Asat markedly decreased, by about 45%, in the rest of growing season. The photosynthetic rates of C. acuta and P. arundinacea reached their highest Asat values after the flood (about 20 µmol m−2 s−1). In the rest of the growing season, Asat decreased and varied between 6 and 13 μmol−2 s−1. C. canescens reached its highest Asat of 17.6 µmol m−2 s−1 after the July flood and then decreased. Asat of C. canescens remained stable after the temperature drop in August and fluctuating around 5 μmol m−2 s−1. After a rise of the groundwater table in September, Asat increased to about 8 µmol m−2 s−1.
Parameters of the PN/I relationships: α apparent maximum quantum yield, Rd- dark respiration, Icom—irradiance compensation point, Asat—light-saturated rate of photosynthesis, of the respective plant species: Ph—Phalaris arundinacea, Car—Carex acuta, Gl—Glyceria maxima, Ac—Acorus calamus, Cal—Calamagrostis canescens). Mean (black dot) and median (thick line) are shown inside each box. Statistically significant between-species differences of each parameter are indicated by different letters above the respective boxes compared.
Apparent maximum quantum yield (α)
The average values of apparent maximum quantum yield (α) ranged from 0.041 µmol CO2 µmol (photon)−1 in C. acuta to 0.078 µmol CO2 µmol (photon)−1 in G. maxima, with average values of the other species in-between. There was only one significant between-species difference, namely that between C. acuta and G. maxima (p < 0.05, Fig. 6). The values of α had a similar seasonal trend in all five plant species studied. They increased after the decrease of air temperature following the flood.
Dark respiration rate
Overall dark respiration (Rd) was higher at the beginning of the growing season and increased also after the flood. Rd increased also at the end of June and at the time of dry soil at the beginning of August. A strongly increasing rate of dark respiration was observed towards the end of the growing season. A. calamus reached the highest seasonal average Rd of all five species studied, but none of the between-species differences was significant. The minimum and also the most stable Rd values out of all five species were found for C. acuta; by contrast, the most variable Rd was that of P. arundinacea (Fig. 6).
Compensation point (I comp)
The value of Icomp was influenced by the input of photosynthetic photon flux density (PPFD). Higher PPFD resulted in a higher Icomp. When PPFD was above 1000 µmol (photon) m−2 s−1 Icomp was about 14–19 µmol (photon) m−2 s−1 and about 20 µmol (photon) m−2 s−1 for P. arundinacea and C. canescens, respectively. When PPFD was less than 500 µmol (photon) m−2 s−1, the Icomp was about 6 (photon) µmol m−2 s−1 for both plant species.
A different trend was observed for A. calamus. It reached higher values of Icomp, of about 50 µmol (photon) m−2 s−1, during days with high PAR irradiance. With decreasing PPFD during August Icomp decreased on average to 27 µmol (photon) m−2 s−1 and another decrease to 7 µmol (photon) m−2 s−1 followed in September. No trend of Icomp was found for G. maxima and C. acuta, with their Icomp varying between 0.01 and 27.4 μmol (photon) m−2 s−1 during the growing season. The seasonal averages of the irradiance compensation point (Icomp) varied between 13.36 μmol (photon) m−2 s−1 and 22.49 μmol (photon) m−2 s−1 for the studied species, except for A. calamus, which reached 29.52 µmol (photon) m−2 s−1. The differences among the five plant species were, however, not significant (Fig. 6).
The PN/I relationships at real and hypothetical plant species compositions
In a real plant community whose species composition is modelled by a non-rectangular hyperbola, the weighted PN/I relationship is different in each period of the growing season (Fig. 7). The curves are sorted in descending order from the curve for the “big-leaf” in the whole growing season, showing the highest values of light-saturated photosynthesis (Asat). The next PN/I curves for the “big-leaf” of the spring, summer and autumn periods followed the curve for the whole growing season with lower maximum values of Asat. Most of the differences among the curve parameters for individual periods were not significant. We found only one difference significant at p < 0.05 probability level, namely that between the irradiance compensation points (Icomp) in the summer and autumn periods (Table S4).
