Abstract
The aim of this study was to reveal the effects of anaesthesia strategies on maternal mean arterial pressure (MAP), heart rate, vasopressor consumption, adverse events, and neonatal resuscitation when women with preeclampsia (PE) undergo caesarean section (CS). Three major databases were searched for randomized controlled trials (RCTs) and prospective controlled studies (PCSs). Two authors independently screened, extracted, and checked eligibility and outcome data. Outcomes involved MAP, vasopressor use, maternal adverse events, APGAR scores, and neonatal resuscitation. Pooled estimates were carried out by contrast-based network meta-analysis, and pooled effect sizes were presented with 95% confidence interval (CI). Eleven RCTs and one PCS (n = 782) formed three-node network meta-analysis, and non-significant differences were observed in MAP, 5-min APGAR score, and neonatal intubation rate among the three anaesthesia strategies. General anaesthesia had significantly lower vasopressor consumption than spinal anaesthesia did (standardised mean difference = − 1.19, 95% confidence interval [CI]: − 1.76 to − 0.63), but it had higher maternal adverse event rate (risk ratio = 2.00, 95% CI 1.16–3.47). Because no optimal anaesthesia strategy has been shown to achieve a balanced maternal and neonatal outcome, therefore a shared decision-making process may be required regarding the most suitable choice of anaesthetic strategy for individual preeclamptic mother undergoing CS. Future larger studies may need to focus on evaluating the role of vasopressors on maternal hemodynamic as well as factors affecting maternal outcomes for different anaesthetic techniques in preeclamptic women undergoing CS.
Similar content being viewed by others
Introduction
Preeclampsia (PE), a multisystem disease during pregnancy, is of concern to both anaesthesiologists and gynaecologists. This is because preeclamptic patients are susceptible to cerebrovascular, cardiopulmonary, renal, and haematological events, and foetal outcomes might be compromised because of the associated poor placental function1,2. Although the prevalence of PE differs by gestational age, a systematic review revealed that the prevalence of PE is approximately 4.6% (95% uncertainty range: 2.7–8.2%) of pregnancies worldwide3. Controversy has surrounded the optimal anaesthesia strategy for preeclamptic patients when they undergo caesarean section (CS) for many years. Until 2015, trial and review studies have investigated the most suitable anaesthetic technique for preeclamptic patients; however, a systematic analysis of their data is lacking. Although a network meta-analysis compared anaesthesia strategies for CS, it did not specified women with preeclampsia4. Moreover, the network meta-analysis admitted that pooling preeclampsia with normal women would be inappropriate due to heterogeneity. A synthesis comparing anaesthesia strategies for preeclampsia in CS is still needed.
Both general anaesthesia and regional anaesthesia (spinal and epidural anaesthesia) have advantages and disadvantages. General anaesthesia is often thought to be unsafe because of several maternal airway-related factors, such as possible difficult airways, failed intubation, and hypertensive response to laryngoscopy and intubation5. However, under certain circumstances, general anaesthesia may be indicated for emergent CS, such as in cases that require a reassuring airway and for patients with severe coagulopathy6. By contrast, reports have claimed that spinal anaesthesia (SA) is associated with less hypotension and less impairment of cardiac function in preeclamptic patients compared with that in healthy patients7,8. Therefore regional anaesthesia has been accepted by many as the superior anaesthetic technique for CS in preeclamptic patients because it appears to have fewer associated complications. Furthermore, it has provided superior analgesia compared with general anaesthesia, resulting in less circulating catecholamine and possible improvement in placental blood flow9,10,11,12. Nevertheless, other clinicians have discouraged SA over the concern that it may cause profound hypotension and worsen new born outcomes.
Epidural anaesthesia (EA) offers the advantages of a more stable hemodynamic profile, lower fluid requirements, and lower neuroendocrine stress compared with general anaesthesia13,14. Furthermore, some studies have shown that, in contrast to SA, EA does not produce sudden hypotension in patients with PE. Recent studies, however, have revealed that the haemodynamic effect of EA is equivalent to that of SA15,16,17. Nevertheless, concerns remain regarding regional anaesthesia in patients with PE because severe PE or Haemolysis, Elevated Liver enzymes, Low Platelet count (HELLP) syndrome may lead to the development of thrombocytopenia, which increases the risk of spinal or epidural haematoma. EA should not be used for patients with a platelet count less than 80 × 109 L−118, because the use of larger needles might induce massive haemorrhage19,20.
Therefore, selecting the optimal anaesthesia strategy for preeclamptic patients is difficult because many clinical points must be considered. The purpose of this study was to conduct a meta-analysis to determine the best available evidence to guide clinical practice.
