Abstract
Insulin aspart (IAsp) is one of the main therapies used to control blood glucose after a meal. This study aimed to compare the pharmacokinetics (PK) and pharmacodynamics (PD) of 2 rapid-acting IAsp products: a new IAsp biosimilar (RD10046) and NovoRapid. In a single-center, randomized, single-dose, 2-period, crossover, euglycemic clamp study (registry number: CTR20180517, registration date: 2018-05-30), healthy Chinese males were randomized to receive 0.2 U/kg of the IAsp biosimilar RD10046 and NovoRapid under fasted conditions on two separate occasions. PK and PD were assessed for up to 10 h. Of the 30 randomized subjects, all 30 completed both treatment periods. The PK (area under the curve [AUC] of total IAsp; maximum observed IAsp concentration [Cmax]) and PD (maximum glucose infusion rate [GIRmax]; total glucose infusion during the clamp [AUCGIR,0–10h]) were similar between the new IAsp biosimilar RD10046 and NovoRapid. In all cases, the 90% CIs for the ratios of the geometric means were completely contained in the prespecified acceptance limits of 0.80–1.25. No hypoglycemic events, allergic reactions, or local injection adverse reactions occurred in this trial. We concluded that the studied IAsp biosimilar (RD10046) was bioequivalent to NovoRapid.
Similar content being viewed by others
Introduction
Insulin is one of the main therapies used to treat diabetes mellitus. Insulin aspart (IAsp), a kind of rapid-acting insulin in which the amino acid proline in the b-chain has been replaced by the amino acid aspartic acid, is manufactured using recombinant DNA technology1,2. The time of onset of action of IAsp is faster, and the duration of action is shorter than that of human insulin despite being equally potent in terms of glucose-lowering effects3,4,5, which leads to greater reductions in postprandial glucose excursions.
RD10046 is a non-innovator recombinant insulin aspart formulation developed by Yichang HEC Changjiang Pharmaceutical Co., Ltd. (HEC Pharm). It was developed in accordance with biosimilar guidelines established by the European Medicines Agency (EMA)6, the US Food and Drug Administration7,8, and the China Food and Drug Administration9. These guidelines recommend a pharmacokinetic (PK) and pharmacodynamic (PD) comparison of a new insulin analog with a reference insulin in glucose clamp studies. This article presents the results of a single-center, randomized, single-dose, 2-period, crossover, euglycemic clamp study in healthy males to evaluate similarities in the pharmacokinetic and pharmacodynamic properties of RD10046 and NovoRapid (Novo Nordisk, Denmark). The main objectives of the study were to (1) demonstrate average bioequivalence (BE) in the PK/PD endpoints between RD10046 and NovoRapid and (2) assess the safety of the two insulin preparations.
Results
Subjects disposition, demographics
In total, 30 healthy male subjects were enrolled after eligibility evaluation; of these, 30 subjects were randomized in a 1:1 ratio to the two treatment arms (sequences) (Fig. 1). The subjects were between 21 and 33 years old with a mean value of 24.7 years old. Subject height, weight, and BMI ranged from 161.5 to 181.0 cm, 52.7 to 77.0 kg, and 19.1 to 24.0 kg/m2, respectively, with mean values of 171.5 cm, 64.3 kg, and 21.8 kg/m2 at the screening visit, respectively. The weight and BMI on the day of dosing were shown in Table 1.
Euglycemic clamp statistics and C-peptide levels
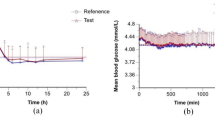
The fasting baseline blood glucose (BG) for each euglycemic clamp was defined as the mean value of several glucose measurements (− 60, − 30, − 20, − 10 min) before IAsp administration. Following drug administration, the onset of insulin action was defined as the time when BG dropped below the target level, which was defined as 5 mg/dL (0.28 mmol/L) less than the subject’s fasting baseline. The baseline BG levels were 4.61 ± 0.31 mmol/L in the RD10046 group and 4.63 ± 0.27 mmol/L in the NovoRapid group (P = 0.93). The target BG levels were 4.33 ± 0.31 and 4.35 ± 0.27 mmol/L in the two groups, respectively. As shown in Fig. 2A, the clamped BGs were comparable (4.36 ± 0.26 mmol/L in the RD10046 group and 4.34 ± 0.23 mmol/L in the NovoRapid group, P = 0.70). The ‘clamped’ BGs were also close to their own target levels [4.36 ± 0.26 vs. 4.33 ± 0.31 mmol/L in the RD10046 group (P = 0.69) and 4.34 ± 0.23 vs. 4.35 ± 0.27 mmol/L in the NovoRapid group (P = 0.88)]. The coefficient of variation in BG (CVBG) was relatively low and comparable in both groups (4.13% ± 0.99% and 4.03% ± 0.82% in the RD10046 group and NovoRapid group, respectively, P = 0.68). The mean absolute relative difference (MARD) at the target BG level was 3.56% ± 1.00% and 3.38% ± 0.75% for the two groups, respectively (P = 0.43).