Average photosynthesis–irradiance relationship (PN/I) of the theoretical “ecosystem big-leaf” modelled by non-rectangular hyperbolas with real plant species composition. The PN/I relationships are presented separately for different periods: spring, summer and autumn. The PN/I relationship including the whole growing season is shown for comparison with those for individual growth periods. All curves were weighted by the plant species´ partial LAI values in individual growth periods (Fig. 3).
The PN/I weighted relationship was different for the two hypothetical plant compositions in the hypothetical “wet” and “dry” hydrological situations (Fig. 4). For all growth periods. Higher Asat was modelled for the “wet” variant as compared with the “dry” variant. Within the “wet” variant, the highest Asat was modelled for the spring period; the other curves are sorted in descending order from the spring one through that for summer to the autumn one. However, in the “dry” variant, the highest Asat was modelled for the summer and autumn periods while the modelled lowest Asat occurred in the spring period. A comparison of whole-season weighted curves for the “big-leaf” shows that the hypothetical “wet” plant species composition variant of the sedge-grass marsh community reaches a higher Asat than the real plant species composition variant. The difference is, however, very small (Fig. 4). Greater differences are evident between the “dry” and “wet” plant species composition variants as well between the “dry” and real variants.
The plants’ photosynthetic response to the flood
The photosynthetic response to water-table fluctuations was measured in P. arundinacea, C. acuta and A. calamus. Photosynthesis was not measured in C. canescens and G. maxima before the flood; therefore their respective reactions to flooding cannot be evaluated. A flood (with a maximum water level of 1.75 m) occurred in June 2013 after a period of extremely high precipitation (Fig. 2B). The vegetation was flooded for about 11 days, when the water level was at least 0.5 m above soil surface (height of C. acuta tussocks). The water level dropped to the height of the sedge tussocks within three days. At the first measurements after the flood, we recorded an increase of α (A. arundinacea, C. acuta and A. calamus) and increasing absolute values of Rd (A. arundinacea and C. acuta). In some of the first measurements after the flood, Asat either increased (P. arundinacea and C. acuta), or decreased (A. calamus). The flooding did not affect the plants’ photosynthetic parameters measured both immediately and later after the flood.
Discussion
Photosynthetic parameters of the studied wetland plant species
Maximum photosynthetic capacity
In general, sedges have a higher net assimilation under waterlogged than under drained conditions with a maximum rate of their net assimilation in the range of 10 to 15 μmol m−2 s−1 35. Ondok and Gloser36 reported Asat of C. acuta at different leaf temperatures (10, 20 and 30 °C) as 13.63, 15.91 and 17.05 μmol m−2 s−1, respectively. Asat of C. acuta was on average 10.83 μmol m−2 s−1. This is the third highest average of the five plant species studied. The last two plant species are not strictly wetland ones, being capable of growing in water-saturated organic soil for a long time. C. canescens grows mainly on tops of sedge tussocks or on raised sites and P. arundinacea grows on the banks of the Central Channel. These plant species showed average Asat 7.02 and 9.41 μmol m−2 s−1, respectively. Ondok and Gloser36 reported Asat of C. canescens in range of 7.02 to 11.36 μmol m−2 s−1. Asat of the moderately flood-tolerant species P. arundinacea, ranged from 5 to 7 μmol m−2 s−1 37 but its Asat measured in container-grown plants was higher: between 12 and 17 μmol m−2 s−1 38. Slightly smaller values were obtained in a flooding experiment, where Asat of P. arundinacea was within the range of 9.9 to 12.5 μmol m−2 s−1 39. Gloser40 reported the net photosynthetic rate of P. arundinacea being 11.41 μmol m−2 s−1. This value is within the range mentioned previously. In our measurements, the Asat was within the range of 2.07 to 15.00 μmol m−2 s−1.
