Abstract
This in vitro study evaluated the protective effect of titanium tetrafluoride (TiF4) varnish and silver diamine fluoride (SDF) solution on the radiation-induced dentin caries. Bovine root dentin samples were irradiated (70 Gy) and treated as follows: (6 h): 4% TiF4 varnish; 5.42% NaF varnish; 30% SDF solution; placebo varnish; or untreated (negative control). Microcosm biofilm was produced from human dental biofilm (from patients with head-neck cancer) mixed with McBain saliva for the first 8 h. After 16 h and from day 2 to day 5, McBain saliva (0.2% sucrose) was replaced daily (37 °C, 5% CO2) (biological triplicate). Demineralization was quantified by transverse microradiography (TMR), while biofilm was analyzed by using viability, colony-forming units (CFU) counting and lactic acid production assays. The data were statistically analyzed by ANOVA (p < 0.05). TiF4 and SDF were able to reduce mineral loss compared to placebo and the negative control. TiF4 and SDF significantly reduced the biofilm viability compared to negative control. TiF4 significantly reduced the CFU count of total microorganism, while only SDF affected total streptococci and mutans streptococci counts. The varnishes induced a reduction in lactic acid production compared to the negative control. TiF4 and SDF may be good alternatives to control the development of radiation-induced dentin caries.
Similar content being viewed by others
Introduction
Head and neck cancer (HNC) represents the sixth most common type of cancer diagnosed worldwide. Unfortunately, more than 66% of HNC cases are diagnosed in advanced stages (III or IV)1. The radiotherapy, associated or not with other therapies, is the main treatment for malignant HNC lesions2, based on the use of high doses of X-rays to destroy tumor cells. However, normal cells are also affected by head and neck radiotherapy, which can cause salivary gland dysfunction and, consequently, hyposalivation that dramatically increases the risk for dental caries. Furthermore, radiotherapy also causes some damage to the dental hard tissue, increasing its susceptibility to demineralization3,4.
Root caries lesions (RCLs) are often diagnosed with advanced age, due to hyposalivation and to the root exposure caused by gingival recession that results from aggressive toothbrushing or chronic periodontitis5. Global annual RCL incidence is reported to vary from 10.1 to 40.6% for healthy people6, while for patients with head and neck radiotherapy is about 16% after the first year, reaching up to 74% after 7 years of treatment7,8. Therefore, interventions to prevent this type of dental disease are needed.
Radiation-induced dental caries is a complex and multifactorial disease, which differs from conventional dental caries due to its sudden progression, rapidly compromising dentin, and reaching surfaces that are not usually affected by carious lesions, such as tips of cusps and smooth surfaces9. Depending on the radiotherapy site, teeth can be significantly affected by the maximum dosage (around 99% in case of tongue tumors, for example)10. Both enamel and dentin are already softened by 10 Gy11,12,13, a dose much lower than those applied in case of HNC. A recent study has shown that radiotherapy decreases odontoblastic cell metabolism, caused by decreased vascularization, as well as induces degradation of collagen fibers9.
Fluorides have been used to prevent RCLs. Annual application of sodium fluoride (5%) varnish or 38% silver diamine fluoride (SDF) solution are able to reduce the emergence of new RCLs by 64 and 71%, respectively14. The application of fluoride products on the teeth has been suggested throughout the period of radiotherapy15,16,17, however, there are few studies on this subject. Wu et al.18 showed in vitro that dentin (exposed to 68.25 Gy of radiation) treated daily with 5% NaF varnish, effectively showed a restored surface and increased microhardness compared to untreated irradiated dentin.
On the other hand, previous studies have shown a promising effect of titanium tetrafluoride (TiF4) varnish to control enamel de-remineralization compared to NaF varnish19,20. The application of TiF4 induces deposition of an acid-resistant layer containing titanium oxide and hydrated titanium phosphate, able to provide mechanical protection, and higher fluoride uptake compared to NaF, increasing the acid resistance of dental hard tissue21.
A recent study also showed a promising effect of TiF4 varnish on the prevention of dentin carious lesions formation compared to NaF varnish, under microcosm biofilm model produced on previous sound dentin22. We do not know if TiF4 varnish would have the same protective effect if applied on previous irradiated dentin, as well as under conditions simulating patients with HNC. The same is assumed for SDF.
