Abstract
The optimal treatment for resectable esophageal squamous cell carcinoma (ESCC) is still a debatable point; however, randomized trials for strategies including neoadjuvant or adjuvant chemotherapy (CT), radiotherapy, or chemoradiotherapy (CRT) are not always available. This network meta-analysis aimed to identify an effective approach through indirect comparisons. An extensive literature search comparing multimodality treatment and surgery was performed, and a network meta-analysis was conducted with the frequentist method. Twenty-three trials including a total of 3636 ESCC patients were included. Neoadjuvant CRT and neoadjuvant CT, which were recommended by most guidelines for esophageal cancer, were associated with an overall survival advantage compared with surgery alone (HR = 0.43, 95% CI 0.26–0.73; HR = 0.71, 95% CI 0.32–1.59). A statistically significant survival benefit from neoadjuvant CRT compared with neoadjuvant CT could not be demonstrated in our study (HR = 0.61, 95% CI 0.32–1.17, P = 0.08). Our network meta-analysis showed that both neoadjuvant CRT and neoadjuvant CT were effective in improving the survival of patients with ESCC. Individual clinical decisions need further study in the future.
Similar content being viewed by others
Introduction
Esophageal carcinoma has the eighth highest incidence and is the sixth leading cause of carcinoma-related deaths worldwide1,2. Esophageal squamous cell carcinoma (ESCC) is the most frequent histological subtype3. The 5-year survival rate of patients with ESCC is still low4.
Surgery is still a potentially curative treatment; however, neoadjuvant or adjuvant therapies have been proven to improve survival5, including chemotherapy (CT), radiotherapy (RT), or synchronous chemoradiotherapy (CRT) performed before or after surgery6,7. Multimodality treatments show overall survival (OS) differences in a large number of trials conducted in the past, even though some conclusions were controversial; for example, preoperative CT followed by surgery was demonstrated to improve survival in one trial8, while another trial had different results9. Presently, there is a paucity of evidence directly comparing neoadjuvant or adjuvant therapies, and the optimal choice among them remains unclear.
When there is a lack of randomized controlled trials (RCTs) directly comparing different treatments, network meta-analysis (NMA) can be used to combine the available evidence and compare the treatments indirectly, under the conditions that heterogeneity and inconsistency are fulfilled10. Although NMAs on multimodality treatments of ESCC have been conducted11,12, the evidence is still not enough, and new evidence has been presented in the last 2–3 years.
Our study aimed to perform a NMA comparing OS for neoadjuvant and adjuvant CT, CRT, and RT in patients with resectable ESCC.
Materials and methods
Literature search
The protocol of this NMA was registered in the PROSPERO database (identification code: 212733).
A systematic search was carried out for available literature published up to July 2018. The searches were limited to articles describing RCTs published in English. RCTs were searched in the EMBASE, MEDLINE, and Cochrane Library databases from 1990. A systematic review of RCTs was conducted following the PRISMA for Network Meta-Analyses (PRISMA-NMA)13 and Cochrane guidelines14. A combination of “[o]esophageal cancer (or neoplasms)” and the following different terms were used: “squamous cell”, “chemotherapy”, “chemoradiotherapy”, “radiotherapy”, “chemoradiation”, “neoadjuvant”, “adjuvant”, “preoperative”, “postoperative”, and “surgery (or esophagectomy)”. We also used the terms searching for systematic reviews and meta-analyses. References of the articles were also searched for potentially available studies. Abstracts or posters of international meetings such as American Society of Clinical Oncology (ASCO) meetings, European Society for Medical Oncology (ESMO) congresses or Chinese Society of Clinical Oncology (CSCO) meetings were also screened using the abovementioned keywords.
Study selection
Only full-text articles were included. We focused on treatments for ESCC, and RCTs published in English were included regardless of histology if the following criteria were met: sufficient data of the ESCC subgroup could be obtained or the majority of the patients had ESCC. Patients were confirmed to undergo radical/curative intent resection without distal metastasis. The latest published studies were chosen if there were data published repeatedly.
The studies were excluded in the following situations: non-RCT design, including patients never undergoing surgery, insufficient data for the ESCC subgroup or less than 80% of patients had ESCC.