Results
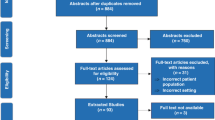
Our search strategy yielded 3244 studies from the Cochrane Database of Systematic Reviews and the CENTRAL (k=216), Embase (k=1943), and New PubMed (k=1085) databases. Moreover, a further study was obtained by hand. After we removed 1112 duplicate and 2109 irrelevant studies through title and abstract screening, we accessed the full texts of the remaining 25 studies and carefully reviewed all the texts. Fifteen articles from 11 randomized controlled trials (RCTs) and a prospective controlled study met the eligibility criteria, and we included all of them in our synthesis (Fig. 1)8,13,16,21,22,23,24,25,26,27,28,29,30,31.
Characteristics and quality of included studies
The 11 RCTs and a prospective nonrandomized controlled trial recruited a total of 782 women with PE from Canada29, India21,22,32, Iran26, the Republic of Macedonia28, Russia23,24,25, South Africa8, Thailand16, the United Kingdom27, and the United States13,30,31 between 1980 and 2015. The mean age of the women ranged from 18.8 to 32 years. Table 1 presents relevant information about maternal mean arterial pressure (MAP) at baseline, gestational age, and baby body weight; furthermore, Appendix 1 shows the quality of the RCTs and a prospective controlled study. These trials formed three-node network for primary outcomes (Fig. 2).
Maternal cardiovascular conditions
Maternal cardiovascular conditions involved MAP, heart rate (HR), systemic vascular resistance (SVR), vasopressor consumption, and vasopressor use rate. Data on HR and SVR were only available from the studies with comparisons of general anaesthesia and SA. Consequently, HR and SVR could only be pooled in a head-to-head meta-analysis. A total of four RCTs (n=251) reported MAP before delivery or intraoperative (Fig. 2A,B)8,13,16,23,24,25. Pooled estimates revealed no significant difference in MAP before delivery or intraoperative among the three anaesthesia strategies (Fig. 3A,B); however, weighted mean difference (WMD) in MAP before delivery between EA and the other anaesthesia strategies raised clinical concerns. The WMD between EA and SA was 21.04 and that between EA and general anaesthesia was 16.66. Therefore, we further performed cumulative probability ranking with 10 000 repetitions, and the results indicated that EA had the highest probability of leading to higher MAP (81.1%; Appendix 2). Loop inconsistency raised some concerns in the pooled estimate of MAP before delivery (chi-square=38.43, P<0.05; Appendix 3), but the funnel plot with Egger’s test did not indicate asymmetry or a significant small study effect in the network meta-analysis of MAP before delivery and intraoperative (Appendix 4 and 5).
A total of two RCTs (n = 130) reported data regarding the mean HR before delivery and intraoperative8,23,24,25. However, these studies only compared general anaesthesia with SA. Based on the available data, patients who received SA had lower HR than those who received general anaesthesia before delivery (WMD = − 31.70, 95% confidence interval [CI] − 39.98 to − 23.42) and intraoperative (WMD = − 16.48, 95% CI − 21.38 to − 11.58). Although the pooled estimates were based on small-scale RCTs (Appendix 6), the included studies consistently exhibited lower HR trends in SA (I-square = 0%). Data on SVR were only available from an RCT with three references23,24,25. Patients who received SA had significantly lower SVR before delivery compared with those who received general anaesthesia, but no difference existed in mean SVR intraoperative between the two anaesthesia strategies (Appendix 7).
Vasopressor use and maternal safety
Vasopressor use rate and vasopressor consumption were presented in seven of the included studies (n = 620)8,16,21,22,27,28,30,31,32. Pooled results revealed that patients who received general anaesthesia seemed to be associated with lower risk of vasopressor use compared with those who received SA (risk ratio [RR]= 0.78, 95% CI 0.66–0.92) and EA (RR= 0.84, 95% CI 0.71–0.99), while there was no significant difference between EA and SA (Fig. 3C). Furthermore, no evidence was detected of inconsistency (chi-square=1.06, P > 0.10) (Appendix 8) or small study effect (coefficient = 1.80, P > 0.10) in the pooled estimate of vasopressor use rate (Appendix 9). Patients who received general anaesthesia was associated with lower vasopressor consumption compared with those who received SA (standardised mean difference [SMD] = − 1.19, 95% CI − 1.76 to − 0.63) and EA (SMD = − 1.33, 95% CI − 2.65 to − 0.01) (Fig. 3D). Loop inconsistency was not tested for because no loop existed in the comparison between the three anaesthesia strategies. Moreover, no evidence of severe small study effects was detected (coefficient = − 0.41, P>0.05) in the pooled estimate of vasopressor consumption (Appendix 10).