Dark respiration
Dark respiration (Rd) is related to the developmental stage of the leaves and the period of the growing season13. Rd depends mainly on leaf temperature41 and physiological condition of leaves (mainly water saturation). The typical range of Rd values is from − 0.7 to − 2.5 μmol m−2 s−1 for C3 plants and from − 1.4 to − 2.5 μmol m−2 s−1 for C4 plants42. Nobel29 presents Rd derived from a generalized hyperbolic function of C3 plants, being about − 1 μmol m−2 s−1 of CO2 at Asat of about 12 μmol m−2 s−1. A wide range of Rd (− 0.41 to − 6.28 μmol m−2 s−1), obtained in our measurements, is usually due to different shapes of light curves of the plant species studied. Maximum absolute values of Rd were recorded for A. calamus (− 6.28 μmol m−2 s−1) and P. arundinacea (− 5.94 μmol m−2 s−1) after the flood and also in October. These Rd values are consistent with those obtained for C. acuta, G. maxima and C. canescens by Ondok and Gloser36. Rd values similar to those of our selected plant species are also consistent with values reported for other wetland plant species such as Schoenoplectus hallii: − 1.3 ± 0.6 μmol m−2 s−1 43, Phragmites australis: − 2.62 ± 0.11 μmol m−2 s−1 and Carex cinerascens − 1.58 ± 0.09 μmol m−2 s−1 44,45. Experimentally measured Rd in P. arundinacea was about − 0.35 μmol m−2 s−1 in the control treatment and − 0.66 μmol m−2 s−1 in the submergence treatment46.
The apparent Rd was surprisingly high after the flood. In principle, this could have been a result of two processes: (1) plant respiration and (2) CH4 formation in fermentative processes in anaerobic soil and its subsequent oxidation to CO2 when it can pass through a vented aerenchymatous plant tissue. Unfortunately, the methodology used does not allow a quantification of the proportionality of the two processes. Nevertheless, we assume that the amount of CO2 vented from the soil was unimportant in this study because the soil was saturated with water (and therefore anaerobic) not only during the high flood pulse, but also before and after it (cf. Fig. 2). If venting of CO2 from the soil had been important, then the measured Rd would have been similar before and after the flood pulse, but this was not the case. Therefore, we assume that the measured Rd reflects mainly increasing respiration rate, indicating resumed growth of aboveground plant parts after their exposure to flooding. Recovery growth requires, among others, energy for transport of storage carbohydrates to the apical meristems, cambium and other growth tissues. This process is also directly reflected in dark respiration47,48. Unfortunately, our photosynthesis measurements did not enable us to distinguish the proportion of recorded Rd reflecting the intensity of fermentative processes from its proportion representing dark (growth/maintenance) respiration of the plants as such42.
Apparent maximum quantum yield
The quantum yield α is represented by the initial linear slope of a generalized hyperbolic relationship between incident photosynthetic photon flux density and the rate of net CO2 uptake by plants (photosynthetic rate). In C3 plants, it is about 0.05 μmol(CO2) μmol(photon)–1 29. The theoretical maximum value of α is 0.1250 μmol(CO2) μmol(photon)–1 as 8 photons are required per one molecule of CO2 fixed. Determination of a correct real maximum quantum yield must be the nearest one to this theoretical value49,50. The value of α has a mathematical definition but it often does not have the desired ecophysiological significance. This vagueness is due to the heterogeneity of methods used for the calculation of this parameter; the results of various calculations of α must therefore be interpreted with caution. Another problem is the curvilinear shape of the PN/I relationship throughout its length, and thus the linear phase is not clearly identifiable51. In our study, the range of apparent maximum quantum yield ranged from 0.051 to its theoretical maximum value of 0.125 μmol μmol−1. The highest average α was calculated for P. arundinacea and A. calamus in August. The lowest one was calculated for C. acuta and C. canescens. This finding is consistent with the shape of their light curves and low Asat.
Light compensation point
The light compensation point (Icomp) is derived from a generalized hyperbolic relationship between the incident photosynthetic photon flux density and the net CO2 uptake rate (photosynthetic rate). In C3 plants, it is 15 μmol m−2 s−1 on the average29, within the average range of 8 to16 μmol mol−2 s−1 29. A comparative review of the genus Carex52 reported Icomp values ranging from 20 to 70 μmol m−2 s−1. Low Icomp values of plants, including sedges, have been reported mainly for herbaceous species occurring in a variable light environment under a deciduous forest canopy53. This Icomp usually increases from sites below a dense canopy towards canopy gaps, ranging from 4.2 ± 2.1 to 17.0 ± 2.9 μmol m−2 s−1 54. Smith and Houpis43 presented Icomp of Schoenoplectus hallii of about 24 μmol m−2 s−1. The Icomp of Phragmites australis and Carex cinerascens were about 48.5 and 37.8 μmol m−2 s−1, respectively44. Flooding usually increased the Icomp, like the effect of canopy gaps44,45.
