Abstract
A significant proportion of the land area of Heilongjiang Province, China, is composed of saline–alkaline soil, which severely inhibits maize growth. Although Trichoderma treatment is widely regarded as a promising strategy for improving the soil environment and promoting plant growth, the mechanism through which Trichoderma asperellum enhances maize resistance to saline–alkaline stress is not clear. In this study, we explored the effect of T. asperellum application at different concentrations to soil saline–alkaline environment on the seedlings of two maize cultivars, assessing the biochemical parameters related to oxidation resistance. Increasing spore densities of T. asperellum suspension effectively regulated the soil ion balance in the rhizosphere of maize seedlings, reduced the soil pH by 2.15–5.76% and sodium adsorption ratios by 22.70–54.13%, increased soil nutrient content and enzyme activity, and improved the soil environment for seedling growth. Additionally, T. asperellum treatment increased the maize seedling content of osmo-regulating substances and rate of glutathione:oxidised glutathione (43.86–88.25%) and ascorbate:oxidised ascorbate (25.26–222.32%) by affecting the antioxidant enzyme activity in the roots, increasing reactive oxygen species scavenging, and maintaining the osmotic balance and metabolic homeostasis under saline–alkaline stress. T. asperellum also improved the saline–alkaline tolerance of maize seedlings by improving the root growth characteristics. Moreover, results showed that Trichoderma applied at high concentration had the greatest effect. In conclusion, improvement in the saline–alkaline tolerance of maize seedlings by T. asperellum under saline–alkaline soil conditions may be achieved through diverse effects that vary among maize cultivars.
Similar content being viewed by others
Introduction
As global human population is expected to increase to nearly 10 billion over the next 50 years, meeting the worldwide food demand, i.e., a fundamental social need, will require at least a 50% increase in global grain production1. Meanwhile, saline–alkaline soil covers over 954 million ha worldwide2 and is rapidly increasing every year. Further, the increase in soil salinization is caused by the current heavy use of fertilizers and causes great economic losses for agricultural productivity3,4,5. This affects the sustainable development of agricultural ecosystems and, ultimately, threatens food security.
In China, Heilongjiang Province is one of the most important maize production areas, with predominantly saline–alkaline soils (2.882 × 106 ha) that seriously restrict the average grain yield6. Maize shows medium sensitivity to salinity and alkalinity, its tolerance to these conditions varies considerably among cultivars, and its yield can be reduced by 20–46% when grown on saline–alkaline soil7. Excess salt concentration degrades the soil, changes soil permeability and substrate potential, and decreases soil microbial activity8. Moreover, soil saline–alkaline stress can cause roots to suffer from ion poisoning and osmotic imbalance, destroy root cell structure, and significantly reduce root vigor, therefore inhibiting crop growth and resulting in a significant reduction in plant biomass, increased plant wilt, and death9.
Under saline–alkaline stress, substantial amounts of reactive oxygen species (ROS) are produced in plants, leading to gradual peroxidation of lipids and changes in antioxidant enzyme activities7. To remove and detoxify excess ROS, plants form both enzymatic and non-enzymatic antioxidant defenses. The ascorbate–glutathione (AsA–GSH) cycle has an important function in eliminating H2O2 and can be stimulated in plants by moderating stress conditions to scavenge ROS10. AsA and GSH are two low molecular weight antioxidants involved in reactions in the AsA–GSH cycle and important for preserving a wide range of metabolic processes11. Hence, maintaining high GSH/oxidized glutathione (GSSG) and AsA/oxidized ascorbic acid (DHA) ratios is crucial for enhancing the saline–alkaline stress tolerance of plants. Furthermore, harmful saline–alkaline soil ions cause a significant decrease in soil enzyme activity by directly inhibiting the number of microorganisms in the soil12. Crops growing in saline–alkaline soils suffer Na+ toxicity and high pH stress caused by excess Na2CO3 and NaHCO3, which are known to cause greater damage than NaCl6. To minimize these negative effects of saline–alkaline soils that have been contaminated owing to intensive farming practices, organic technology can be combined with microbes for synergistic plant-growth enhancement or soil bioremediation13.