Date extraction
Three authors independently reviewed the full text of the enrolled studies. The study endpoint was OS, and the outcome measure was the hazard ratio (HR) with 95% confidence interval (CI). However, HRs and CIs were not available at most times, and they could be estimated using a previously introduced method15,16,17. The HR, 95% CI and standard error of lnHR were carefully extracted from the survival curve.
Quality of evidence assessment
All enrolled articles underwent quality assessment, and the quality of evidence can be graded to 4 levels: high, moderate, low, and very low18. The quality can be downgraded due to publication bias, indirectness, inconsistency or imprecision.
Risk of bias assessment
All articles were assessed for risk of bias using the Cochrane risk of bias tool for RCTs19. Enrolled RCTs were classified into 3 categories: high risk, low risk, or unclear risk. Yongqiang Wang and Lei Shan extracted the data, and Wenhao Zhang verified the data independently.
Statistical analysis
OS was used as the study endpoint. Standard pairwise meta-analysis was performed for direct comparisons using the inverse variance DerSimonian-Laird random effects model20. If a direct comparison was based on 2 or more studies, the study heterogeneity was quantified using the I-squared statistic, and it was considered low, moderate, or high for I-squared values of < 25%, 25% to 50%, and > 50%, respectively21. A network of evidence can be constructed in the absence of direct comparisons between treatments22. The methods for indirect treatment comparisons are categorized as Bayesian or frequentist23. The frequentist method was used in our study, and it was also known as the adjusted indirect treatment comparison (AITC) method, which has been described previously22,24. A random effects NMA was carried out with a frequentist setting23,24,25. More information about the research synthesis methods could be obtained from the article26,27,28. The use of Stata in dealing with more complicated networks is introduced in the article29, including the evidence for publication bias (as assessed by means of a funnel plot dedicated to network meta-analysis). The adjusted indirect method we also used was introduced by Branko Miladinovic et al.30. A heterogeneity parameter (tausquared) was assumed across all comparisons. Each summary effect is presented along with 95% CI and predictive interval. The predictive interval was calculated using the between-study variance tau-squared, providing information on the magnitude of heterogeneity. Contribution matrix is generated during the direct and indirect analysis using Stata 12.029.
Transitivity is one of the key assumptions of NMA31; for example, if the information of surgery versus neoadjuvant chemoradiation and surgery versus neoadjuvant chemotherapy is available, then the information of the neoadjuvant chemoradiation versus neoadjuvant chemotherapy comparison can be obtained for the NMA. Usually, the treatment of surgery alone is assumed to be the common treatment as the transitivity assumption and is regarded reasonably consistent among all studies. Participants could be principlely randomized to any of the treatments in the network. Lack of transitivity can present as inconsistency between direct and indirect estimates (loop inconsistency) or between estimates deriving from different study designs (design inconsistency). The inconsistency between direct and indirect estimates or between estimates from different study designs can be studied using the design-by-treatment interaction model26,32.
Statistical tests were 2-sided. The statistical analysis and graph drawing were performed with Stata 12.0 (StataCorp LP, College Station, TX, USA).
Results
Search results
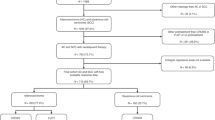
More than 5000 articles were identified from the literature search, and 42 potentially eligible articles were retrieved for detailed analysis. In the next step, 14 articles were excluded because of duplicates, the unavailability of the full text, the lack of the outcome of interest, or the lack of subgroup analysis. Twenty-four reports of RCTs8,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55 (published from 1990 to 2018) were intended to be included in the NMA, and 23 RCTs were enrolled after an article from the 2018 CSCO Meeting55 was excluded because of limited stage and therapy (only N1 patients were enrolled and there was only one article for adjuvant radiotherapy) (Fig. 1, Supplementary Table 1). Most trials were two-arm trials, and the rest were three-arm trials. The following treatments were compared in the trials: surgery with neoadjuvant CT, surgery with neoadjuvant CRT, surgery with neoadjuvant RT, surgery with adjuvant CT, surgery with adjuvant CRT, neoadjuvant CT with neoadjuvant RT, adjuvant CT with adjuvant CRT, and neoadjuvant CT with adjuvant CT. These are presented in the network plot (Fig. 2).