As shown in Fig. 2B and Table 1, the mean values of basal C-peptide were 300 and 288 pmol/L for the RD10046 and NovoRapid groups, respectively. The overall C-peptide levels after dosing were 206 ± 63.3 and 200 ± 63.0 pmol/L, respectively. A mean reduction of 30.2% and 30.7% from baseline was observed, respectively. The AUC0–10h of C-peptide were 139 ± 11.8 and 135 ± 9.77 nmol/L × min in groups RD10046 and NovoRapid respectively (P = 0.16). The mean C-peptide concentration profiles after RD10046 and NovoRapid subcutaneous injection were similar, indicating comparable (Fig. 2B) endogenous insulin production between the two treatments. For this reason, the interference of endogenous insulin to the observed GIR was speculated to be equal between the two treatments. Only one experiment in the RD10046 group showed significant increases in postdosing C-peptide from baseline (e.g., > 200 pmol/L)10, and as a result, this experiment was excluded from PD analysis.
Pharmacokinetics
The subjects received a subcutaneous injection of RD10046 or NovoRapid (0.2 U/kg) on two separate occasions with an interval of 7–14 days. Subjects were weighed on the morning of the experiment day under fasting conditions. The doses of IAsp were 12.7 ± 1.1 U for the RD10046 group and 12.7 ± 1.2 U for the NovoRapid group (P = 0.91).
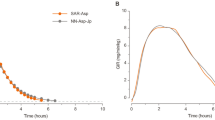
The mean insulin concentration-versus-time profiles were similar between formulations (Table 2, Fig. 2C). The similarity in PK profiles was confirmed by the ratio of least squares (LS) means for AUCIAsp,0–10h, and Cmax values. Moreover, the equivalent PK of RD10046 relative to NovoRapid was demonstrated by the 90% CIs for the exposure ratios within 0.80–1.25. The time to the observed maximum drug concentration (Tmax) was almost the same between formulations, with a median difference of 0 min and a 95% CI of the difference from − 10 to 10 min.
Pharmacodynamics
The glucose infusion rate (GIR) was adjusted in accordance with the glucose measurement (every 5 min from 0 to 4 h and every 10 min from 4 to 10 h) to maintain the BG at the target level after dosing. The GIR reflected the glucose-lowering effect of IAsp. The GIR profiles (mean and 95% CIs) after 0.2 U/kg IAsp injection were comparable between RD10046 and NovoRapid (Fig. 2D). No difference was detected in onset time, maximum glucose infusion rate (GIRmax), time to GIRmax (tGIRmax), the area under the curve (AUC) of GIR from 0 to 10 h (AUCGIR,0–10h), the time for GIR to rise from 0 to half of GIRmax (Tearly50%), and the time for GIR to decline from GIRmax to half of GIRmax (Tlate50%) (Table 2). Median differences for PD time parameters were small (less than 15 min), and all 95% CIs encompassed zero. The 90% CI of the geometric mean ratios for AUCGIR,0–10h and GIRmax were also entirely contained within the bioequivalent interval of 80–125%.
Safety evaluation
Both IAsp formulations were well tolerated, and no injection-site reactions were identified. No clinically significant alterations in laboratory values, urinalysis values, or vital signs were identified. No subjects discontinued the study because of an adverse event (AE). A total of 7 mild AEs, all unlikely to be related to the study drug, were reported in 7 subjects and were unevenly distributed between the two treatments (6 in subjects treated with RD10046 vs. 1 in those treated with NovoRapid) (Table 3).