Trichoderma is an important fungus that can spread rapidly in the soil, colonizing and surviving on the surface of plant root systems for prolonged periods, proliferating, and forming effective groups for biocontrol in crops and soils. Trichoderma effectively promotes root growth and the secretion of organic compounds that induce local or systemic resistance in plants14 and has a strong ability to mobilize and absorb soil nutrients. Thus, compared to other soil microbes, Trichoderma is more efficient and competitive in effectively improving soil structure, increasing the nutrient utilization efficiency by crops, and promoting crop growth15,16. For example, Trichoderma treatments improves the growth of Arabidopsis thaliana seedlings under salt stress by increasing root development and producing osmotic substances to eliminate Na+17. Similarly, Trichoderma harzianum significantly alleviates the salinity effects on tomato growth under saline irrigation and has a positive effect on the effective phosphorus concentration in the soil, which effectively reduces the need for phosphorus fertilizer18. Furthermore, Yasmeen and Siddiqui indicated the ameliorative effects of T. harzianum (Th-6) on maize and rice under a hydroponic saline environment, showing that Trichoderma promotes plant growth and increases environmental stress tolerance19. They also identified several interaction mechanisms that take place among the plants, soil, and Trichoderma under salt stress, such as Trichoderma increasing the activity of antioxidative defense systems in plants to resist salt stress and enhancing the relative antioxidant gene expression levels in stressed plants20,21.
Abiotic stress in Trichoderma-treated plants has been studied on vegetables, maize, wheat, and soybean, among others, with most studies artificially simulating the abiotic stress conditions of interest, such as drought or salinity. Owing to the complexity of natural saline–alkaline soil conditions, it is difficult to effectively simulate such conditions artificially. Thus, in this study, an experiment was performed in a natural saline–alkaline soil. To our knowledge, there are no reports on the effect of Trichoderma on soil characteristics and AsA–GSH cycle in maize under saline–alkaline soil stress conditions. This study evaluated the soil salt ion content, sodium adsorption ration (SAR), and pH value; soil enzyme and nutrients contents, antioxidant enzyme activities, non-enzymatic antioxidant (i.e., AsA and GSH) contents and redox ratios, and degree of lipid peroxidation to (1) assess the effects of Trichoderma asperellum on the physical and chemical characteristics of the maize seedling rhizosphere in a saline–alkaline soil and (2) evaluate the effects of T. asperellum on the AsA–GSH cycle antioxidant and growth characteristics of maize seedling roots under saline–alkaline soil stress and elucidate the mechanism underlying the improvement of the rhizosphere environment and promotion of plant AsA–GSH cycle antioxidant defense system by T. asperellum.
Materials and methods
Experimental materials and design
The experiment was conducted at the Key Laboratory of Modern Agricultural Cultivation and Crop Germplasm Improvement of Heilongjiang Province, Daqing (46° 58′ N; 125° 03′ E, 150 m a.s.l.), China. Two typical and widely-grown commercial maize hybrids with differing performance on saline–alkaline soils were screened in the laboratory: ‘Jiangyu 417’ (‘JY417’) is a highly salt-tolerant cultivar, and ‘Xianyu 335’ (‘XY335’) is a salt-sensitive cultivar. Unbroken maize seeds (germination rate > 90%) of uniform size were selected and surface-sterilized with 10% NaClO solution for 10 min. Then, the seeds were rinsed with sterile distilled water, air-dried, and placed in an incubator at 25 °C in the dark for two days to promote germination. Seeds with the same sprout length were selected and transplanted into plastic pots (12 × 11 cm, width × height)7,22. Soil (pH 9.30) obtained from a typical in situ saline–alkaline area of Daqing was used to fill the pots after air-drying. Each plastic pot was filled with 700 g of the experimental soil and each treatment included ten replicate pots with each pot containing five seedlings. T. asperellum was used to treat the seedlings. First, T. asperellum (Genbank accession: KJ541741) was activated in PDA media 28 ± 2 °C at 185 r min−1 and then prepared as a spore suspension (1 × 109 colony-forming units mL−1), which was inoculated onto a sterilized solid matrix (1:20, v/w) and incubated at 28 °C for 10 days. A concentrated T. asperellum spore suspension (1 × 109 spores L−1) was prepared by adding 200 mL of the suspension to each liter of soil to obtain the following treatments: 1 × 103 (T1), 1 × 106 (T2), and 1 × 109 (T3) spores L−1. A control was prepared, which consisted of 200 mL of the vehicle without spores. The strains used in this work were selected according to their biocontrol and/or plant-growth promotion activities as determined in previous experiments carried out in our laboratory7,22. The experiment was conducted in a semi-controlled growth chamber adjusted to 25/20 °C day/night temperature and a 16 h photoperiod under a photosynthetic photon flux density of 1000 µmol m−2 s−1. Tap water was supplied daily to maintain soil moisture. The basic physicochemical properties of the experimental soil are listed in Table S1.