Our calculated Icomp values are within the range reported for Carex species52 and are highly variable. The range was from 7.35 to 31.84 μmol m−2 s−1. The lowest Icomp was calculated for C. acuta and the highest was for A. calamus. Icomp can characterize plant adaptation to sunny and shady habitats. Leaves acclimated to shade respire less and are less stressed by light than leaves acclimated to high irradiance (sunny leaves). The low Icomp of C. acuta is related to the structure of its leaves and stand with the leaf bases being self-shaded in sedge tussocks. A contrasting situation develops in stands of A. calamus, whose straight leaves shade the leaf bases less than it is the case in C. acuta. Icomp estimated from the PN/I curves of the five selected wetland species is consistent with the ecophysiological principle that Icomp is lower in leaves adapted to shade than in leaves exposed to direct sunlight13.
Photosynthesis of the sedge-grass community: effect of the flood
Wetland plants tolerate fluctuating water levels and can also survive submergence during floods. Photosynthesis of emergent plants under water is inhibited due to reduced stomatal conductance45, decreased availability of PPFD55, high photorespiration56 and decrease of chlorophyll content45. In our case the flood effect was not significant due to the short duration of the flooding (only 11 days). The plants resumed their growth without visible long-term damage immediately after the flood retreated. Undeveloped wetland plant species can withstand short floods due to their tolerance to flooding13. Some species were still without leaves or with only partially developed leaves at the end of spring. The young leaves are usually not damaged at all or very little by a short-time flood. The plants have sufficient carbohydrate reserves57 in their belowground parts (stem bases and rhizomes) at this time. They can use them for a—fast recovery of their growth after the short-time flood subsides. Similar results were reported by Li et al.44, who analysed the effects of flooding on gas exchange in leaves of Phragmites australis, Carex cinerascens, and Hemarthria altissima.
Photosynthetic parameters of the big-leaf: effect of plant species composition
Sedge-grass marshes belong to fens with plant communities formed by a mixture of herbaceous plant species dominated by rhizomatous graminoids58, which determine the physiological characteristics of the whole fen plant community36,59. Different physiological properties of the plant species are usually linked with the plants´ structure creating spatial ecological niches60. These niches are closely associated with the physiological functioning of the individual plant species as well as the whole plant community, and vice versa.
Canopy structures of G. maxima and C. acuta are more effective in the competition for PPFD thanks to their more erectophile foliage than that of C. canescens36. In G. maxima, the greatest proportion of its foliage is concentrated in its higher canopy layers and this species thus tolerates shading. A. calamus is a heliophyte with its assimilatory surface placed mainly in its lower canopy layers61,62,63. Leaves of A. calamus expand earlier than those of the other species61, thus compensating the poorer competitive ability of A. calamus64.
Based on the above mentioned canopy structure and physiological properties of the G. maxima and A. calamus, we assume that plant communities with dominant G. maxima and/or A. calamus reach higher Asat especially under optimal hydrological situations. This assumption is confirmed by the real species composition of the plant community, and also by the hypothetical “wet” variant of its species composition. The “wet” variant reached Asat of 13.27 μmol m−2 s−1, which is close to the lower values of Asat in the real in situ plant species composition variant (Fig. 4).
The PN/I relationship of the “big-leaf” was different in individual growth periods (Fig. 7). The comparison of these periods shows a relatively large difference between the spring and the other periods in both the real and the “wet” variants. The highest Asat of the PN/I relationship during spring was predictable on the basis of the PN/I relationship of A. calamus, a heliophyte64 which is photosynthetically highly active in spring, when its growth starts early and its leaves are not shaded by other dominant plant species61,62,64. Higher achieved Asat also corresponds to the higher LAI proportion of Glyceria maxima and Acorus calamus in both the real variant and the “wet” one during the spring period.