Due to the need for finding alternatives to minimize the development of radiation-induced dentin caries, the aim of this work was to evaluate: (1) the protective effect of TiF4 varnish compared to NaF varnish and SDF solution on irradiated root dentin with respect to demineralization; and (2) the antimicrobial action of TiF4 varnish compared to NaF varnish and SDF solution on microcosm biofilm produced from biofilm collected from patients subjected to head and neck radiation.
Results
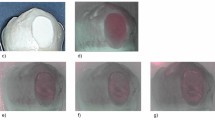
TiF4 varnish and SDF solution significantly reduced the integrated mineral loss compared to NaF varnish, placebo varnish and negative control (p < 0.0001). With respect to lesion depth, no protective effect of fluorides was found (Table 1). Figure 1 shows the TMR image and the lesion profile of a representative dentin sample per group.
TiF4 and SDF significantly reduced biofilm viability compared to negative control, while SDF further differed from placebo varnish (p < 0.0001). NaF had no effect on biofilm viability (Fig. 2). TiF4 and NaF varnishes-treated biofilms had reduced thickness (17.9 ± 3.1 and 18.7 ± 3.3 μm, respectively) compared to negative control (24.3 ± 4.3 μm), but not to placebo varnish (20.3 ± 3.6 μm) (p = 0.0032). SDF had no effect on biofilm thickness (20.0 ± 3.7 μm).
In agreement with viability assay, TiF4 was the only one able to reduce the CFU counts of total microorganisms (p = 0.003). No effect of fluoride treatments was seen on Lactobacillus sp. SDF significantly reduced the total streptococci (p < 0.0001) and mutans streptococci (p = 0.0001) CFU numbers compared to placebo varnish and negative control, while TiF4 and NaF did not (Table 2).
All varnishes (TiF4, NaF and placebo) significantly reduced lactic acid biofilm production compared to negative control and SDF (p < 0.0001), which in turn did not differ from each other (Fig. 3).
Discussion
Our recent study has shown irrelevant antimicrobial effect of TiF4 varnish, but a significant ability to reduce carious lesions development in sound dentin under microcosm biofilm model22. One other recent study evaluated the effect of fluorides on irradiated dentin, but not under cariogenic conditions18.
Considering the high prevalence of HNC and the side-effects of radiotherapy, efforts to find a good alternative to avoid the development of radiation-dentin caries lesions are extremely relevant. Accordingly, our study confirmed the anti-caries effect of TiF4 varnish compared to NaF varnish, as shown by Dos Santos et al.22, which was comparable to those found for SDF. An annual application of SDF is able to prevent 71% root caries lesions development14. However, tooth discoloration induced by SDF application is of major concern, while no significant staining potential by TiF4 varnish was found23.
Interestingly, carious lesions created under conditions simulating irradiated patients were 1.5–2-folder greater compared to those induced on sound dentin under a similar microcosm biofilm22 and, despite it, TiF4 varnish still had a protective effect. This result is also supported by an in vivo study24, which further showed penetration of F and Ti into the dentin caries lesion. According to Tveit et al.25, a titanium-rich layer remained on the dentin surface even after 3 weeks of TiF4 application in vivo.
Microcosm biofilm induced using human biofilm from irradiated patients also presented a bacterial load 1.2–1.4 times higher compared to microcosm biofilm growth under health conditions on dentin samples22. Signori et al.26 have discussed that the bacteria source (saliva vs. biofilm, caries vs. free-caries patient) has no influence on the potential of microcosm biofilm to induce demineralization under sucrose exposure. However, in the present study, the bacterial load may have had a significant influence on radiation-induced dentin caries. We thus speculate that a high bacterial load could have impaired the anti-caries effect of NaF, found in our previous work22, but not demonstrated here.
Therefore, we are planning to conduct future studies analyzing the biofilm behavior and the degree of dentin demineralization, comparing the sources of bacteria (biofilm from irradiated vs. non irradiated patients) and the quality of dentin substrate (irradiated vs. non irradiated), to better address the effect of both factors on the caries development.