Sample collection and pretreatment
Maize seedlings were taken when the three heart-shaped leaf stage had been reached and the fourth leaf was still developing to show the persistent effect of T. asperellum on growth behavior (the 27th day after T. asperellum application). At this point, plant height, leaf dry weight, and leaf relative water content were measured. The seedlings were five plants were randomly selected and removed from the soil. Rhizosphere soil was sampled from each treatment and transferred to the laboratory, sieved through a 2 mm mesh, and air-dried for analysis of rhizosphere soil physicochemical properties and enzyme activity. We defined rhizosphere soil as that soil 0.5 cm away from any root structure (i.e., fine or coarse) that remained attached to the root zone after the plant/soil complexes were excavated, laterally shaken, and moderate pressure was applied by hand to the soil aggregates. If the soil aggregates remained attached to the roots after this procedure, they were considered rhizosphere soil23.
Determination of soil characteristics
Data on soil characteristics were collected as previously described in Fu et al.24, by measuring pH, organic matter (OM), available nitrogen (AN), available phosphorous (AP), and available potassium (AK). Soil pH was determined using a glass combination electrode with a soil:water ratio of 1:125. Soil OM was determined using the K2Cr2O7-H2SO4 digestion method; AN was extracted with 1 M KCl and analyzed using the cadmium reduction method, while AP was extracted with a 0.5 M NaHCO3 solution with the pH adjusted to 8.5, and AK was extracted with neutral 1 N NH4OAc26.
Soil hydrogen peroxidase activity was determined using the KMnO4 titration method27. The activities of sucrase, urease, and alkaline phosphatase were assayed based on the release and quantitative determination of the product glucose, NH3-N, and P2O5, respectively. Briefly, approximately 5 g of air-dried soil samples were incubated in 15 mL of 8% (w/v) sucrose solution27, 15 mL of 10% (w/v) urea solution27, and 20 mL of 0.5% (w/v) disodium phenyl phosphate solution28, as required, in a suitable buffer for 24 h at 37 °C, and spectrophotometric measurements were performed at 508, 578, or 660 nm, respectively27.
The soil:water ratio was adjusted to 1:5 for all crushed soil samples in preparation for the leaching solution. Each glass bottle containing the soil–water mixture was placed on an oscillator for 30 min to thoroughly dissolve the soil salts. Then, the bottle contents were allowed to settle for 24 h to obtain a clarified solution. Soil HCO3− concentrations were determined by titrimetric hydrochloric acid methods, soil Cl− concentration titration was carried out using standard AgNO3, and soil SO42− concentrations were measured using an Hucoa-Elsoss dionex 2000I/SP ionic chromatographer29. Soil Mg2+ and Ca2+ concentrations were measured with disodium dihydrogen ethylenediamine tetraacetate (EDTA) using a murexide indicator for calcium and an eriochrome black indicator for calcium and magnesium together29. Soil Na+ and K+ concentrations were determined in the filtrate using flame photometry29. SAR was calculated using the following equation:
where SAR was the sodium adsorption ratio (cmol kg−1)0.5, and [Na+], [Ca2+], and [Mg2+] were the respective concentrations in solution18.
Analysis of maize roots
To separate the soil from the roots, the XY335 and JY417 maize seedling root samples described in the Sample collection and pretreatment section were soaked and rinsed with water over three layers of gauze cloth laid out at the bottom of a sink, and fine roots were then collected from the gauze cloth. A portion of each root sample was dried and frozen in liquid nitrogen. Roots were stored at − 80 °C until the antioxidant enzyme activity assays7. Frozen root tissue (1 g) was homogenized with 10 mL 0.1 M potassium phosphate buffer (pH 7.0), containing 0.1 mM EDTA-Na2, 0.5 mM ascorbate, and 1% polyvinyl polyvinylpyrrolidone in an ice bath7. The homogenate was filtered and centrifuged at 28,710 × g and 4 °C for 10 min, and the supernatant was used for the protein content and antioxidant enzyme activity determination7.
The activities of monodehydroascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR) were determined as described by Li et al.30. Meanwhile, on the 27th day after T. asperellum application the hydrogen peroxide (H2O2), malondialdehyde (MDA), superoxide anions (O2−), soluble sugars, soluble protein, proline, ascorbate peroxidase (APX), glutathione reductase (GR), AsA, and GSH contents in XY335 and JY417 roots were determined as described by Fu et al.7. Antioxidant enzyme activities were indicated as U mg−1 (enzyme activity unit number per mg protein).