NMA results
The network of eligible comparisons for NMA is shown in Fig. 2, and the contribution matrix of the NMA is shown in Fig. 3. In Fig. 3, direct comparisons are represented in the columns of the matrix, and network estimates are represented in the rows of the matrix. Each direct comparison contributes differently to the network summary effects. The matrix is useful to identify the most influential comparisons of the entire network. The inconsistency test chart is shown in Fig. 4. The values of the ratio of two HRs were all close to 1, implying that the direct evidence and the indirect evidence were generally consistent. The network comparisons for OS are shown in Table 1 (Supplementary Table 2). In our model, neoadjuvant CRT, neoadjuvant CT, neoadjuvant RT, and adjuvant CRT offered an OS advantage over surgery alone with HR and CIs showed in Table 1 and Fig. 5. There was also a similar trend for adjuvant CT; however, distinct significance could not be demonstrated (HR 0.80; 0.47–1.36; P = 0.401), which indicated that adjuvant CT did not provide a further benefit compared with surgery. There were no significant differences between neoadjuvant CRT and neoadjuvant CT, neoadjuvant CRT and neoadjuvant RT, neoadjuvant CRT and adjuvant CRT, neoadjuvant CRT and adjuvant CT, neoadjuvant CT and neoadjuvant RT, neoadjuvant CT and adjuvant CRT, neoadjuvant CT and adjuvant CT, neoadjuvant RT and adjuvant CRT, neoadjuvant RT and adjuvant CT, and adjuvant CRT and adjuvant CRT.
The common treatment (surgery) was reasonably consistent across trials, and no evidence of violation of the transitivity assumption was observed. The participants could be randomized to any treatments being compared in the network due to the equal distribution of the modifiers across studies.
Quality assessment of trials, publication bias and evidence grading
There was no severe risk of bias in the eligible RCTs (Supplementary Table 3). Funnel plot analysis also did not indicate any prominent risk of publication bias (Fig. 6). These findings, coupled with the absence of inconsistency and the lack of violation of the transitivity assumption, allowed us to grade the RCTs as high or moderate.
Discussion
ESCC still has a poor 5-year survival, and individualized clinical decisions are difficult to make due to inadequate evidence. High-quality adequately sized trials are few because of the complexity of the surgery and the inconsistency of the clinical decisions made in different medical centers. The potential aggressiveness of this tumor also increases the risk of R1 resection. More reliable evidence is still needed.
Our study analyzed 23 RCTs, and neoadjuvant treatments were demonstrated to be more effective for ESCC. The efficacy in 3636 patients was assessed using OS. Neoadjuvant CRT, neoadjuvant RT, adjuvant CRT and neoadjuvant CT conferred better OS. Adjuvant CT failed to show an OS benefit for ESCC.