We did not apply methods to demonstrate morphological dentin alterations induced by radiation and its relation to the caries lesions formation. We have support from previous studies that showed morphological dentin alterations (presence of cracks on surface and dentin tubules occlusion) after irradiation, by using scanning electron microscopy-SEM18,27. The damage of dentin surface induced by irradiation can have interfered on the CaF2 deposition produced by NaF varnish, and consequently on the F− release from this reservoir, justifying its lack of antimicrobial and anti-caries effects in the present study either.
In contrast to the report of Dos Santos et al.22, the present study found that TiF4 was able to reduce total microorganism CFU, however, with no effect on the numbers of cariogenic species. A microbiome study of microcosm biofilm growth under health and radiation conditions are needed, since other species not studied here may be part of the microcosm biofilm, contributing to the sudden caries lesion development in irradiated dentin28 and justifying the antimicrobial effect of TiF4 varnish found on total microorganisms.
SDF, on the other hand, had an antimicrobial effect on total streptococci and mutans streptococci, in agreement with the literature29,30. Zhao et al.30 demonstrated that SDF hinders dentin collagen degradation. SDF also induces the formation of products such as CaF2, Ag3PO4 and NH4OH, which interact with dentin hydroxyapatite forming fluorapatite31, providing an acid-resistant structure. A clinical trial also confirms that SDF is more effective in stopping caries lesions in dentin when compared to NaF32. Therefore, the anti-caries effect of SDF may be due to its antimicrobial action and also to its chemical interaction with dentin surface. It is likely that the interaction with the tooth surface is more important than its antimicrobial effect, since SDF did not reduce lactate production, despite it had significantly decreased the cariogenic microorganism CFU counting.
In principle, we expected to see low lactate production by the biofilm after SDF application, since this fluoride significantly decreased mutans streptococci CFU counting33, which was not observed. We speculate that other aciduric bacteria could be presented in the biofilm, which in turn were not affected by SDF. Also, SDF did not reduce the biofilm thickness. The high amount of extracellular matrix, involved in the biofilm thickness, could have retained more acid in the biofilm, justifying our findings. This hypothesis needs to be confirmed in future studies.
NaF varnish has previously shown to reduce the production of lactic acid under microcosm biofilm34, mono-species biofilm35 and multispecies biofilm36. Both NaF and TiF4 significantly reduced biofilm thickness and lactic acid production, despite the fact that they were not different from placebo varnish, in disagreement with the findings of Dos Santos et al.22 This finding deserves further attention. Somehow component(s) of our varnish (except F) might have acted as glycolytic enzyme inhibitor(s), reducing the amount of lactate produced by the biofilm.
The caries protective effect of TiF4 seems to be more due to dentin surface modification than to the antimicrobial effect. Interestingly, the TMR profile of TiF4-treated dentin showed an intermediated highly mineralized layer (around 90 μm depth), which might contain F and Ti. For both TiF4 and SDF, their chemical interaction could have improved mineral gain especially in the intermediate layer (arrows in Fig. 1), but they were not able to impair bacterial acid penetration and, consequently, no reduction in lesion depth was seen.
In conclusion, both TiF4 varnish and SDF solution were similarly able to reduce the development of radiation-induced dentin caries in vitro. The result of the present study needs to be confirmed by randomized clinical trials in patients affected by HNC and treated with radiotherapy.
Methods
Tooth sample preparation and treatment groups
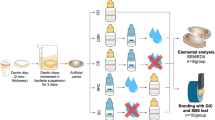
The bovine teeth were collected from cattle slaughtered in the food manufacturing industry (Frigol S.A, Lençóis Paulista-SP, Brazil). The study was approved by Ethics committee on animal research (CEUA, Number: 004/2018, Bauru School of Dentistry, University of São Paulo, Bauru, Brazil) following the guidelines of the CONCEA (National Council for Control of Animal Experimentation). No animals were harmed in order to conduct this study. One hundred and eighty bovine root dentin samples (4 mm × 4 mm) were prepared22. Dentin samples were submitted to X-rays from linear accelerator with an energy of 6 meV (Varian, Clinac 6EX, USA) in total dose of 70 Gy and, thereafter, sterilized using ethylene oxide for 4 h under a pressure of 0.5 ± 0.1 kgF/cm.