The remaining root samples were used for the analysis of root activity, root volume, weight, and root superficial area as previously described24. Root activity was determined in a portion of the root sample using the 2,3,5-triphenyl tetrazolium chloride method31. The total volume of the root system was determined using the drainage method, and the superficial area of the root system was determined using the methylene blue adsorption test24. To determine the dry weight, root samples were weighed to determine the fresh weight, dried in a forced air oven at 105 °C for 30 min, and then dried to a constant weight at 75 °C. All of the above indicators were assessed five times.
Statistical analysis
In this study, data were presented as means (n = 5) ± standard deviation (SD). To detect differences among treatments, the least significant difference (LSD) test was used at a significance level of P = 0.05, with C and T indicating cultivars and treatments of the tables, respectively. LSD tests and Pearson's correlations were calculated in SPSS 21.0 software package (Chicago, IL).
Study statement
This study was conducted in compliance with relevant institutional, national, and international guidelines and legislation.
Results
Effects of T. asperellum on salt ion content, sodium adsorption ration, and pH of maize seedlings under saline–alkaline stress
After applying spore suspensions of T. asperellum at different concentrations, we observed significant increases in the soil contents of Ca2+, Mg2+, and K+ relative to those in the control, whereas, Na+, HCO3−, Cl−, and SO42− contents significantly decreased (Table 1). Thus, increasing T. asperellum spore densities in suspension effectively regulated the soil ion balance in the rhizosphere of maize seedlings, and all ions showed significant differences under treatment T3. Compared with those in the control, T3 significantly reduced the Na+ and HCO3− contents by 19.46% and 35.87% in XY335, and 20.02% and 36.29% in JY417, respectively, with an effect more pronounced than that with treatments T1 and T2. Although the Cl− and SO42− contents were low, their variation patterns were similar to that of HCO3− content. Overall, however, the composition of ions in the rhizosphere of maize seedlings was improved by the T. asperellum treatment.
As shown in Table 1, compared with those in the control, T. asperellum treatment significantly reduced the soil pH and SAR values, although with no significant cultivar × treatment interaction effects (P < 0.05). Under saline–alkaline stress, pH was 9.26 and 9.15 in soils planted with XY335 and JY417, respectively, and we found that for both maize cultivars, the pH and SAR values in the rhizosphere soil decreased with an increasing concentration of T. asperellum spores. Under treatment T3, pH was 8.73 and 8.66, while SAR was 3.45 and 3.15 for XY335 and JY417, respectively, which were all significantly different from those recorded under treatments T1 and T2. However, although soil pH and SAR for XY335 did not differ significantly between treatments T1 and T2, we detected significantly different responses between these two treatments in JY417. Further, pH and SAR values in JY417 were lower than those in XY335, indicating that JY417 showed a certain degree of tolerance to saline–alkaline stress.
Effects of T. asperellum on the nutrient contents of maize seedlings in rhizosphere soil
As shown in Table 2, compared with those in the control, T. asperellum treatment significantly increased soil nutrient parameters, although without any significant cultivar × treatment interaction effects (P < 0.05), and with the rhizosphere soil nutrient content for JY417 being higher than that for XY335. Similarly, soil chemical parameters in the rhizosphere soil were significantly improved, being higher after treatment with T. asperellum than those in the control. Furthermore, nutrient contents increased with increasing T. asperellum spore concentration, showing notable differences between treatments T1 and T2. Compared with those in the control, there were significant increases in the soil contents of OM, AN, AP, and AK: 65.32%, 23.80%, 123.60%, and 46.09% in XY335, and 67.42%, 21.14%, 109.94%, and 48.50% in JY417, respectively, in response to treatment T3.