Discussion
The hyperinsulin euglycemic clamp is regarded as the gold standard for insulin sensitivity evaluation11,12. Further, it is widely accepted and used to investigate the PK/PD of insulin preparations13,14,15. The quality of the euglycemic clamp is of great importance because it is related to the accuracy of the assessment of PK/PD16. In this trial, the overall CVBG was less than 5%, and the MARD was relatively low. The clamped BG levels were also close to their target glucose (4.36 ± 0.26 vs. 4.33 ± 0.31 mmol/L for RD10046 and 4.34 ± 0.23 vs. 4.35 ± 0.27 mmol/L for NovoRapid). These findings are indicative of the successful performance of the euglycemic clamp technique with blood glucose control close to the clamp target throughout the study. Furthermore, comparable baselines of blood glucose and C-peptide levels and a crossover study allowed a valid foundation for comparison of RD10046 and NovoRapid.
According to the EMA guideline6, either healthy subjects or type 1 diabetes patients could be used to evaluate the PK/PD bioequivalence of the two formulations. Healthy volunteers usually exhibit lower intraindividual variability than patients with type 1 diabetes mellitus, while the presence of type 1 diabetes mellitus could ensure the absence of interference of endogenous insulin. Studies in patients with type 1 diabetes would require standardized and well-controlled conditions (e.g., a long fasting period, washout of current insulin, absence of basal insulin, administration of a fixed-dose, and a run-in period before the euglycemic clamp)17. A study conducted in healthy subjects could reduce the pharmacokinetic variability not related to the differences between pharmaceutical products by avoiding underlying disease or concomitant medication. Thus, the present study was conducted in healthy participants. The frequently used insulin doses in clamp studies are 0.2–0.3 U/kg bodyweight for rapid-/short-acting insulins6. A recent study reported the PK/PD of a rapid-acting analog18, and the experimental procedure was similar to that in our study. The results showed that the degree of C-peptide suppression had little relationship with the injection dose of exogenous insulin (7 U, 15 U, 30 U). In the present study, the average IAsp injection dose was approximately 12 U, and the suppression of endogenous insulin secretion was approximately 30%. Although a C-peptide suppression of over 50% would have been more powerful for indicating freedom from the interference of endogenous insulin, the bioequivalence of PK/PD could still be thoroughly evaluated as long as the C-peptide concentrations were comparable between the two formulations in all periods without a C-peptide suppression of more than 50%19.
An overall maximum observed drug concentration appearing at approximately 50 min accompanied by a maximum GIR of 7.64 mg/kg/min and an overall glucose-lowering effect of 1542 mg/kg after 0.2 U/kg NovoRapid administration during a 10-h euglycemic clamp were observed in the present study, which was consistent with previous reports with a Tmax of 48–70 min3,20,21, a GIRmax of 6–9 mg/kg/min22,23,24,25, and a total glucose infusion of approximately 1300–1500 mg/kg23,24. The maximum observed drug concentration and total drug exposure were higher than those in the previous report, which might be due mainly to different analysis methods. All the PK/PD parameters were similar between the new IAsp biosimilar RD10046 and NovoRapid, and the 90% CIs for the ratios of the geometric means were completely contained in the prespecified acceptance limits of 0.80–1.25. These results indicated that RD10046 was bioequivalent to NovoRapid.
The evaluation of the safety of the two formulations in this study indicated no safety concerns. A different safety profile for biosimilar insulin may arise because of a different molecular entity, a change in the structure, or different impurity profiles in the manufacturing process. Although the frequency of AEs was not equal between the two treatments, no clinically significant alterations in vital signs or laboratory results were reported, and none of the AEs were likely to be related to the two formulations.
Conclusion
In summary, the study demonstrated that the PK (AUCIAsp,0–10h and Cmax) and PD (GIRmax and AUCGIR,0–10h) properties of the biosimilar IAsp and NovoRapid were similar after single 0.2 units/kg s.c. doses in healthy Chinese subjects.
Subject and method
Subject
This research started on 27 Jun 2018 and ended on 25 Oct 2018. A total of 36 Chinese males were recruited and finally according to the inclusion criteria, 30 subjects were included in this clinical trial. Subjects were required to be healthy Chinese men, aged 18–45 years, with a BMI between 19.0 and 24.0 kg/m2, a fasting glucose level less than 6.1 mmol/L, and normal glucose tolerance. They were also required to be nonsmokers and without a family history of diabetes mellitus or hypertension and no abnormalities were found in ECG, routine blood and urine, or liver and renal function.