In addition, data on adverse events were reported in five studies (n=331)21,26,27,29,30. Three of them compared general anaesthesia with SA21,26, one of them compared SA with EA27, and the other two studies compared general anaesthesia with EA (Appendix 11)29,30. Pooled estimates showed that general anaesthesia had significantly higher adverse event than SA did (RR = 2.00, 95% CI 1.16–3.47), but no difference in maternal adverse event existed in other comparisons (Appendix 12). Furthermore, no evidence existed for loop inconsistency (chi-square = 0.16, P > 0.05; Appendix 13) or small study effects (coefficient = 0.62, P > 0.05; Appendix 14).
Neonatal outcomes
Although appearance, pulse, grimace, activity, and respiration (APGAR) score and resuscitation are two critical neonatal outcomes during delivery, only seven of the included studies reported APGAR scores (n = 479)21,22,25,26,27,29,32. Five of them compared general anaesthesia with SA21,22,26,32, two of them compared SA with EA27,32, and other studies compared general anaesthesia with EA29,32. Both of 5-min APGAR scores (Fig. 2E) and 1-min APGAR scores (Fig. 2F) were contributed by six RCTs. Only one significant difference could be observed among the estimates for APGAR scores, and babies in general anaesthesia group had significantly lower 1-min APGAR score as compared with those in SA group (Weighted mean difference = − 0.48, 95% CI − 0.94 to − 0.02) (Fig. 3E,F). The Lu–Ades method did not reveal significant loop inconsistency in the pooled estimates of 5-min APGAR scores (chi-square = 0.49, P > 0.05; Appendix 15) and design-by-treatment interaction model did not show significant inconsistency in the result of 1-min APGAR scores (chi-square = 0.80, P > 0.10; Appendix 16). Moreover, no evidence indicated small study effects in the pooled estimates of 5-min APGAR scores (coefficient = − 0.95, P > 0.05; Appendix 17) or 1-min APGAR scores (coefficient = − 0.76, P > 0.05; Appendix 18).
Neonatal resuscitations were mainly reported in three studies with comparisons of general and SA (n = 303)8,21,28, and only one trial with a comparison of SA and EA reported neonatal intubation (Appendix 19)30. Based on the available data, no significant difference was observed in neonatal intubation between the three anaesthesia strategies (Appendix 20 to 21). However, SA had a significantly lower rate of facemask use than did general anaesthesia (RR = 0.59, 95% CI 0.41–0.86; Figure 4). This result also had very low heterogeneity (I-square = 0%, P>0.10).
Discussion
For preeclamptic patients, neuraxial anaesthesia (SA and EA) has been considered the optimal choice for CS due to its advantages of attenuation of hypertensive response and superior pain relief. Because of concerns over difficult airway management, coagulopathy, postpartum haemorrhage, renal failure, the need for postoperative intensive care, and postoperative complications, many reports have recommended against general anaesthesia for patients undergoing CS21,33,34,35. However, our findings did not support these recommendations, and post hoc evaluation of confidence in the findings of our meta-analysis were shown in Table 2. Some studies have revealed that general anaesthesia does not pose higher risks to neonates, yet other studies have indicated that general anaesthesia may be associated with adverse neonatal outcomes, higher rates of low 1- and 5-min APGAR scores, a greater requirement for advanced resuscitation, and even mortality21,33,34,35.
Maternal outcomes
According to our data set, we found that SA led to more frequent use of vasopressors than did general anaesthesia, but it had relatively more stable maternal HR and maternal SVR. By contrast, EA seemed to have a more stable MAP. Notably, general anaesthesia did not result in less favourable haemodynamic parameters, including MAP and SVR; the overall complication rate was slightly higher but it did not reach statistical significance. During the intraoperative period, no type of anaesthesia exhibited clinical or statistical differences in terms of MAP or SVR, which was probably because of the appropriate intraoperative management steps taken, including the use of vasopressors and fluid administration.
Current evidence has suggested that maternal blood pressure should be controlled within 160/110 mmHg36,37,38,39,40,41,42,43. Furthermore, the blood pressure of preeclamptic patients during the perioperative period, especially the intraoperative period, should be maintained close to their baseline blood pressure instead of a textbook normal blood pressure. To prevent severe systolic hypertension with subsequent loss of cerebral vasculature autoregulation, diastolic and systolic blood pressures are recommended to be controlled within 90–105 and 140–155 mm Hg, respectively; alternatively, MAP should be controlled between 105 and 125 mm Hg44. Based on the primary articles, in patients receiving general anaesthesia, the MAP before foetal delivery was higher than the ideal MAP. By contrast, the MAP before foetal delivery of the patients receiving SA was closer to the lower limit of the ideal MAP range, and some even had a MAP less than the suggested MAP range following the phase of anaesthetic induction16. This might explain why preeclamptic patients under SA required more frequent use of vasopressors as well as larger does. One prospective study45, however, indicated that preeclamptic patients had less hypotension than patients without PE during SA. Nevertheless, to avoid hypotension under SA, vasopressors are often necessary for preeclamptic patients when their MAP is less than 60 mmHg or if their blood pressure decreases by more than 10%.