The sedge C. acuta is a species which is characteristic of the studied fen community. Its PN/I relationship curve is very similar to that of the whole growing season´s PN/I relationship of the “big-leaf” derived from the real species composition of the Wet Meadows´ plant community (Fig. 5). C. acuta is thus a keystone species creating the unique spatial structure of the sedge-grass marsh by forming its hummocks and hollows structure59. If we reduce the LAI proportion of C. acuta together with that of such species as G. maxima and A. calamus in favour of C. canescens, which we assume to occur in the “dry” variant of plant species composition, the PN/I relationship reaches lower Asat values for individual growth periods (5.457 to 7.862 μmol m−2 s−1 on the average) and also for the whole growing season: Asat = 9.079 μmol m−2 s−1 (Table S4; Fig. 4). C. canescens is most abundant in the least wet habitats, mainly on tops of sedge tussocks, in our case those of C. acuta. Competition is decisive for the balance between C. acuta and C. canescens, being influenced mainly by the water régime65. C. canescens thrives in the terrestrial ecophase while C. acuta takes advantage of the limosal ecophase59. In wet years, C. acuta prevailed in the stand whereas C. canescens prevailed mainly in dry years65.
Phalaris arundinacea is a grass forming communities occupying habitats along stream and river banks62. In the Wet Meadows, P. arundinacea occupies the nutrient-rich banks of the Central Channel and forms either monodominant stands or stands in which it is mixed with the other plant species studied. The PN/I relationship of P. arundinacea itself attains a lower Asat (9.411 μmol m−2 s−1) than C. acuta (Asat = 10.825 μmol m−2 s−1). The importance of P. arundinacea is relatively greatest in the spring period, when its stems and leaves expand early, together with those of A. calamus, and are photosynthetically highly active. P. arundinacea thus contributes significantly to the photosynthesis of the “big-leaf” mainly during the spring period. G. maxima also plays an important role at this time, thanks to its rate of photosynthesis reaching its seasonal maximum in spring.
We can conclude that the real plant species composition is mixed at the study site suitably for reaching an optimum physiological state of the local vegetation for its rapid growth and a high PN/I relationship. The growth optima of the studied plant species are different, these differences being evident also in their different PN/I relationships (Fig. 5). Ecological niches of these plant species are therefore also different and overlap only partly. The relatively poorer growth of the sedges is competitively disadvantageous under the “dry” conditions of a long-lasting terrestrial ecophase59,66. In reality, a drop of their photosynthesis under the “dry” conditions, associated with low Asat values, can probably be compensated by the photosynthesis of other plant species, especially non-wetland ones. These plants tend to invade the Wet Meadows site during a long-lasting terrestrial ecophase. The effect of invasions was not an object of the present study, but it may represent a challenging task for future studies.
Conclusions
The parameters of the PN/I relationship significantly differed among all five plant species studied. These differences indicate that ecological niches of these species are different and overlap only partly. The “big-leaf” concept used for upscaling the parameters obtained for individual species to the scale of both partial and overall growth periods (with respect to the whole growing season) was very convenient and useful. Differences in the PN/I relationship among the growth periods can be related to structural differences among individual species and also to the keystone role of C. acuta in the spatial structure of the whole plant community. Species composition (diversity) is essential for reaching a light-saturated rate of photosynthesis (Asat) of the “big-leaf” which represents the sedge-grass marsh in different growth periods. In this respect, our study is leaving open the question of the effect on the PN/I parameters of the sedge-grass marsh as a “big-leaf” of non-wetland plants spontaneously invading the Wet Meadows site.
All parameters of the PN/I relationship of the individual plant species studied and individual growth periods are summarized in the Supplementary Information (Tables S3, S4 and S5) for possible use in growth and carbon sequestration models based on the “big-leaf” approach to modelling canopy carbon fluxes. We hope that these parameters can be widely used in this way in both current and future studies.
Data availability
The datasets from in situ measurements of the current study are available from the corresponding author on reasonable request.
References
IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. (Cambridge University Press, Cambridge, 2013).
Reichstein, M. et al. Climate extremes and the carbon cycle. Nature 500, 287–295 (2013).
Matson, P. A., Harriss, a. R. C. (ed.) Biogenic Trace Gases: Measuring Emissions from Soil and Water. Methods in ecology (Blackwell Science, Oxford [England]; Cambridge, Mass., USA, 1995).
Baldocchi, D. et al. FLUXNET: a new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull. Am. Meteorl. Soc. 82, 2415–2434 (2001).