Before sterilization, the average surface roughness was measured by using a contact profilometer (Mahr Perthometer, Göttingen, Germany) and the software MarSurf XCR-20 (Mahr Perthometer, Göttingen, Germany) (5 readings for the calculation of the mean), for samples randomization into the groups (n = 36/group, n = 12 for each biofilm assay): 4% TiF4 varnish (pH 1.0, 2.45% F); 5.42% NaF varnish (pH 5.0, 2.45% F); 30% SDF solution (pH 8.5, 3.54% F); placebo varnish (pH 5.0) or untreated (negative control)37. The dentin roughness may influence biofilm formation22 and, therefore, it was applied to standardize the initial conditions of the groups (mean: 0.34 ± 0.03 μm). Before treatment, 2/3 of the dentin surface was protected using nail polish to allow to have 2 control areas (untreated and non-demineralized areas).
The F and placebo varnishes contained the same artificial resin as base and ethanol as solvent; SDF solution contained hydrofluoric acid, silver nitrate, ammonium hydroxide and deionized water. The treatments were applied using microbrush on the samples surfaces for 6 h. During this period, dentin samples were stored in remineralizing solution37. The treatments were then removed using cotton swab and acetone solution22,37 and the nail polish was reapplied at the same sites, before biofilm formation.
Microcosm biofilm formation
A mixed solution containing thawed inoculum (compound from human biofilm from 2 donors who received a total radiation dose of 70 Gy in the head and neck region, mixed with 1% saline solution in the proportion 2 g: 1 mL, and diluted for freezing in 30% of glycerol26) and McBain saliva (proportion 1:50) was added to each well containing a treated dentin samples (24-well microplate, 1.5 mL/well), and incubated for 8 h (5% CO2 and 37 °C)22,38. Thereafter, the medium was removed and fresh McBain saliva containing 0.2% sucrose was added to the wells for further 16 h22,37. The medium was replaced daily for more 4 days and incubated under the same conditions described above22,37.
Demineralization analysis: Transverse microradiography (TMR)
After 5 days of biofilm growth, dentin samples (except those from the lactic acid assay) were cleaned, transversally sectioned, polished and submitted to microradiograph exposure (20 kV and 20 mA, Softex, Tokyo, Japan) as previously described22. The developed plate was analyzed using a transmitted light microscope fitted with a 20× objective. Two images per sample were obtained using data acquisition (version 2012) and interpreted using calculation (version 2006) software from Inspektor Research System (Amsterdam, Netherlands). The mineral content was calculated based on the work of Dos Santos et al.22, assuming 50 vol% of mineral content for sound dentin and that the lesion depth ends when dentin contains around of 47.5% of mineral volume. The integrated mineral loss (ΔZ, vol% μm) and lesion depth (LD, μm) were calculated for the mean of the 2 images per sample.
Biofilm viability analysis
Biofilm was stained using nucleic acid marker (2 µM SYTO 9 green fluorescent nucleic acid stain, Thermo Fisher Scientific, USA) (v = 10 μL/well) for 15 min in a dark environment22. Confocal laser scanning microscopy (Leica TCS SPE, Mannheim, Germany) and Leica Application Suite-Advanced Fluorescence software (LAS AF, Mannheim, Germany) were used to analyze the biofilm surface. Three images (275 μm2) were captured and analyzed using BioImage L 2.0 software. The percentage of live bacteria-% and the biofilm thickness-μm were obtained.
Analysis of colony forming units (CFU)
Four different types of agar were used to the CFU counting: 1) brain heart infusion agar (BHI; Difco, Detroit, USA) for total microorganisms; (2) mitis salivarius agar (MSA; Neogen, Indaiatuba, Brazil) for total streptococci; (3) SB-20 M for mutans streptococci; and (4) rogosa (MRS agar; Kasvi, Curitiba, Brazil) for Lactobacillus sp.22. Bacterial suspensions were diluted (10−4) and spread on Petri dishes (25 μL/dish) and then, the dishes incubated under 5% CO2 and 37 °C for 48 h. The CFU numbers were counted by two examiners and converted to log10 CFU/mL.