Pregnancy induces several maternal physiological and biochemical adjustments to ensure sufficient delivery of oxygen and nutrients to the foetus through the placenta. To compensate for SVR decreasing, HR usually increases by 20%, and the maternal cardiac output increases by 30% to 40% for the purpose of maintaining an adequate placenta perfusion46. PE is characterised by a pathological increase of systemic vascular resistance in response to endogenous angiotensin II and catecholamine secretions47. According to our primary studies, the SVR of patients receiving SA was significantly lower than that of patients receiving general anaesthesia between the time of anaesthetic induction to foetal delivery; however, no SVR differences existed during the rest of the intraoperative period. We postulated that this was because of the use of vasopressors, and evidently from our analysis, the rate of the use and dosage of vasopressors were higher in the SA group.
Vasopressors are known to be used to increase the vascular tone through constricting blood vessels, leading to an elevated SVR and increased MAP. Some vasopressors also demonstrate inotropic and chronotropic properties, resulting in both raised HR and cardiac contractility48,49. In our study, the greater use of vasopressors in the SA group might have increased maternal cardiac output and SVR through increased cardiac contractility and HR, which would explain the lower SVR fluctuation during most of the intraoperative period.
Collectively, SA might not be the ideal anaesthetic strategy because it causes less haemodynamic stability through the rapid onset of sympathectomy, which may result in severe hypotension. With fine-tuned coordination between obstetricians, anaesthetists, and paediatricians, general anaesthesia may provide a shorter anaesthetic time, which might accelerate foetal delivery—and this would ultimately provide the relief for maternal hypertension. As for the effect of EA on maternal outcomes, the lack of data and primary articles may have rendered the analysis nonsignificant.
Neonatal safety
An ideal anaesthetic technique for CS should have the fewest side effects, such as haemodynamic compromise and neonatal depression. Controversy exists concerning the choice of anaesthetic technique for CS in patients with PE. And according to our analysis, the choice may not be as relevant as no differences were observed in the 5-min APGAR scores, which is known to predict the requirement for neonatal resuscitation. We further divided neonatal resuscitation into three parts, namely mask ventilation, intubation, and full CPR for comparison purposes Comparing general anaesthesia with SA, only the demand for mask ventilation was significantly higher in the general anaesthesia group. The reason for this is unknown as the details about the indication for mask ventilation were lacking in the primary articles. Furthermore, no significant difference was observed for advanced resuscitation between the anaesthesia groups.
Neonatal mortality is another crucial outcome to consider. However, remarkably few studies have recorded neonatal mortality. Moreover, mortality cannot be appropriately pooled because of insufficient data in the articles and issues such as foetuses with extremely low body weight (<1500 g) and hydrops foetalis. Currently, the effect of anaesthetic strategy on neonatal mortality is difficult to measure or estimate.
Limitations
Our study had several limitations. First, our synthesis had a limited sample size (n = 721), and a complete network meta-analysis could only be performed for maternal MAP, vasopressor use rate, maternal adverse events, and APGAR scores. Results of HR, SVR, and neonatal resuscitation were only based on limited comparisons of the three anaesthesia strategies. The limited number of trials in the present synthesis may result in unrobust and underpowered estimates. Second, no further related studies have been conducted since 2015. With only one small-scale trial (n = 60) from the Republic of Macedonia that compared general anaesthesia and SA, we may not fully evaluate the effect of recent anaesthetic advances on the outcomes of our study. Third, wide CIs appeared in the estimates of MAP and CPR. The wide CIs may be due to the nature of wide range of MAP in patients with hypertension and small sample size with rare events of CPR. Increasing the study sample size is necessary for studies in the future. Fourth, poor methodologies in our primary studies may have contributed to the overall bias and affected the results of our study.
Conclusions
This is the first network meta-analysis to provide an overview of the maternal and foetal effects of different mono-strategy of anaesthesia for women with PE undergoing CS. No single anaesthetic strategy was found to be superior in terms of overall neonatal outcome. When comparing spinal and general anaesthesia, spinal anaesthesia was found to be associated with lower heart rate, SVR and more vasopressor use, yet general anaesthesia was found to have higher maternal adverse events and neonatal facemask use. A shared decision-making process may be required regarding the most suitable choice of anaesthetic strategy for individual preeclamptic mother undergoing CS. Future larger studies may need to focus on evaluating the role of vasopressors on maternal hemodynamic as well as factors affecting maternal outcomes for different anaesthetic techniques in preeclamptic women undergoing CS.
Methods
In this study, we adhered to the Cochrane Handbook for Systematic Reviews of Interventions and the PRISMA guidelines for conducting and reporting meta-analyses50,51. Thus, the present study did not approach patients or any human. The answerable question for this study was structured according to the following PICO framework:
Population: patients with PE who underwent CS.