As poor outcomes are presented with surgery alone, combined therapies are required. Neoadjuvant therapy has been compared with postoperative therapy or surgery alone for resectable ESCC for curative intent. Stage I patients have not yet been evaluated. The expected advantages of neoadjuvant therapy are as follows. 1. The patients’ response to chemotherapy or radiotherapy can be evaluated through neoadjuvant therapy, and downstaging is expected to make the surgical procedure easier and improve the radical resection rate. 2. Neoadjuvant therapy is expected to kill the micrometastasis, which may be potentially useful to prolong the long-term survival of patients. 3. Patients more easily complete the treatment plan before surgery, and chemotherapeutic drugs can reach the target before the local blood supply is destroyed. Most studies have focused on neoadjuvant CRT and neoadjuvant CT. The recent National Comprehensive Cancer Network (NCCN) guidelines suggest preoperative chemoradiation for patients with stage cT1b-T4a and N0 or N + (RT, 41.4–50.4 Gy + concurrent chemotherapy), and its multidisciplinary recommendation is that combined modality therapy is effective for patients with localized esophageal cancer. Three articles are cited56,57,58; however, the majority of the patients enrolled in the research had adenocarcinoma. A few RCTs that verified the effectiveness of neoadjuvant CRT have been reported since the 1990s; though few studies contributed to OS, the pathologic complete response (pCR) rate was higher in these patients. A high-quality study by Bosset et al.40 reported that recurrence-free survival was significantly improved in patients with squamous cell carcinoma with no benefit of OS. The patient characteristics, including histological type and stage, and CRT protocols varied greatly in the meta-analysis performed in North America and Europe, and radical surgery was regarded as a vital factor for long-term survival. Guidelines for the diagnosis and treatment of carcinoma of the esophagus in Japan59 recommended neoadjuvant chemotherapy with cisplatin + 5-fluorouracil (5-FU) in patients with resectable stage II or III thoracic esophageal carcinoma according to JCOG920443 and JCOG99078. Postoperative irradiation was only suggested for use when there was local residual tumor or local recurrence. However, few cases presented pCR60, and few meta-analyses demonstrated its survival benefit over surgery alone61. The role of neoadjuvant RT remains unsatisfactory, and its impact can be affected by several elements, such as the volume of irradiation, daily fractions, total dose, and interaction with other treatments. It has been reported that daily fractionation > 40 Gy with radiation could increase the late toxicity risk62, and as a result, it may be harmful to long-term survival. The value of neoadjuvant RT remains controversial, and no recommendation has been made in the clinical guidelines until now.
The value of postoperative adjuvant therapy for stage I patients has not yet been studied. The expected advantages of postoperative adjuvant therapy are to address the residual tumor, lymph node metastasis, and possible micrometastasis. However, disadvantages also existed, as no visible target could be used to evaluate the effect of postoperative adjuvant therapy and lower compliance compared with that of neoadjuvant therapy. More evidence suggests better survival benefits for neoadjuvant therapy than for adjuvant therapy63,64.
Our analysis showed that neoadjuvant CRT may have the best OS benefit and seems to significantly improve OS compared with surgery alone (HR = 0.43,95% CrI 0.26–0.73, P = 0.008). This may be due to four mechanisms: 1. radiation damage can be enhanced by cell repair inhibition; 2. radioresistant-phase tumor cells can be cleared and radiosensitive-phase cells can be accumulated; 3. hypoxic cells can be cleared; and 4. the repopulation of tumor cells can be inhibited65. Theoretically, chemotherapy can control micrometastatic tumor cells, and radiotherapy can control regional tumors; therefore, the effect of spatial cooperation was presented. Sometimes it can lead to downstaging with a higher radical resection rate, which may improve OS66. Nonetheless, it could not be denied that neoadjuvant CRT was also associated with a higher risk of postoperative mortality than neoadjuvant CT or surgery alone67. As mentioned before, treatment outcomes differ according to the individual cases, the administration of the drug, the dosage of the radiation and so on. Additional high-quality randomized trials with larger sample sizes are needed.
In our study, we also suggest that neoadjuvant CT had a survival benefit over surgery alone (HR = 0.710, 95% CI 0.32–1.59, P = 0.009); however, a statistically significant survival benefit of neoadjuvant CRT compared with neoadjuvant CT could not be demonstrated in our study (HR = 0.61, 95% CI 0.32–1.17, P = 0.08). The result was similar to that of Sjoquist et al.’s study63. Neoadjuvant CT was reported to have a survival benefit over surgery, but they were not able to demonstrate a statistically significant survival benefit for neoadjuvant CRT against neoadjuvant CT after enrolling patients with both squamous cell carcinoma and adenocarcinoma (2049 patients and 1291 patients, respectively). It is always difficult to conduct a direct comparison of neoadjuvant CRT against neoadjuvant CT because of the limited sample size, and our NMA seemed to provide some of the latest evidence for the multiple-disciplinary treatment for patients with ESCC.