Sellers, P. J., Berry, J. A., Collatz, G. J., Field, C. B. & Hall, F. G. Canopy reflectance, photosynthesis, and transpiration. III. A reanalysis using improved leaf models and a new canopy integration scheme. Remote Sens. Environ. 42, 187–216 (1992).
de Pury, D. & Farquhar, G. D. Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ. 20, 537–557 (1997).
Lasslop, G. et al. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: critical issues and global evaluation. Glob. Change Biol. 16, 187–208 (2010).
Naeem, S. & Li, S. Biodiversity enhances ecosystem reliability. Nature 390, 507–509 (1997).
Tilman, D. Biodiversity: population versus ecosystem stability. Ecology 77, 350–363 (1995).
Walker, B., Kinzig, A. & Langridge, J. Plant attribute diversity, resilience, and ecosystem function: the nature and significance of dominant and minor species. Ecosystems 1999, 95–113 (1999).
Isbell, F. I., Polley, H. W. & Wilsey, B. J. Biodiversity, productivity and the temporal stability of productivity: patterns and processes. Ecol. Lett. 12, 443–451 (2009).
Tilman, D. Functional diversity. Ecyclopedia Biodivers. 2001, 109–121 (2001).
Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups (Springer, Berlin, 2003).
Crawford, R. M. M. Studies in Plant Survival: Ecological Case Histories of Plant Adaptation to Adversity (Blackwell Scientific Publications, Oxford, 1989).
Kaplan, Z. (ed.) Klíč ke květeně: České republiky. Second editions. (Academia, Prague, 2019).
Jeník, J., Kurka, R. & Husák, Š. Wetlands of the Třeboň Basin Biosphere Reserve in the central European context, in Freshwater Wetlands and their Sustainable Future. A Case Study of the Třeboň Basin Biosphere Reserve 11–18 (CRC Press, New York, 2002).
Holubičková, B. Příspěvek ke studiu rašeliništní vegetace. I. Mokré louky u Třeboně (A contribution to the study of peatland vegetation. I. Mokré louky near Třeboň). (1959).
Blažková, D. Pflanzensoziologische Studie über die Wiesen der Südböhmischen Becken. Stud. CSAV 73, 1–172 (1973).
Prach, K. Vegetational changes in a wet meadow complex, south-bohemia, Czech Republic. Folia Geobot. Phytotaxon. 28, 1–13 (1993).
Prach, K. Vegetation changes in a wet meadow complex during the past half-century. Folia Geobot. 43, 119–130 (2008).
Prach, K. & Soukupová, L. Alterations in the Wet Meadows vegetation pattern, in Freshwater Wetlands and their Sustainable Future. A Case Study of the Třeboň Basin Biosphere Reserve 243–254 (CRC Press, 2002).
Balátová-Tuláčková, E. Die Nass- und Feuchtwiesen Nordwest-Böhmens mit besonderer Berücksichtigung Der Magnocaricetalia-Gesellschaften. Rozpr. Českoslov. Akad. Věd, Řada Mat. Přír. Věd 1978.
Květ, J. (ed.) Freshwater Wetlands and their Sustainable Future: A Case of the Třeboň Basin Biosphere Reserve, Czech Republic. Man and the biosphere series 28. (UNESCO, Paris, 2002).
Honissová, M. et al. Seasonal dynamics of biomass partitioning in a tall sedge Carex acuta L. Aquat. Bot. 125, 64–71 (2015).
Hejný, S. Dynamic changes in the macrophyte vegetation of South Bohemian fishponds after 35 years. Folia Geobot. Phytotaxon. 25, 245–255 (1990).
Káplová, M., Edwards, K. R. & Květ, J. The effect of nutrient level on plant structure and production in a wet grassland: a field study. Plant Ecol. 212, 809–819 (2011).
Chambers, J. M. Software for Data Analysis: Programming with R (Springer, Berlin, 2008).
Koyama, K. & Takemoto, S. Morning reduction of photosynthetic capacity before midday depression. Sci. Rep. 4, 4389 (2015).
Nobel, P. S. Physicochemical and Environmental Plant Physiology (Academic Press, New York, 2009).
Gilmanov, T. G. et al. Gross primary production and light response parameters of four Southern Plains ecosystems estimated using long-term CO2-flux tower measurements: GPP OF SOUTHERN PLAINS ECOSYSTEMS. Glob. Biogeochem. Cycles 17, 1–15 (2003).