Intervention: general anaesthesia.
Comparison: SA or EA.
Outcomes: maternal cardiovascular status; maternal adverse events; neonatal resuscitation; and APGAR score.
To comprehensively understand the effects of anaesthesia strategies on patients with PE undergoing CS, we not only applied head-to-head meta-analysis but also constructed a contrast-based consistency model to pool quantitative data. Protocol of this study had been registered on PROSPERO before we initiated this synthesis in 2019, and the registry number is CRD42020152390.
Eligibility criteria and evidence selection
Two authors (C.C. and Y.N.K.) defined selection criteria according to the proposed PICO framework prior to a comprehensive search, and selected evidence if a study (a) recruited patients with PE undergoing CS, (b) compared different anaesthesia strategies, and (c) was a RCT or prospective nonrandomised clinical study with two or more groups. Because PE, CS, and anaesthesia were the three core elements of this study, we searched the Cochrane Database of Systematic Reviews (including Cochrane Central Register of Controlled Trials, CENTRAL), Embase, and the New PubMed using terms related to PE, CS, and anaesthesia in free text, medical subject headings (MeSH or Emtree), and abbreviations. In this search strategy, we did not restrict language or date of publication. The final search was done for reference before September, 2020 (Appendix 22).
The study selection process involved two steps: (a) title and abstract screening and (b) full-text review. The two authors (C.C. and Y.C.L.) independently excluded studies after screening titles and abstracts. Then, they retrieved the full texts of the potential studies for an extensive review. In addition to the abovementioned primary criteria, they further removed studies according to the following exclusion criteria: (a) studies that recruited healthy pregnant women and patients with PE without conducting a stratified analysis; (b) studies that mainly compared anaesthesia regimens; (c) studies that mainly compared anaesthesia doses; (d) studies with no comparison between anaesthesia strategies; and (e) grey literature without details about study designs or outcomes. An experienced researcher (Y.N.K.) made the final decisions if the first two authors had disagreed in the study selection process. Flowchart of selection was illustrated using Microsoft PowerPoint.
Data extraction and quality assessment
The two authors also individually extracted data regarding study information, baseline characteristics, and outcomes. Study information included study location, recruitment duration, allocation method, blinding, loss to follow-up, type of data analysis, sponsors, and conflicts of interest. Baseline characteristics were anaesthesia strategy, mean age, gestational age, baseline HR, and baseline blood pressure. Outcomes were maternal HR, maternal MAP, maternal SVR, maternal vasopressor use, maternal adverse events, neonatal resuscitation, and 1-min and 5-min APGAR scores. The two authors extracted the number of cases and total cases for maternal vasopressor use, maternal adverse events, and neonatal resuscitation because these outcomes are commonly presented as rates. Furthermore, they determined the means and standard deviations, along with the total sample size, for maternal HR, maternal MAP, maternal SVR, maternal vasopressor use, and 1-min and 5-min APGAR scores because these were continuous variables. Based on the study information, the two authors assessed the quality of the RCTs using the Cochrane Risk of Bias Tool.
Data synthesis and analysis
Quantitative synthesis in this study mainly relied on contrast-based network meta-analysis and head-to-head meta-analysis. RRs were calculated for maternal vasopressor use, maternal adverse events, and neonatal resuscitation. RD was also calculated when the RR indicated clinical concern (0.5 or 2) without significance. WMD was estimated for maternal HR, maternal MAP, maternal SVR, and 1-min and 5-min APGAR scores. Because vasopressors could vary between trials, we used SMD for maternal vasopressor use. In the results, we present effect size (RR, RD, WMD, or SMD) with the 95% CI. Heterogeneity in the pooled results was examined using the I-square statistics. An estimate was considered highly heterogeneous when the I-square statistic was higher than 50% or the P value of the heterogeneity test was higher than 0.10. Funnel plots and Egger’s test were also used to reveal small study effects. When the p value of Egger’s test is lower than 0.05, the pooled estimate may be affected by a small study effect. We further employed surface under the cumulative ranking curve (SUCRA) to numerically present the overall ranking for each anaesthesia strategy. By ranking the probability of each anaesthesia strategy in terms of efficacy, the SUCRA illustrated echelons for identifying the optimal strategy. In addition, we tested inconsistency in each network meta-analysis by using the inconsistency test, which is a method for reflecting transitivity and consistency52,53,54. Inconsistency test was mainly done using loop inconsistency method by Lu and Ade55. Inconsistency test for vasopressor use rate and 1 minute APGAR score was based on design-by-treatment interaction model since these outcomes consisted of two-arm and three-arm RCTs56. Pooled results should be interpreted with care when high heterogeneity, a small study effect, or inconsistencies are detected in the synthesis. Network meta-analysis was done using “network” package57, and head-to-head meta-analysis was carried out by “metan” package58. The “network” package is based on packages “mvmeta,” “metareg,” and “networkplot”57. These packages can be run in STATA version 14 for Microsoft Windows (Texas, USA). Network plots were generated by STATA, and further polished in Microsoft PowerPoint. Overall quality of results of network meta-analysis were further evaluated for clinical implication confidence based on the concept of relevant rules59.