Effects of T. asperellum on the enzyme activity of maize seedlings in rhizosphere soil
As shown in Table 3, there was a significant increase in soil enzyme activities in XY335 and JY417 rhizosphere soil after T. asperellum application compared to those in the control, although no significant cultivar × treatment interaction effects were detected, except for alkaline phosphatase activity (P < 0.05). Soil enzyme activities in JY417 rhizosphere soil tended to be generally higher than those in XY335 soil. Moreover, we noted that soil enzyme activities were differently enhanced by an increase in T. asperellum concentration from T1 and T2. However, the highest soil enzyme activities were recorded under treatment T3 in each case, which were significantly higher than those in the other treatments. Compared with those in the control, the T3 treatment significantly increased soil urease, phosphatase, sucrase, and hydrogen peroxidase activities by 38.24%, 43.48%, 38.13%, and 55.40% in XY335, and 37.83%, 51.89%, 29.43%, and 52.79% in JY417, respectively. Thus, T. asperellum treatment can significantly increase soil enzyme activities and improve the soil physical and chemical environment in the rhizosphere of maize seedlings.
Growth promotion in maize seedlings treated with T. asperellum under saline–alkaline soil stress
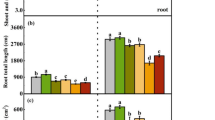
Compared to those in the control, T. asperellum treatment significantly increased the maize seedling growth under saline–alkaline stress by significantly enhancing all measured seedling variables, although we detected no significant cultivar × treatment interaction effects (Table 4, Table S2). Moreover, the dry weight of the root system and root relative water content, volume, superficial area, and activity in seedlings treated with T. asperellum were significantly higher than those in the control, and the effect was concentration dependent.
The recorded values for all variables increased with an increasing concentration of T. asperellum, with the growth-enhancing effects of the fungus on XY335 being more apparent than those on JY417 seedlings.
Effects of T. asperellum on non-enzymatic system contents in the roots of maize seedlings cultured in a saline–alkaline soil
Compared with those control, the soluble sugar and proline contents significantly increased in the roots of T. asperellum-treated maize seedlings (P < 0.05), particularly in those receiving the T3 treatment, which promoted the largest accumulation of these substances. These increases in osmoregulatory substances may explain how treatment with T. asperellum induces systemic resistance to saline–alkaline stress in maize seedlings (Table 5).
To elucidate the effect of the AsA–GSH cycle on alleviating oxidative stress in maize seedling roots under saline–alkaline stress, we examined the GSH/GSSG and AsA/DHA ratios (Table 5), showing that these two ratios were significantly reduced under saline–alkaline stress, but significantly increased in response to treatment with increasing concentrations of T. asperellum spores. We also detected significant cultivar × treatment interaction effects on AsA/DHA. Thus, the AsA–GSH cycle appeared to play an important role in controlling the saline–alkaline tolerance induced by T. asperellum, with XY335 performing better than JY417 in this regard.
Effects of T. asperellum on antioxidant enzyme activities in the roots of maize seedlings cultured in saline–alkaline soil
To determine whether the antioxidant enzyme system plays a role in the T. asperellum-induced saline–alkaline tolerance of maize seedlings, we determined the activities of enzymes involved in the AsA–GSH cycle (Table 6). APX, MDHAR, DHAR, and GR significantly increased in the XY335 and JY417 maize seedling roots with increasing concentration of T. asperellum under saline–alkaline stress, with a significant cultivar × treatment interaction effect on APX. Under treatment T3, APX, MDHAR, DHAR, and GR activities increased by 75.02%, 37.66%, 40.26%, and 76.09% in XY335, and 67.18%, 34.41%, 38.95%, and 58.38% (P < 0.05) in JY417, respectively, compared with those of the control. Thus, overall, T. asperellum can promote the seedlings of both cultivars by enhancing antioxidation enzyme activities, which is beneficial in terms of coping with the generation of excess ROS associated with saline–alkaline stress.
Effects of T. asperellum on the reactive oxygen species accumulation and oxidation parameters in the roots of maize seedlings grown in saline–alkaline soils
When analyzing the effect of T. asperellum on ROS clearance, we found that the contents of H2O2, O2−, and MDA in the roots of maize seedlings growing under control conditions were significantly higher than those in the other treatments, with significant cultivar × treatment interaction effects (P < 0.05; Table S3). After 27 days of treatment, H2O2, O2−, and MDA contents in the roots of maize seedlings had decreased significantly in response to treatment with increasing concentrations of T. asperellum, indicating that T. asperellum can enhance ROS clearance in the roots of maize seedlings by affecting both enzymatic and non-enzymatic systems (i.e., antioxidant enzyme activity and osmoregulation, respectively).
Relationships between the AsA–GSH cycle enzyme activity and soil characteristics
Pearson’s correlation analysis was used to evaluate the relationships between the AsA–GSH cycle enzyme activity and soil characteristics (Table 7). The AsA–GSH cycle enzyme activity was significantly correlated with soil characteristics, exhibiting a positive correlation with soil nutrient and a negative correlation with soil pH and SAR.