Several considerations should be kept in mind about our study. Single-patient data could not be obtained, and the survival data were extracted from the survival curve. Some errors may exist compared with the original data. Patients with adenocarcinoma were included in one of the studies enrolled (with 137 ESCC patients and 57 adenocarcinoma patients), and there was no subgroup analysis53. Some of the trials were found to have a high or unclear risk of bias, though the test of heterogeneity and inconsistency was negative. The studies included crossed nearly three decades, over which the CT drugs, implementation of radiotherapy, or even the surgery procedure changed considerably, and OS might be affected in different studies. The staging system and technology have advanced, and more sensitive disease staging is provided; inevitably, the distribution of stage varied greatly among the studies in different periods. This may affect the determination of the evaluation of the survival benefit. Although the AITC method we used has been found to be more favorable to the Bayesian method because of its simplicity considering that direct treatment comparisons are absent and networks are less complex68, the AITC method cannot handle the correlations well in multiarm trials. Therefore, our study cannot totally substitute high-quality multicenter, prospective, randomized controlled clinical trials directly comparing the different treatments, and readers should interpret the results with caution.
In summary, regional variability exists in the use of multimodality therapy in patients with ESCC, neoadjuvant CRT is preferred in Europe and the United States, neoadjuvant CT is recommended in Asian countries, and there is no clear recommendation in China due to the controversy. Our NMA suggests that neoadjuvant CRT or neoadjuvant CT may prolong survival in patients with ESCC. Individualized molecular markers may provide some information for clinical decisions in the future.
References
Ferlay, J. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127(12), 2893–2917 (2010).
Torre, L. A. et al. Global cancer statistics, 2012 CA Cancer. J. Clin. 65(2), 87–108 (2015).
Pennathur, A., Gibson, M. K., Jobe, B. A. & Luketich, J. D. Oesophageal carcinoma. Lancet 381(9864), 400–412 (2013).
Graham, A. J. et al. Defining the optimal treatment of locally advanced esophageal cancer: a systematic review and decision analysis. Ann. Thorac. Surg. 83(4), 1257–1264 (2007).
Mariette, C., Piessen, G. & Triboulet, J. P. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 8, 545–553 (2007).
Van, M. E. & Van, G. A. Systemic treatment for oesophageal cancer. Eur. J. Cancer. 41, 664–672 (2005).
Cohen, D. J. & Leichman, L. Controversies in the treatment of local and locally advanced gastric and esophageal cancers. J. Clin. Oncol. 33, 1754–1759 (2015).
Ando, N. et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann. Surg. Oncol. 19, 68–74 (2012).
Ajani, J. A. & Swisher, S. G. Preoperative chemotherapy for localized squamous cell carcinoma of the esophagus? We should go back to the drawing board!. Ann. Surg. Oncol. 19, 3–4 (2012).
Caldwell, D. M., Ades, A. E. & Higgins, J. P. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331(7521), 897–900 (2005).
Huang, Y. et al. A systematic review and network meta-analysis of neoadjuvant therapy combined with surgery for patients with resectable esophageal squamous cell carcinoma. Int. J. Surg. 38, 41–47 (2017).
Montagnani, F. et al. Multimodality treatment of locally advanced squamous cell carcinoma of the oesophagus: a comprehensive review and network meta-analysis. Crit. Rev. Oncol. Hematol. 114, 24–32 (2017).
Hutton, B. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162(11), 777–784 (2015).
Higgins, J. P. T. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions (Wiley, Hoboken, 2011).
Parmar, M. K., Torri, V. & Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 17(24), 2815–2834 (1998).
Williamson, P. R., Smith, C. T., Hutton, J. L. & Marson, A. G. Aggregate data meta-analysis with time-to-event outcomes. Stat. Med. 21(22), 3337–3351 (2002).
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S. & Sydes, M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 8, 16 (2007).
Salanti, G. et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE 9, e99682 (2014).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin. Trials. 7, 177–188 (1986).
Higgins, J. P. et al. Measuring inconsistency in meta- analyses. BMJ 327, 557–560 (2003).
Glenny, A. M. et al. Indirect comparisons of competing interventions. Health Technol. Assess. 9, 1–134 (2005).
Hong, H. et al. Comparing Bayesian and frequentist approaches for multiple outcome mixed treatment comparisons. Med. Decis. Making 33, 702–714 (2013).