Trial procedures were carried out in accordance with the Declaration of Helsinki and the principles of Good Clinical Practice. All participants gave informed consent, and the study was approved by the ethics committee of West China Hospital of Sichuan University. The trial’s information was available on the website http://www.chinadrugtrials.org.cn and the registration number of this trial was CTR20180517.
Study design
There was a phase I, single-site, randomized, single-dose, two-period, crossover, euglycemic clamp study, evaluating the similarity in PK and PD of two IAsp products in healthy subjects (a new IAsp biosimilar RD10046 by HEC Pharm vs. NovoRapid by Novo Nordisk). In this study, subjects were randomly allocated to one of two treatment sequences (Fig. 1), in which they received 0.2 units/kg s.c. doses of two different IAsp products (as listed above) on two occasions.
Subjects were admitted to the phrase I unit on day-1 of each treatment period. On day 1, subjects received a single dose of the IAsp biosimilar RD10046 or NovoRapid after an overnight fast of at least 10 h, and a euglycemic clamp procedure that lasted up to 10 h postdose was performed. Subjects were discharged when they had had carbohydrates at the end of the euglycemic clamp. An interval of 7–14 days existed between the two doses.
Clamp procedure
The euglycemic clamp study was performed after inserting two catheters (an antecubital venous catheter for infusing 20% dextrose and a retrograde venous catheter in the hand heated in a box at 55–65℃ for measuring blood glucose). After collecting the basal blood glucose level [defined as the mean of the glucose measurement of several times (− 60, − 30, − 20, − 10 min) before dosing], the subjects received a 0.2 U/kg dose of NovoRapid or RD10046 by s.c. injection into a lifted abdominal skin-fold. Blood samples were obtained at the bedside for immediate determination of whole blood glucose concentrations and then monitored every 5 min from 0 to 240 min and every 10 min from 240 to 600 min after dosing. During the euglycemic clamp, the GIR was varied as necessary to maintain the blood glucose concentration within approximately ± 10% of the target level (defined as 0.28 mmol/L below the subject’s basal glucose level). The investigator made the GIR adjustment based on glucose measurements and experience. If the GIR fell to zero for at least 30 min, the clamp was discontinued. A 4-mL blood sample was collected at the following points for analysis of C-peptide and IAsp concentration: − 30, 0 (before dosing), 10, 20, 30, 40, 50, 60, 90, 120, 150, 180, 210, 240, 270, 300, 360, 420, 480 and 600 min. The baseline of C-peptide was defined as the mean value of two measurements at − 30 and 0 min.
Analytical techniques
Whole blood glucose concentrations were tested with a glucose analyzer (Biosen C_line GP+, Neckar Healthcare. Co. Ltd., Magdeburg, Germany) using an automated glucose oxidase technique. Serum C-peptide levels were analyzed using an ELISA (Cat. No. 80‐CPTHU‐E01.1; ALPCO, Salem, NH), and plasma IAsp concentrations were assessed by means of a validated, ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method at Covance Laboratories in Shanghai. The ranges of quantification were 20–3000 pmol/L for C-peptide and 0.2–10 ng/mL for IAsp.
Statistical methods
The primary endpoint parameters of PK were shown as follows derived from time-profiles of IAsp: (1) maximal concentration of IAsp (Cmax), (2) the AUC of IAsp from 0 to 10 h (AUCIAsp, 0-10h). The time of Cmax (Tmax) and terminal half-life (t1/2) were also recorded. Those parameters mentioned above were calculated by PKsolver (version 2.0)26. Log-transformed AUCIAsp,0–10h and Cmax were evaluated with a linear mixed-effects model including subject as a random effect with the period, sequence, and treatment as fixed effects. For each parameter, the difference in the LS means along with the 90% CIs was back-transformed to produce the ratio of geometric means and the CI comparing treatments. The pharmacokinetic similarity was to be concluded if the 90% CIs for both AUCIAsp,0–10h and Cmax were completely contained within the interval of 0.80–1.25.