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- APGAR:
-
Appearance, pulse, grimace, activity, and respiration
- CI:
-
Confidence intervals
- HR:
-
Heart rate
- MAP:
-
Mean arterial pressure
- PE:
-
Preeclampsia
- RCT:
-
Randomized clinical trial
- RR:
-
Risk ratio
- SMD:
-
Standardized mean difference
- SUCRA:
-
Surface under the cumulative ranking
- SVR:
-
Systemic vascular resistance
- WMD:
-
Weighted mean difference
References
Ramanathan, J. Pathophysiology and anesthetic implications in preeclampsia. Clin. Obstet. Gynecol. 35, 414–425 (1992).
Hariharan, N., Shoemaker, A. & Wagner, S. Pathophysiology of hypertension in preeclampsia. Microvasc. Res. 109, 34–37. https://doi.org/10.1016/j.mvr.2016.10.002 (2017).
Abalos, E., Cuesta, C., Grosso, A. L., Chou, D. & Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 170, 1–7. https://doi.org/10.1016/j.ejogrb.2013.05.005 (2013).
Kim, W. H. et al. Comparison between general, spinal, epidural, and combined spinal-epidural anesthesia for cesarean delivery: A network meta-analysis. Int. J. Obstet. Anesth. 37, 5–15. https://doi.org/10.1016/j.ijoa.2018.09.012 (2019).
Izci, B. et al. The upper airway in pregnancy and pre-eclampsia. Am. J. Respir. Crit. Care Med. 167, 137–140. https://doi.org/10.1164/rccm.200206-590OC (2003).
Roopa, S. et al. Anesthetic management of combined emergency cesarean section and craniotomy for intracerebral hemorrhage in a patient with severe pre-eclampsia. Curr. Anaesth. Crit. Care 21, 292–295. https://doi.org/10.1016/j.cacc.2010.06.001 (2010).
Aya, A. G. M. et al. Patients with severe preeclampsia experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients: A prospective cohort comparison. Anesth. Analg. 97, 867–872. https://doi.org/10.1213/01.ane.0000073610.23885.f2 (2003).
Dyer, R. A., Piercy, J. L. & Reed, A. R. The role of the anaesthetist in the management of the pre-eclamptic patient. Curr. Opin. Anaesthesiol. 20, 168–174. https://doi.org/10.1097/ACO.0b013e328136c1ac (2007).
Jones, L. et al. Pain management for women in labour: An overview of systematic reviews. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD009234.pub2 (2012).
Shnider, S. M. et al. Maternal catecholamines decrease during labor after lumbar epidural anesthesia. Am. J. Obstet. Gynecol. 147, 13–15. https://doi.org/10.1016/0002-9378(83)90076-5 (1983).
Lederman, R. P., Lederman, E., Work, B. Jr. & McCann, D. S. Anxiety and epinephrine in multiparous women in labor: Relationship to duration of labor and fetal heart rate pattern. Am. J. Obstet. Gynecol. 153, 870–877. https://doi.org/10.1016/0002-9378(85)90692-1 (1985).
Jouppila, R., Jouppila, P., Hollmén, A. & Kuikka, J. Effect of segmental extradural analgesia on placental blood flow during normal labour. Br. J. Anaesth. 50, 563–567. https://doi.org/10.1093/bja/50.6.563 (1978).
Ramanathan, J., Coleman, P. & Sibai, B. Anesthetic modification of hemodynamic and neuroendocrine stress responses to cesarean delivery in women with severe preeclampsia. Anesth. Analg. 73, 772–779. https://doi.org/10.1213/00000539-199112000-00016 (1991).
Hood, D. D. & Curry, R. Spinal versus epidural anesthesia for cesarean section in severely preeclamptic patients: A retrospective survey. Anesthesiology 90, 1276–1282. https://doi.org/10.1097/00000542-199905000-00009 (1999).
Clark, V. A., Sharwood-Smith, G. H. & Stewart, A. V. G. Ephedrine requirements are reduced during spinal anaesthesia for caesarean section in preeclampsia. Int. J. Obstet. Anesth. 14, 9–13. https://doi.org/10.1016/j.ijoa.2004.08.002 (2005).
Visalyaputra, S. et al. Spinal versus epidural anesthesia for cesarean delivery in severe preeclampsia: A prospective randomized, multicenter study. Anesth. Analg. 101, 862. https://doi.org/10.1213/01.ANE.0000160535.95678.34 (2005).