Miladinovic, B. Indirect treatment comparison. The Stata 14(1), 76–86 (2014).
Lumley, T. Network meta-analysis for indirect treatment comparisons. Stat. Med. 21, 2313–2324 (2002).
White, I. R. et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res. Synth. Methods 3, 111–125 (2012).
White, I. R. Multivariate random-effects meta-regression: updates to mvmeta. Stata J. 11, 255–270 (2011).
Salanti, G. & Schmid, C. H. Research synthesis methods special issue on network meta-analysis: introduction from the editors. Res. Synth. Methods 3, 69–70 (2012).
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P. & Salanti, G. Graphical tools for network meta- analysis in STATA. PLoS ONE 8, e76654 (2013).
Branko, M., Anna, C., Iztok, H. & Benjamin, D. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J. Clin. Epidemiol. 64, 163–171 (2011).
Donegan, S. et al. Assessing key assumptions of network meta-analysis: a review of methods. Res. Synth. Methods 4, 291–323 (2013).
Higgins, J. P. et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res. Synth. Methods 3, 98–110 (2012).
Kelsen, D. P. et al. Preoperative therapy for esophageal cancer: a randomized comparison of chemotherapy versus radiation therapy. J. Clin. Oncol. 8(8), 1352–1361 (1990).
Schlag, P. M. Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The ChirurgischeArbeitsgemeinschaftFuerOnkologie der DeutschenGesellschaftFuerChirurgie Study Group. Arch. Surg. 127(12), 1446–1450 (1992).
Nygaard, K. et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy The second Scandinavian trial in esophageal cancer. World J. Surg. 16(6), 1104–1109 (1992).
Maipang, T. et al. Induction chemotherapy in the treatment of patients with carcinoma of the esophagus. J. Surg. Oncol. 56(3), 191–197 (1994).
Prise, L. E. et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 73(7), 1779–1784 (1994).
Pouliquen, X. et al. 5-Fluorouracil and cisplatin therapy after palliative surgical resection of squamous cell carcinoma of the esophagus. A multicenter randomized trial. French associations for surgical research. Ann. Surg. 223(2), 127–133 (1996).
Ando, N. et al. A randomized trial of surgery with and without chemotherapy for localized squamous carcinoma of the thoracic esophagus: the Japan Clinical Oncology Group Study. J. Thorac. Cardiovasc. Surg. 114(2), 205–209 (1997).
Bosset, J. F. et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N. Engl. J. Med. 337(3), 161–167 (1997).
Law, S., Fok, M., Chow, S., Chu, K. M. & Wong, J. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J. Thorac. Cardiovasc. Surg. 114(2), 210–217 (1997).
Ancona, E. et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 91(11), 2165–2174 (2001).
Ando, N. et al. Japan Clinical Oncology Group. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J ClinOncol. 21(24), 4592–4596 (2003).
Tachibana, M. et al. Postoperative chemotherapy vs chemoradiotherapy for thoracic esophageal cancer: a prospective randomized clinical trial. J. Eur. J. Surg. Oncol. 29(7), 580–587 (2003).
Lee, J. L. et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann. Oncol. 15(6), 947–954 (2004).
Burmeister, B. H. et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 6, 659–668 (2005).
Natsugoe, S. et al. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Dis. Esophagus. 19(6), 468–472 (2006).
Allum, W. H., Stenning, S. P., Bancewicz, J., Clark, P. I. & Langley, R. E. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J. Clin. Oncol. 27(30), 5062–5067 (2009).
Lv, J. et al. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World. J. Gastroenterol. 16(13), 1649–1654 (2010).
Cao, X. F., He, X. T., Ji, L., Xiao, J. & Lv, J. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. J. Dis. Esophagus. 22(6), 477–481 (2009).
Boonstra, J. J. et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long term results of a randomized controlled trial. BMC Cancer 11, 181–190 (2011).
Van, H. P. et al. Preoperative chemoradiotherapy from esophageal or junctional cancer. N. Engl. J. Med. 366(22), 2074–2084 (2012).
Mariette, C. et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J. Clin. Oncol. 32(23), 2416–2422 (2014).
Shapiro, J. et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 16(9), 1090–1098 (2015).