GIR profiles were smoothed by a locally weighted regression technique (LOESS, factor 0.10) using SAS software (version 9.4). The primary glucose-lowing effectiveness endpoints were the following parameters derived from the smoothed GIR profiles during the time interval from 0 to 10 h: (1) maximal GIR (GIRmax), (2) AUC of GIR from 0 to 10 h (AUCGIR, 0–10h). The time-related parameters including the time of GIRmax (tGIRmax), the onset of time (Tonset) (defined as the first time when BG declining at least 0.28 mmol/L from baseline), the time for GIR to rise from 0 to half of GIRmax (Tearly50%), and the time for GIR to decline from GIRmax to half of GIRmax (Tlate50%) were also recorded. A similar analysis was performed for GIRmax and AUCGIR, 0–10h.
Time-related PK/PD parameters were evaluated by a nonparametric approach based on the Hodges-Lehmann method.
Considering a 20% within-subject variability in primary endpoint parameters of PK/PD following IAsp administration, and assuming a difference of 5% between test and reference, and a type-I error rate of 2.5%, a sample size of 30 subjects was planned (after considering 20% dropout rate) for the replicate crossover study to have 90% power to reject the null hypothesis that PK/PD of RD10046 was not bioequivalent to NovoRapid.
The safety assessments included the vital signs, physical examination, laboratory tests (complete blood cell count, liver and renal function, urinalysis, etc.), 12-lead ECG, injection site reaction, hypoglycemia, and other AEs.
Ethics approval
Written informed consent will be obtained from each participant at the time of enrolling in the study. The trial has been approved by the Ethics Committee of West China Hospital of Sichuan University.
Data availability
The reasonable requests for data and materials should be addressed to Prof Yu.
Change history
05 August 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-95661-9
References
Listed, N. A. Insulin aspart. Asp-B28 NovoRapid. Drugs R D 2, 103–106 (1999).
Whittingham, J. L., Edwards, D. J., Antson, A. A., Clarkson, J. M. & Dodson, G. G. Interactions of phenol and m-cresol in the insulin hexamer, and their effect on theassociation properties of B28 pro → asp insulin analogues. Biochemistry 37, 11516–11523 (1998).
Mudaliar, S. R. et al. Insulin aspart (B28 asp-insulin): A fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care 22, 1501–1506. https://doi.org/10.2337/diacare.22.9.1501 (1999).
Nosek, L. et al. Insulin aspart has a shorter duration of action than human insulin over a wide dose-range. Diabetes Obes. Metab. 15, 77–83. https://doi.org/10.1111/j.1463-1326.2012.01677.x (2013).
Ma, Z. et al. A comparison of pharmacokinetics and pharmacodynamics of insulin aspart, biphasic insulin aspart 70, biphasic insulin aspart 50, and human insulin: a randomized, quadruple crossover study. Diabetes Technol. Ther. 14, 589–595. https://doi.org/10.1089/dia.2011.0299 (2012).
Agency, E. M. Guideline on non‐clinical and clinical development of similar biological medicinal products containing recombinant human insulin and insulin analogues. (2015).
Food and Drug Administration. Guidance for industry: statistical approaches to establishing bioequivalence. (2001).
Food and Drug Administration. Guidance for industry: bioavailability and bioequivalence studies submitted in NDAs or INDs-general considerations-draft guidance. (2014).
China Food and Drug Administration. Draft Guideline on Development and Evaluation of Biosimilars (Chinese Version). (2014).
Heise, T. et al. Euglycaemic glucose clamp: What it can and cannot do, and how to do it. Diabetes Obes. Metab. 18, 962–972 (2016).
Andres, R., Swerdloff, R., Pozefsky, T., Coleman, D. & Coleman, D. Manual feedback technique for the control of blood glucose concentration. Automation in Analytical Chemistry (1966).
DeFronzo, R. A., Tobin, J. D. & Andres, R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am. J. Physiol. 237, E214-223. https://doi.org/10.1152/ajpendo.1979.237.3.E214 (1979).
Becker, R. H. A. Pharmacodynamic Evaluation: Diabetes Methodologies (Springer, 2011).
Bequette, B. W. Glucose clamp algorithms and insulin time-action profiles. J. Diabetes Sci. Technol. 3, 1005–1013. https://doi.org/10.1177/193229680900300503 (2009).
Linnebjerg, H. et al. Comparison of the pharmacokinetics and pharmacodynamics of LY2963016 insulin glargine and EU- and US-approved versions of lantus insulin glargine in healthy subjects: Three randomized euglycemic clamp studies. Diabetes Care 38, 2226–2233. https://doi.org/10.2337/dc14-2623 (2015).