Bates, D. M. & Watts, D. G. Nonlinear Regression Analysis and Its Applications (Wiley, New York, 1988).
Ogren, E. Convexity of the photosynthetic light-response curve in relation to intensity and direction of light during growth. Plant Physiol. 101, 7 (1993).
Hollander, M., Wolfe, D. A. & Chicken, E. Nonparametric Statistical Methods (Wiley, New York, 2014).
Best, D. J. & Roberts, D. E. Algorithm AS 89: the upper tail probabilities of Spearman’s Rho. Appl. Stat. 24, 377 (1975).
Busch, J. & Losch, R. The gas exchange of Carex species from eutrophic wetlands and its dependence on microclimatic and soil wetness conditions. Phys. Chem. Earth Part B Hydrol. Oceans Atmos. 24, 117–120 (1999).
Ondok, J. & Gloser, J. Leaf photosynthesis and dark respiration in a sedge-grass marsh. 1. model for mid-summer conditions. Photosynthetica 17, 77–88 (1983).
Caudle, K. L. & Maricle, B. R. Physiological relationship between oil tolerance and flooding tolerance in marsh plants. Environ. Exp. Bot. 107, 7–14 (2014).
Ge, Z.-M. et al. Measured and modeled biomass growth in relation to photosynthesis acclimation of a bioenergy crop (Reed canary grass) under elevated temperature, CO2 enrichment and different water regimes. Biomass Bioenerg. 46, 251–262 (2012).
Waring, E. F. & Maricle, B. R. Photosynthetic variation and carbon isotope discrimination in invasive wetland grasses in response to flooding. Environ. Exp. Bot. 77, 77–86 (2012).
Gloser, J. Net photosynthesis and dark respiration of reed estimated by gas-exchange measurements, in Pond Littoral Ecosystems. Structure and Functioning, vol. 1978, 227–234 (Springer, Berlin, 1978).
Zhou, X., Liu, X., Wallace, L. L. & Luo, Y. Photosynthetic and respiratory acclimation to experimental warming for four species in a tallgrass prairie ecosystem. J. Integr. Plant Biol. 49, 270–281 (2007).
Jones, H. G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology (Cambridge University Press, Cambridge, 1992).
Smith, M. & Houpis, J. L. J. Gas exchange responses of the wetland plant Schoenoplectus hallii to irradiance and vapor pressure deficit. Aquat. Bot. 79, 267–275 (2004).
Li, M., Yang, D. & Li, W. Leaf gas exchange characteristics and chlorophyll fluorescence of three wetland plants in response to long-term soil flooding. Photosynthetica 45, 222–228 (2007).
Li, M., Hou, G., Yang, D., Deng, G. & Li, W. Photosynthetic traits of Carex cinerascens in flooded and nonflooded conditions. Photosynthetica 48, 370–376 (2010).
Vervuren, P., Beurskens, S. & Blom, C. Light acclimation, CO2 response and long-term capacity of underwater photosynthesis in three terrestrial plant species. Plant Cell Environ. 22, 959–968 (1999).
Bouma, T. J., De Visser, R., Van Leeuwen, P. H., De Kock, M. J. & Lambers, H. The respiratory energy requirements involved in nocturnal carbohydrate export from starch-storing mature source leaves and their contribution to leaf dark respiration. J. Exp. Bot. 46, 1185–1194 (1995).
McCutchan, C. L. & Monson, R. K. Night-time respiration rate and leaf carbohydrate concentrations are not coupled in two alpine perennial species. New Phytol. 149, 419–430 (2002).
Emerson, R. The quantum yield of photosynthesis. Annu. Rev. Plant Physiol. 9, 1–24 (1958).
Singsaas, E. L., Ort, D. R. & DeLucia, E. H. Variation in measured values of photosynthetic quantum yield in ecophysiological studies. Oecologia 128, 15–23 (2001).
de Lobo, F. A. et al. Fitting net photosynthetic light-response curves with Microsoft Excel—a critical look at the models. Photosynthetica 51, 445–456 (2013).
Busch, J. Characteristic values of key ecophysiological parameters in the genus Carex. FLORA 196, 405–430 (2001).