Discussion
In our study sites, we showed that soluble salt contents in the soil contributed to high soil pH and SAR values. However, compared to those of the control, Na+, HCO3−, Cl−, and SO42− contents in soil were significantly reduced through treatment with increasing concentrations of T. asperellum spore suspensions. Concomitantly, the contents of Ca2+, Mg2+, and K+ in the rhizosphere soil of treated seedlings were significantly higher than those in the control. Moreover, T. asperellum treatment promoted significant reductions in soil pH and SAR values and alleviated Na+ toxicity to maize seedlings, particularly at the higher spore concentrations examined. These results were in line with the reports by Vinale et al.32, who found that, as part of their normal metabolism, several Trichoderma strains produce organic acids with a certain buffering effect on soil pH. These effects could be attributed to a promotion of the soil microbiota metabolic processes by T. asperellum, whereby OM is converted to humus, which can adsorb excess salt ions in the soil to a certain extent33,34. Furthermore, T. asperellum improved the chemical structure of the saline–alkaline soil, thereby increasing its permeability and promoting water–salt balance in the saline–alkaline soil, subsequently reducing salt accumulation and lowering soil pH. Notably, soluble salt contents in the soil differed between the two examined maize cultivars, with XY335 salt contents performing better than those in JY417, indicating that different maize cultivars differed in their capacity to absorb salt during growth for use in biosynthesis.
Trichoderma can increase soil OM and improve plant absorption of soil available nutrients35, with soil enzymes playing an important role in the transformation and circulation of soil nutrients36. Our results revealed significant reductions in soil OM and available nutrient contents in the rhizosphere soil of the two maize cultivars under control condition, whereas the contents of these soil constituents increased upon treating seedlings with suspensions of T. asperellum spores. Moreover, we noted that the soil OM and available nutrient contents increased gradually with increasing concentrations of T. asperellum. In addition, the increase in AP was higher than that of alkali-hydrolyzed nitrogen and AK under T. asperellum treatment, these observations indicated that Trichoderma can convert unavailable soil inorganic phosphorus to AP, which was consistent with the findings of previous studies37. Furthermore, we observed an increase in soil nitrogen in response to Trichoderma treatment, which may be related to an increase in microbial nitrogen fixation promoted by Trichoderma38. Additionally, saline–alkaline stress significantly inhibited soil enzyme activity, and this effect was significantly alleviated in response to the application of increasing T. asperellum spore concentrations. Notably, the soil nutrient contents and enzyme activities in the rhizosphere of maize cultivar JY417 were higher than those in XY335, thereby highlighting the cultivar dependence of T. asperellum effects on the physicochemical properties of rhizosphere soils. Therefore, T. asperellum application effectively promoted soil enzyme activities in the rhizosphere, increased soil nutrient content, and improved the rhizosphere soil chemical properties, thereby alleviating saline–alkaline soil stress on maize seedlings.
Fungi in the genus Trichoderma have been reported to promote the growth of Triticum aestivum L.39, Brassica juncea L.40, Zea mays L., and Oryza sativa L.19. Moreover, the application of T. asperellum spore suspensions increased the dry weight of maize seedlings in this study (Table S2). In addition to improving plant growth (e.g., plant height), T. asperellum treatment can promote root development (Table 4). Given that the root tips and surface are the sites of nutrient uptake in maize, T. asperellum could be promoting maize seedling growth via stimulation of the root system growth, leading to an increase in the nutrient uptake capacity. Root growth and vigor directly affect plant growth, nutrition, and crop yield, and root activity serves as one of the main indicators of root function41. Therefore, we analyzed the root activity of maize seedlings treated with T. asperellum and found a significant rhizosphere interaction between T. asperellum and maize seedlings. Moreover, treatment with T. asperellum enhanced root features in both maize cultivars examined (Table 4).
We also demonstrated that T. asperellum application can enhance maize seedling resistance to saline–alkaline stress by inducing non-enzymatic changes in soluble sugars and proline in the roots (Table 5). Plants accumulate osmoregulatory substances and promote osmotic balance under saline–alkaline stress, which leads to enhanced tolerance to dehydration21,42. Thus, T. asperellum improves osmotic regulation by decreasing osmotic pressure and maintaining the water absorption capacity of the cells, thereby reducing the adverse effects of saline–alkaline stress by balancing osmotic potential, ultimately enhancing the saline–alkaline tolerance of maize seedlings.