Benesch, C., Heise, T., Klein, O., Heinemann, L. & Arnolds, S. How to assess the quality of glucose clamps? Evaluation of clamps performed with ClampArt, a novel automated clamp device. J. Diabetes Sci. Technol. 9, 792–800. https://doi.org/10.1177/1932296815576957 (2015).
Kapitza, C., Nosek, L., Schmider, W., Teichert, L. & Nowotny, I. Single-dose euglycemic clamp study demonstrating pharmacokinetic and pharmacodynamic similarity between SAR341402 insulin aspart and US- and EU-approved versions of insulin aspart in subjects with type 1 diabetes. Diabetes Technol. Ther. 22, 278–284. https://doi.org/10.1089/dia.2019.0351 (2020).
Leohr, J. et al. Pharmacokinetic and glucodynamic responses of ultra rapid lispro vs lispro across a clinically relevant range of subcutaneous doses in healthy subjects. Clin. Ther. 42, 1762–1777. https://doi.org/10.1016/j.clinthera.2020.07.005 (2020).
Bhatia, A. et al. Comparative evaluation of pharmacokinetics and pharmacodynamics of insulin glargine (Glaritus((R))) and Lantus((R)) in healthy subjects: a double-blind, randomized clamp study. Acta Diabetol. 55, 461–468. https://doi.org/10.1007/s00592-018-1113-3 (2018).
Heinemann, L., Weyer, C., Rauhaus, M., Heinrichs, S. & Heise, T. Variability of the metabolic effect of soluble insulin and the rapid-acting insulin analog insulin aspart. Diabetes Care 21, 1910–1914. https://doi.org/10.2337/diacare.21.11.1910 (1998).
Heinemann, L., Kapitza, C., Starke, A. A. & Heise, T. Time-action profile of the insulin analogue B28Asp. Diabet Med. 13, 683–684. https://doi.org/10.1002/(SICI)1096-9136(199607)13:7%3c683::AID-DIA144%3e3.0.CO;2-1 (1996).
Engwerda, E. E. C., Tack, C. J. & de Galan, B. E. A comparison of the pharmacodynamic profiles of jet-injected regular human insulin versus conventionally administered insulin aspart in healthy volunteers. Diabetes Res. Clin. Pract. 121, 86–90. https://doi.org/10.1016/j.diabres.2016.09.001 (2016).
Heise, T. et al. Faster-acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes. Metab. 17, 682–688. https://doi.org/10.1111/dom.12468 (2015).
Heise, T. et al. Pharmacokinetic and pharmacodynamic properties of faster-acting insulin aspart versus insulin aspart across a clinically relevant dose range in subjects with type 1 diabetes mellitus. Clin. Pharmacokinet. 56, 649–660. https://doi.org/10.1007/s40262-016-0473-5 (2017).
Heise, T., Eckers, U., Kanc, K., Nielsen, J. N. & Nosek, L. The pharmacokinetic and pharmacodynamic properties of different formulations of biphasic insulin aspart: A randomized, glucose clamp, crossover study. Diabetes Technol. Ther. 10, 479–485. https://doi.org/10.1089/dia.2008.0019 (2008).
Zhang, Y., Huo, M., Zhou, J. & Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 99, 306–314. https://doi.org/10.1016/j.cmpb.2010.01.007 (2010).
Funding
This project was sponsored by Yichang HEC Changjiang Pharmaceutical Co., Ltd (HEC Pharm).
Author information
Authors and Affiliations
Contributions
S.W., S.L, and Y.Y. conceived and designed the experiments; H.L., H.Y., L.S., J.Q., J.L., and H.T. performed the experiments; H.L., S.W., S.L., and Y.Y. analyzed the data; H.L and Y.Y.wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
S.W. and S.L. were employees of Yichang HEC changjiang Pharmaceutical Co., Ltd (HEC Pharm).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the spelling of the author Sainan Wan which was incorrectly given as Sainan Wai.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, H., Yu, H., Sun, L. et al. Similar pharmacokinetics and pharmacodynamics of a new biosimilar and reference insulin aspart in healthy Chinese males. Sci Rep 11, 9495 (2021). https://doi.org/10.1038/s41598-021-88782-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88782-8