Hull, J. C. Photosynthetic induction dynamics to sunflecks of four deciduous forest understory herbs with different phenologies1. Int. J. Plant Sci. 163, 913–924 (2002).
Wayne, E. R. & Van Auken, O. W. Light responses of Carex planostachys from various microsites in a Juniperus community. J. Arid Environ. 73, 435–443 (2009).
Colmer, T. D. & Pedersen, O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytol. 177, 918–926 (2008).
Mommer, L. & Visser, E. J. W. Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Ann. Bot. 96, 581–589 (2005).
Dušek, J. Seasonal dynamic of nonstructural saccharides in a rhizomatous grass Calamagrostis epigeios. Biol. Plant. 45, 383–387 (2002).
Mitsch, W. J. (ed.) Wetland Ecosystems (Wiley, Hoboken, NJ, 2009).
Soukupová, L. Life strategy of graminoid populations in the wet meadows, in Freshwater Wetlands and their Sustainable Future. A Case Study of the Třeboň Basin Biosphere Reserve 255–267 (CRC Press, New York, 2002).
Polechová, J. & Storch, D. Ecological Niche, in Encyclopedia of Ecology 72–80 (Elsevier, Amsterdam, 2019). https://doi.org/10.1016/B978-0-12-409548-9.11113-3.
Dykyjová, D. Production ecology of Acorus calamus. Folia Geobot. Phytotaxon. 15, 29–57 (1980).
Westlake, D. F., Květ, J., Andrzej Szczepański, a International Biological Programme. (ed.) The Production Ecology of Wetlands: The IBP Synthesis (Cambridge University Press, Cambridge, UK; New York, NY, USA, 1998).
Pai, A. & McCarthy, B. C. Variation in shoot density and rhizome biomass of Acorus calamus L. With respect to environment. Castanea 70, 263–275 (2005).
Pai, A. & McCarthy, B. C. Suitability of the medicinal plant, Acorus calamus L, for wetland restoration. Nat. Areas J. 30, 380–386 (2010).
Květ, J., Lukavská, J. & Tetter, M. Biomass and net primary production in graminoid vegetation. in Freshwater Wetlands and their Sustainable Future. A Case Study of the Třeboň Basin Biosphere Reserve 293–299 (CRC Press, Boca Raton, 2002).
Hejný, S. The dynamic characteristics of littoral vegetation with respect to changes of water level. Hidrobiol. Bucur. 1971, 71–85 (1971).
Acknowledgements
The authors gratefully acknowledge the financial support provided by the Project Nos. P504/11/1151 of the Grant Agency of the Czech Republic and No. 081/2016/Z of the Grant Agency of the University of South Bohemia. The present study used the research infrastructure of the wetland (Wet Meadows) associate ecosystem site of the Integrated Carbon Observation System (ICOS). The infrastructure was supported by the Ministry of Education, Youth and Sports of the Czech Republic within the National Sustainability Programme I (NPU I), grant number LO1415 and by the project for national infrastructure support CzeCOS/ICOS Reg.No. LM2015061. Special thanks are due to Ing. Stanislav Stellner for his technical assistance and support during the in situ measurements and to Dr. Jan Květ for helpful discussions and language-check of this paper.
Author information
Authors and Affiliations
Contributions
M.M. carried out the field measurements, analysed the data and contributed to writing the manuscript; J.D. contributed to writing the manuscript and reviewed the manuscript and he is also the author of all the pictures; L.F. and L.M. statistically processed data and reviewed the manuscript; H.Č. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mejdová, M., Dušek, J., Foltýnová, L. et al. Photosynthetic parameters of a sedge-grass marsh as a big-leaf: effect of plant species composition. Sci Rep 11, 3723 (2021). https://doi.org/10.1038/s41598-021-82382-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82382-2
This article is cited by
-
Plant species as ecological engineers of microtopography in a temperate sedge-grass marsh
Scientific Reports (2025)
-
Environmental Effects on Assimilated Carbon Quantity and Quality in Two Different Wet Grassland Plants
Wetlands (2024)
-
Addressing Effects of Environment on Eddy-Covariance Flux Estimates at a Temperate Sedge-Grass Marsh
Boundary-Layer Meteorology (2023)