Trichoderma treatment also induced antioxidant enzyme activities, and thereby minimized the oxidative damage caused by ROS under conditions of saline–alkaline stress. Enhanced antioxidation enzyme activity can protect plant tissues from oxidative damage to membranes under saline–alkaline stress, thereby reducing saline–alkaline toxicity and enhancing plant growth. In this study, different T. asperellum concentrations increased the activities of APX, MDHAR, DHAR, and GR in the roots of maize seedlings (Table 6). We suspect that enhanced MDHAR and DHAR activities may have contributed to an increase in AsA content and a decrease in DHA content under saline–alkaline stress. Under normal conditions, ROS, including H2O2, O2−, and MDA, act as signal molecules at low concentrations. However, excessive ROS accumulation under saline–alkaline stress is detrimental to plant tissues43. Consistent with the changes observed in enzyme activities, T. asperellum treatment promoted significant reductions in H2O2, O2−, and MDA contents under saline–alkaline stress conditions. Our data indicated that T. asperellum treatment enhanced the saline–alkaline tolerance of maize seedlings by inducing the root antioxidant enzyme system to remove excess ROS.
The AsA–GSH cycle is one of the major pathways through which excess H2O2 is removed from chloroplasts to neutralize the toxic effects of oxidative stress generated by this ROS44. AsA and GSH are two important antioxidants in plants subjected to environmental stress, whereas APX, MDHAR, DHAR, and GR are important ROS scavenging enzymes45. Collectively, AsA, GSH, APX, MDHAR, DHAR, and GR constitute the AsA–GSH cycle responsible for H2O2 detoxification, with stable GSH/GSSG and AsA/DHA ratios being crucial for maintaining cellular redox homeostasis under environmental stress46. In this study, T. asperellum treatment increased GSH/GSSG and AsA/DHA ratios (Table 5) in the root system of maize seedlings. Relative to those in the control, the observed ratio differences were significant at different T. asperellum concentrations in each of the maize cultivars examined, with XY335 performing better than JY417, which may explain Trichoderma promoting saline–alkaline tolerance in maize seedlings, although probably the underlying mechanisms differ for each cultivar. Nonetheless, T. asperellum treatment significantly alleviated all measured indices of oxidative damage caused by saline–alkaline stress in maize seedlings.
In this study, maize growth in Trichoderma-treated soils was better than that in the control soil and was significantly correlated with soil characteristics and AsA–GSH cycle, whose combined effect enhanced saline–alkaline stress tolerance in maize plants. These observations provide further evidence for associations of maize growth with soil characteristics and AsA–GSH cycle, which will be useful for improving crop yields in challenging saline–alkaline soils.
Conclusions
This study investigated the role of exogenous T. asperellum in improving the components of the antioxidant defence system and remediation the saline–alkaline soil to enhance saline–alkaline stress tolerance in maize plants. First, the application of T. asperellum to saline-alkali soil reduced soil pH and SAR values, improved the nutrient content and enzyme activity of soil, which could directly promote the growth of maize. Second, T. asperellum activated more efficient antioxidant systems with enhanced antioxidant enzymes and improved the cellular redox status, which may be a useful strategy for lessening the accumulation of H2O2 and O2−, ensuring the structural and functional integrity of the cell membrane, and alleviating the inhibition of plant growth. The associated changes in AsA–GSH cycle enzyme activity were attributed to the general improvement in the saline–alkaline soil environment, which suggested that the effect of different doses of Trichoderma on maize growth might be cooperatively driven by changes in the AsA–GSH cycle enzyme and soil characteristics. Hence, T. asperellum can efficiently act as plant grow promoting fungi (PGPF) are in maize for successful avoidance of saline–alkaline stress damage. Accordingly, we believe that this fungus can be developed as an effective bio-fertilizer and be used to promote sustainable agricultural practices. However, there are some issues that need to be addressed in future studies, such as the efficacy of the strain of plant-growth-promoting fungi T. asperellum interactions with other plant species and other abiotic stresses.
References
Godfray, H. C. J. et al. Food security: The challenge of feeding 9 billion people. Science 327, 812–818 (2010).
An, Y. M., Song, L. L., Liu, Y. R., Shu, Y. J. & Guo, C. H. D. novo transcriptional analysis of alfalfa in response to saline–alkaline stress. Front. Plant Sci. 7, 931 (2016).