Analysis of lactic acid production
Dentin samples with 5-day microcosm biofilm were incubated in a buffered peptone water (BPW) (Synth, Diadema, Brazil) supplemented with 0.2% sucrose (v = 1 mL/sample) for 3 h, under 5% CO2 and 37 °C22. Lactate concentrations were evidenced via enzymatic method (Enzymatic assay for d- and l-Lactic acid—Ref. 8240; R-Biopharm, Darmstadt, Germany) following the manufacturer’s guidelines. The absorbance was measured at 340 nm using a microplate reader (Fluorstar Optima—BMG Labtech, Ortenberg, Germany) and the values converted to g/L.
Statistical analysis
The biofilm assays (viability, UFC counting and lactic acid assay) were performed in triplicate with four data points for each replicate (n = 12). Dentin samples from all biofilm analysis, except from the lactic acid assay, were analyzed by TMR (n = 24). Data were statistically compared using GraphPad Prism software for Windows (GraphPad Software, San Diego, USA). The normal distribution and homogeneity were checked using Kolmogorov–Smirnov and Bartlett tests, respectively. All data were compared using Analysis of Variance (ANOVA) followed by Tukey–Kramer test. The level of significance was set at 5%.
Ethics aspects and Saliva collection
The local ethical committee (CAAE: 97497318.00000.5417) of Bauru School of Dentistry-USP (Bauru-Brazil) approved this study. The study was conducted in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study. Biofilm was collected from two donors (1 male: 65 years old with 20 teeth and 1 female: 57 years old with 24 teeth) who received the total head and neck 3D radiotherapy (final dose: 70 Gy), 5 months previously to the study, and met the inclusion criteria: (1) low salivary flow (stimulated saliva flow < 1 mL/min and non-stimulated saliva flow < 0.3 mL/min), (2) without acute gingivitis, (3) not using antibiotics or (4) being submitted to professional fluoride application in the last 3 months neither. Biofilm was collected from the cervical area of all roots without active caries lesions, by using a periodontal curette. Biofilm collection and storage were performed as described by Signore et al.26.
References
Cohen, N., Fedewa, S. & Chen, A. Y. Epidemiology and demographics of the head and neck cancer population. Oral Maxillofac. Surg. Clin. N. Am. 30(4), 381–395. https://doi.org/10.1016/j.coms.2018.06.001 (2018).
Likhterov, I. et al. Objective and subjective hyposalivation after treatment for head and neck cancer: long-term outcomes. Laryngoscope 128(12), 2732–2739. https://doi.org/10.1002/lary.27224 (2018).
Gupta, N. et al. Radiation-induced dental caries, prevention and treatment—a systematic review. Natl. J. Maxillofac. Surg. 6(2), 160–166. https://doi.org/10.4103/0975-5950.183870 (2015).
Pereira, I. F. et al. Radiation-induced oral mucositis in Brazilian patients: prevalence and associated factors. In Vivo 33(2), 605–609. https://doi.org/10.21873/invivo.11517 (2019).
Saunders, R. H. Jr. & Meyerowitz, C. Dental caries in older adults. Dent. Clin. N. Am. 49(2), 293–308. https://doi.org/10.1016/j.cden.2004.10.004 (2005).
Hayes, M., Burke, F. & Allen, P. F. Incidence, prevalence and global distribution of root caries. Monogr. Oral Sci. 26, 1–8. https://doi.org/10.1159/000479301 (2017).
Siala, W. et al. Toxicité neurologique tardive après traitement des carcinomes nasopharyngés [Late neurotoxicity after nasopharyngeal carcinoma treatment]. Cancer Radiother. 13(8), 709–714. https://doi.org/10.1016/j.canrad.2009.05.006 (2019).
Hamilton, S. N. et al. Documentation and incidence of late effects and screening recommendations for adolescent and young adult head and neck cancer survivors treated with radiotherapy. Support Care Cancer 27(7), 2609–2616. https://doi.org/10.1007/s00520-018-4559-5 (2019).
Campi, L. B. et al. Effect of radiotherapy on the chemical composition of root dentin. Head Neck 41(1), 162–169. https://doi.org/10.1002/hed.25493 (2019).