Wenjie, N., et al. Survival Benefit of Adjuvant Radiotherapy in pT3–4N1M0 Esophhageal Carcinoma: Results From a Retrospective Chinese two- centre Study Using Propensity Score—matched. C. Chinese Society of Clinical Oncology (CSCO) Poster (2018).
Cunningham, D. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355, 11–20 (2006).
Cooper, J. S. et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01) Radiation Therapy Oncology Group. JAMA 281, 1623–1627 (1999).
Macdonald, J. S. et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N. Engl. J. Med. 345, 725–730 (2001).
Hiroyuki, K. et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus 12, 1–30 (2015).
Dixit, S., Tilston, M. & Peter, W. M. Risk stratification for recurrence in patients with esophageal and junctional carcinoma treated with neoadjuvant chemotherapy and surgery. Med. Oncol. 27(2), 242–248 (2010).
Kidane, B., Coughlin, S., Vogt, K. & Malthaner, R. Preoperative chemotherapy for resectable thoracic esophageal cancer. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD001556.pub3 (2015).
Martel, M. K., Sahijdak, W. M., Haken, R. K., Kessler, M. L. & Turrisi, A. T. Fraction size and dose parameters related to the incidence of pericardial effusions. Int. J. Radiat. Oncol. Biol. Phys. 40(1), 155–161 (1998).
Sjoquist, K. M. et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 12, 681–692 (2011).
Sandro, P. et al. Survival after neoadjuvant and adjuvant treatments compared to surgery alone for resectableesophageal carcinoma: a network meta-analysis. Ann Surg. 265, 481–491 (2017).
Brunner, T. B. The rationale of combined radiotherapy and chemotherapy—Joint action of Castor and Pollux. Best Pract. Res. Clin. Gastroenterol. 30(4), 515–528 (2016).
Pultrum, B. B. et al. A critical appraisal of circumferential resection margins in esophageal carcinoma. Ann. Surg. Oncol. 17(3), 812–820 (2010).
Jin, H. L., Zhu, H., Ling, T. S., Zhang, H. J. & Shi, R. H. Neoadjuvant chemoradiotherapy for resectable esophageal carcinoma: a meta-analysis. World J. Gastroenterol. 15(47), 5983–5991 (2009).
O’Regan, C., Ghement, I., Eyawo, O., Guyatt, G. H. & Mills, E. J. Incorporating multiple interventions in meta-analysis: an evaluation of the mixed treatment comparison with the adjusted indirect comparison. Trials 10, 86 (2009).
Funding
The present study was funded by the National Key Research and Development Program of China (Grant No. 2016YFC0106005), Clinical Research Center of Shandong University (2020SDUCRCC002).
Author information
Authors and Affiliations
Contributions
Y.Z.: Conducting a research and investigation process, creation and presentation of the published work, specifically writing the initial draft (including substantive translation), preparation, creation and presentation of the published work by those from the original research group, specifically critical review, commentary or revision- including pre- or post-publication stages. Application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data. Yongqiang Wang: Management activities to annotate (produce metadata), scrub data and maintain research data. L.S.: Preparation, creation and presentation of the published work, specifically visualization/data presentation. C.P.: Provision of study materials, computing resources. W.Z.: Preparation, creation and presentation of the published work, specifically visualization/data presentation. X.Z.: Ideas; formulation of overarching research goals and aims. Acquisition of the financial support for the project leading to this publication. Oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Y., Wang, Y., Shan, L. et al. A network meta-analysis for neoadjuvant and adjuvant treatments for resectable squamous cell carcinoma of esophagus. Sci Rep 11, 6800 (2021). https://doi.org/10.1038/s41598-021-86102-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86102-8
This article is cited by
-
Effect of Time to Minimally Invasive Esophagectomy After Neoadjuvant Chemotherapy for Esophageal Squamous Cell Carcinoma
Journal of Gastrointestinal Cancer (2023)
-
Ginsenoside compound K inhibits the proliferation, migration and invasion of Eca109 cell via VEGF-A/Pi3k/Akt pathway
Journal of Cardiothoracic Surgery (2022)