Chiu, C. L., Mansor, M., Ng, K. P. & Chan, Y. K. Retrospective review of spinal versus epidural anaesthesia for caesarean section in preeclamptic patients. Int. J. Obstet. Anesth. 12, 23–27. https://doi.org/10.1016/s0959-289x(02)00137-1 (2003).
Izak, M. & Bussel, J. B. Management of thrombocytopenia. F1000Prime Rep. 6, 45–45. https://doi.org/10.12703/P6-45 (2014).
Santos, A. C. & Birnbach, D. J. Spinal anesthesia in the parturient with severe preeclampsia: Time for reconsideration. Anesth. Analg. 97, 621–622. https://doi.org/10.1213/01.ane.0000085017.03397.59 (2003).
Santos, A. C. Spinal anesthesia in severely preeclamptic women: When is it safe?. Anesthesiology 90, 1252–1254 (1999).
Chattopadhyay, S., Das, A. & Pahari, S. Fetomaternal outcome in severe preeclamptic women undergoing emergency cesarean section under either general or spinal anesthesia. J. Pregnan. 325098–325098, 2014. https://doi.org/10.1155/2014/325098 (2014).
Dasgupta, S., Chakraborty, B., Saha, D. & Ghosh, D. Comparison of neonatal outcome in women with severe pre-eclampsia undergoing caesarean section under spinal or general anaesthesia. J. Indian Med. Assoc. 109, 166–170 (2011).
Kinzhalova, S., Makarov, R. & Davidova, N. Comparison of general and spinal anaesthesia on haemodynamic parameters in severe preeclamptic pregnancy undergoing caesarean section. Eur. J. Anaesthesiol. 30, 178 (2013).
Kinzhalova, S. D. & Makarov, R. Haemodynamic parameters of pregnant women with chronic arterial hypertension during Caesarean section: Comparison of general and spinal anaesthesia: 11AP3–6. Eur. J. Anaesthesiol. 29, 167–168 (2012).
Kinzhalova, S. V., Makarov, R. A. & Davydova, N. S. Central haemodynamics parameters during different anaesthesia methods of surgical delivery in preeclampsia women. Anesteziol. Reanimatol. 5, 52–54 (2012).
Moslemi, F. & Rasooli, S. Comparison of spinal versus general anesthesia for cesarean delivery in patients with severe preeclampsia. J. Med. Sci. 7, 1044–1048. https://doi.org/10.3923/jms.2007.1044.1048 (2007).
Sharwood-Smith, G., Clark, V. & Watson, E. Regional anaesthesia for caesarean section in severe preeclampsia: Spinal anaesthesia is the preferred choice. Int. J. Obstet. Anesth. 8, 85–89 (1999).
Sivevski, A. G., Sholjakova, M. V., Kartalov, A. B., Kuzmanovska, B. K. & Durnev, V. M. Comparison of low dose spinal anesthesia with general anesthesia in pre-eclamptic parturients undergoing emergency cesarean section. Anaesth. Pain Intens. Care 19, 37–43 (2015).
Hodgkinson, R., Husain, F. J. & Hayashi, R. H. Systemic and pulmonary blood pressure during caesarean section in parturients with gestational hypertension. Can. Anaesth. Soc. J. 27, 389–394 (1980).
Wallace, D. H. et al. Randomized comparison of general and regional anesthesia for cesarean delivery in pregnancies complicated by severe preeclampsia. Obstet. Gynecol. 86, 193–199 (1995).
Wallace, D. H. et al. Randomized study of general anesthesia vs epidural or spinal-epidural analgesia for Cesarean section in pregnancies complicated by severe preeclampsia. Am. J. Obstet. Gynecol. 166, 302 (1992).
Mathur, V., Nagrath, A., Saxena, S. & Maheshwari, M. Study of the maternal and neonatal outcome in caesarean deliveries in pregnancies complicated by pre-eclampsia conducted under general anaesthesia and regional anaesthesia. J. Obstet. Gynaecol. India 52, 28–32 (2002).
Saygı, A. İ et al. Comparison of maternal and fetal outcomes among patients undergoing cesarean section under general and spinal anesthesia: A randomized clinical trial. Sao Paulo Med. J. 133, 227–234. https://doi.org/10.1590/1516-3180.2014.8901012 (2015).
Chumpathong, S. et al. Maternal and neonatal outcomes in women with severe pre-eclampsia undergoing cesarean section: A 10-year retrospective study from a single tertiary care center: Anesthetic point of view. J. Matern. Fetal Neona 29, 4096–4100. https://doi.org/10.3109/14767058.2016.1159674 (2016).
Algert, C. S. et al. Regional block versus general anaesthesia for caesarean section and neonatal outcomes: A population-based study. BMC Med. 7, 20–20. https://doi.org/10.1186/1741-7015-7-20 (2009).