Morais-Faria, K. et al. Dosimetric distribution to the teeth of patients with head and neck cancer who underwent radiotherapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 120(3), 416–419. https://doi.org/10.1016/j.oooo.2015.05.009 (2015).
Gonçalves, L. M. et al. Radiation therapy alters microhardness and microstructure of enamel and dentin of permanent human teeth. J. Dent. 42(8), 986–992. https://doi.org/10.1016/j.jdent.2014.05.011 (2014).
Reed, R. et al. Radiotherapy effect on nano-mechanical properties and chemical composition of enamel and dentine. Arch. Oral Biol. 60(5), 690–697. https://doi.org/10.1016/j.archoralbio.2015.02.020 (2015).
Liang, X., Zhang, J. Y., Cheng, I. K. & Li, J. Y. Effect of high energy X-ray irradiation on the nano-mechanical properties of human enamel and dentine. Braz. Oral Res. 30, S1806. https://doi.org/10.1590/1807-3107BOR-2016.vol30.0009 (2016).
Magalhães, A. C. Conventional preventive therapies (fluoride) on root caries lesions. Monogr. Oral Sci. 26, 83–87. https://doi.org/10.1159/000479349 (2017).
Epstein, J. B., van der Meij, E. H., Lunn, R. & Stevenson-Moore, P. Effects of compliance with fluoride gel application on caries and caries risk in patients after radiation therapy for head and neck cancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 82(3), 268–275 (1996).
Deng, J., Jackson, L., Epstein, J. B., Migliorati, C. A. & Murphy, B. A. Dental demineralization and caries in patients with head and neck cancer. Oral Oncol. 51(9), 824–831. https://doi.org/10.1016/j.oraloncology.2015.06.009 (2015).
Hong, C. H. L. et al. A systematic review of dental disease management in cancer patients. Support. Care Cancer 26(1), 155–174. https://doi.org/10.1007/s00520-017-3829-y (2018).
Wu, L., Geng, K. & Gao, Q. Effects of different anti-caries agents on microhardness and superficial microstructure of irradiated permanent dentin: an in vitro study. BMC Oral Health 19(1), 113. https://doi.org/10.1186/s12903-019-0815-4 (2019).
Comar, L. P. et al. In situ effect of sodium fluoride or titanium tetrafluoride varnish and solution on carious demineralization of enamel. Eur. J. Oral Sci. 120(4), 342–348. https://doi.org/10.1111/j.1600-0722.2012.00968.x (2012).
Comar, L. P. et al. Response of carious enamel to TiF4 varnish treatment under diverse cariogenic activities in situ. J. Dent. 63, 81–84. https://doi.org/10.1016/j.jdent.2017.05.023 (2017).
Comar, L. P. et al. Mechanism of action of TiF4 on dental enamel surface: SEM/EDX, KOH-soluble F, and X-ray diffraction analysis. Caries Res. 51(6), 554–567. https://doi.org/10.1159/000479038 (2018).
Dos Santos, D. M. S. et al. Protective effect of 4% titanium tetrafluoride varnish on dentin demineralization using a microcosm biofilm model. Caries Res. 53(5), 576–583. https://doi.org/10.1159/000499317 (2019).
Mosquim, V., Rodrigues Pereira Santi, L., Martines de Souza, B. & Magalhães, A. C. Can TiF4 varnish or TiF4/NaF solution stain eroded and sound enamel?. J. Dent. 85, 11–17. https://doi.org/10.1016/j.jdent.2019.04.006 (2019).
Dérand, T., Lodding, A. & Petersson, L. G. Effect of topical F-solutions on caries-like lesions in root surfaces. Caries. Res. 23(3), 135–140. https://doi.org/10.1159/000261166 (1989).
Tveit, A. B., Tötdal, B., Klinge, B., Nilvéus, R. & Selvig, K. A. Fluoride uptake by dentin surfaces following topical application of TiF4, NaF and fluoride varnishes in vivo. Caries Res. 19(3), 240–247. https://doi.org/10.1159/000260850 (1985).