ACOG Practice Bulletin No. 202: Gestational hypertension and preeclampsia. Obstet. Gynecol. 133, e1–e25. https://doi.org/10.1097/AOG.0000000000003018 (2019).
Butalia, S. et al. Hypertension Canada’s 2018 guidelines for the management of hypertension in pregnancy. Can. J. Cardiol. 34, 526–531. https://doi.org/10.1016/j.cjca.2018.02.021 (2018).
Regitz-Zagrosek, V. et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 39, 3165–3241. https://doi.org/10.1093/eurheartj/ehy340 (2018).
Magee, L. A. et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J. Obstet. Gynaecol. Can. 36, 416–441. https://doi.org/10.1016/s1701-2163(15)30588-0 (2014).
Tranquilli, A. L. et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnan. Hypertens. 4, 97–104. https://doi.org/10.1016/j.preghy.2014.02.001 (2014).
Brown, M. A. et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 72, 24–43. https://doi.org/10.1161/HYPERTENSIONAHA.117.10803 (2018).
Lowe, S. A. et al. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust. N. Z. J. Obstet. Gynaecol. 55, e1–e29. https://doi.org/10.1111/ajo.12399 (2015).
Redman, C. W. G. Hypertension in pregnancy: The NICE guidelines. Heart 97, 1967–1969. https://doi.org/10.1136/heartjnl-2011-300949 (2011).
Turner, J. A. Severe preeclampsia: Anesthetic implications of the disease and its management. Am. J .Ther. 16, 284–288. https://doi.org/10.1097/MJT.0b013e31816f6fd8 (2009).
Aya, A. G. M. et al. Spinal anesthesia-induced hypotension: A risk comparison between patients with severe preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth. Analg. 101, 869. https://doi.org/10.1213/01.ANE.0000175229.98493.2B (2005).
Soma-Pillay, P., Nelson-Piercy, C., Tolppanen, H. & Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 27, 89–94. https://doi.org/10.5830/CVJA-2016-021 (2016).
Lindheimer, M. D. & Katz, A. I. Preeclampsia: Pathophysiology, diagnosis, and management. Annu. Rev. Med. 40, 233–250. https://doi.org/10.1146/annurev.me.40.020189.001313 (1989).
Kee, V. R. Hemodynamic pharmacology of intravenous vasopressors. Crit. Care Nurse 23, 79–82. https://doi.org/10.4037/ccn2003.23.4.79 (2003).
Overgaard Christopher, B. & Džavík, V. Inotropes and vasopressors. Circulation 118, 1047–1056. https://doi.org/10.1161/CIRCULATIONAHA.107.728840 (2008).
Hutton, B. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 162, 777–784. https://doi.org/10.7326/m14-2385 (2015).
Higgins, J. P. et al. Cochrane Handbook for Systematic Reviews of Interventions (John Wiley & Sons, 2019).
Cipriani, A., Higgins, J. P., Geddes, J. R. & Salanti, G. Conceptual and technical challenges in network meta-analysis. Ann. Intern. Med. 159, 130–137 (2013).
Salanti, G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: Many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synth. Methods 3, 80–97 (2012).
Tonin, F. S., Rotta, I., Mendes, A. M. & Pontarolo, R. Network meta-analysis: A technique to gather evidence from direct and indirect comparisons. Pharmacy Pract. 15, 2 (2017).
Lu, G. & Ades, A. Assessing evidence inconsistency in mixed treatment comparisons. J. Am. Stat. Assoc. 101, 447–459 (2006).
Higgins, J. et al. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 3, 98–110 (2012).
White, I. R. Network meta-analysis. Stata J. 15, 951–985 (2015).
Harris, R. J. et al. Metan: Fixed-and random-effects meta-analysis. Stata J. 8, 3–28 (2008).
Nikolakopoulou, A. et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 17, e1003082 (2020).
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Author information
Authors and Affiliations
Contributions
C.C.: This author helped conceptualization, data curation, investigation, original draft. A.H.-W.L.: This author helped conceptualization, investigation, critical revision. C.-Y.C.: This author helped supervision and critical revision. Y.-C.L.: This author helped conceptualization, data curation, investigation, methodology, supervision, and critical revision. Y.-N.K.: This author helped formal analysis, methodology, visualization, critical revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, C., Liao, A.HW., Chen, CY. et al. A systematic review with network meta-analysis on mono strategy of anaesthesia for preeclampsia in caesarean section. Sci Rep 11, 5630 (2021). https://doi.org/10.1038/s41598-021-85179-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-85179-5
This article is cited by
-
Norepinephrine vs. phenylephrine for spinal hypotension in cesarean section: a network meta-analysis
Journal of Anesthesia (2025)