Signori, C., van de Sande, F. H., Maske, T. T., de Oliveira, E. F. & Cenci, M. S. Influence of the inoculum source on the cariogenicity of in vitro microcosm biofilms. Caries Res. 50(2), 97–103. https://doi.org/10.1159/000443537 (2016).
Velo, M. M. A. C. et al. Radiotherapy alters the composition, structural and mechanical properties of root dentin in vitro. Clin. Oral Investig. 22(8), 2871–2878. https://doi.org/10.1007/s00784-018-2373-6 (2018).
Larsen, T. & Fiehn, N. E. Dental biofilm infections—an update. APMIS 125(4), 376–384. https://doi.org/10.1111/apm.12688 (2017).
Shah, S. et al. Efficacy of silver diamine fluoride as an antibacterial as well as antiplaque agent compared to fluoride varnish and acidulated phosphate fluoride gel: an in vivo study. Indian J. Dent. Res. 24(5), 575–581. https://doi.org/10.4103/0970-9290.123374 (2013).
Zhao, I. S. et al. Mechanisms of silver diamine fluoride on arresting caries: a literature review. Int. Dent. J. 68(2), 67–76. https://doi.org/10.1111/idj.12320 (2018).
Rosenblatt, A., Stamford, T. C. & Niederman, R. Silver diamine fluoride: a caries “silver-fluoride bullet”. J. Dent. Res. 88(2), 116–125. https://doi.org/10.1177/0022034508329406 (2009).
Chu, C. H., Lo, E. C. & Lin, H. C. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J. Dent. Res. 81(11), 767–770. https://doi.org/10.1177/0810767 (2002).
Ishiguro, T. et al. Sodium fluoride and silver diamine fluoride-coated tooth surfaces inhibit bacterial acid production at the bacteria/tooth interface. J. Dent. 84, 30–35. https://doi.org/10.1016/j.jdent.2018.12.017 (2019).
Fernandez, Y. et al. Effect of mouthwashes on the composition and metabolic activity of oral biofilms grown in vitro. Clin. Oral Investig. 21(4), 1221–1230. https://doi.org/10.1007/s00784-016-1876-2 (2017).
Deng, D. M., van Loveren, C. & ten Cate, J. M. Caries-preventive agents induce remineralization of dentin in a biofilm model. Caries Res. 39(3), 216–223. https://doi.org/10.1159/000084801 (2005).
Cheng, X. et al. Comparative effect of a stannous fluoride toothpaste and a sodium fluoride toothpaste on a multispecies biofilm. Arch. Oral Biol. 74, 5–11. https://doi.org/10.1016/j.archoralbio.2016.10.030 (2017).
Souza, B. M. et al. Analysis of the antimicrobial and anti-caries effects of TiF4 varnish under microcosm biofilm formed on enamel. J. Appl. Oral Sci. 26, e20170304. https://doi.org/10.1590/1678-7757-2017-0304 (2018).
McBain, A. J. Chapter 4: In vitro biofilm models: an overview. Adv. Appl. Microbiol. 69, 99–132. https://doi.org/10.1016/S0065-2164(09)69004-3 (2009).
Acknowledgments
We thank to The São Paulo Research Foundation (FAPESP) for a grant provided to the last author (FAPESP 2019/21797-0) and for the scholarships provided to the first author (FAPESP 2019/07241-0). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
B.M.S, M.S.S., A.S.B. and P.S.K.B. performed the experiments. A.C.M. supervised the experiments. A.C.M., M.A.R.B. and P.S.S.S. conceived the ideas. B.M.S. and A.C.M. analyzed the data and wrote the paper. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors B.M.S, M.S.S., A.S.B., P.S.K.B and P.S.S.S. declare no potential conflict of interests, while M.A.R.B. and A.C.M. have patent of TiF4 varnish.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Souza, B.M., Silva, M.S., Braga, A.S. et al. Protective effect of titanium tetrafluoride and silver diamine fluoride on radiation-induced dentin caries in vitro. Sci Rep 11, 6083 (2021). https://doi.org/10.1038/s41598-021-85748-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-85748-8
This article is cited by
-
The damage and remineralization strategies of dental hard tissues following radiotherapy
BMC Oral Health (2024)
-
SDF in radiation-induced caries
British Dental Journal (2023)