Jamil, A., Riaz, S., Ashraf, M. & Foolad, M. R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 30, 435–458 (2011).
Zhu, Y. & Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 34, 455–472 (2013).
Zhang, W., Wang, C., Lu, T. & Zheng, Y. Cooperation between arbuscular mycorrhizal fungi and earthworms promotes the physiological adaptation of maize under a high salt stress. Plant Soil 423, 125–140 (2017).
Luo, S. S. et al. Aggregate-related changes in soil microbial communities under different ameliorant applications in saline-sodic soils. Geoderma 329, 108–117 (2018).
Fu, J., Liu, Z., Li, Z., Wang, Y. & Yang, K. Alleviation of the effects of saline–alkaline stress on maize seedlings by regulation of active oxygen metabolism by Trichoderma asperellum. PLoS ONE 12, e0179617 (2017).
Oo, A. N., Iwai, C. B. & Saenjan, P. Soil properties and maize growth in saline and nonsaline soils using cassava-industrial waste compost and vermicompost with or without earthworms. Land Degrad. Develop. 26, 300–310 (2015).
Guo, R. et al. Germination, growth, chlorophyll fluorescence and ionic balance in linseed seedlings subjected to saline and alkaline stresses. Plant Prod. Sci. 17, 20–31 (2014).
Wang, J., Zeng, Q., Zhu, J., Liu, G. & Tang, H. Dissimilarity of ascorbate–glutathione (ASA–GSH) cycle mechanism in two rice (Oryza sativa L.) cultivars under experimental free-air ozone exposure. Agric. Ecosyst. Environ. 165, 39–49 (2013).
Drazkiewicz, M., Polit, E. S. & Krupa, Z. Response of the ascorbate-glutathione cycle to excess copper in Arabidopsis thaliana (L.). Plant Sci. 164, 195–202 (2003).
Tripathi, S., Chakraborty, A., Chakrabarti, K. & Bandyopadhyay, B. K. Enzyme activities and microbial biomass in coastal soils of India. Soil Biol. Biochem. 39, 2840–2848 (2007).
Baez-Rogelio, A., Yolanda, E. M. G., Verónica, Q. H. & Jesús, M. R. Next generation of microbial inoculants for agriculture and bioremediation. Microb. Biotechnol. 10, 19–21 (2017).
Harman, G. E., Owell, C. R., Viterbo, A., Chel, I. & Lorito, M. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2, 43–56 (2004).
Saravanakumar, K., Shanmuga, A. V. & Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of avicennia marina. Aquat. Bot. 104, 101–105 (2013).
Tripathi, P. et al. Trichoderma: a potential bioremediator for environmental clean up. Clean Technol. Environ. Policy 15, 541–550 (2013).
Contreras-Cornejo, H., Macías-Rodríguez, L. I., Alfaro, C. R. & López-Bucio, J. Trichoderma spp. improve growth of arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant Microbe Interact. 27, 503–514 (2014).
Daliakopoulos, I. N. et al. Effectiveness of Trichoderma harzianum in soil and yield conservation of tomato crops under saline irrigation. CATENA 175, 144–153 (2019).
Yasmeen, R. & Siddiqui, Z. S. Ameliorative effects of Trichoderma harzianum on monocot crops under hydroponic saline environment. Acta Physiol. Plant. 40, 4 (2018).
Zhang, S. W., Gan, Y. T. & Xu, B. L. Application of plant-growth promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 7, 1405 (2016).
Zhang, F. et al. Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol. Environ. Saf. 170, 436–445 (2019).
Fu, J., Wang, Y. F., Liu, Z. H., Li, Z. T. & Yang, K. J. Trichoderma asperellum alleviates the effects of saline–alkaline stress on maize seedlings via the regulation of photosynthesis and nitrogen metabolism. Plant Growth Regul. 85, 1–12 (2018).
Bell, C. et al. Rhizosphere stoichiometry: are C:N:P ratios of plants, soils, and enzymes conserved at the plant species-level?. New Phytol. 201, 505–517 (2014).
Fu, J., Xiao, Y., Wang, Y., Li, Z. & Yang, K. Trichoderma affects the physiochemical characteristics and bacterial community composition of saline–alkaline maize rhizosphere soils in the cold-region of Heilongjiang Province. Plant Soil 436, 211–227 (2019).
Li, X. et al. Shifts of functional gene representation in wheat rhizosphere microbial communities under elevated ozone. ISME J. 7, 660–671 (2013).
Bao, S. D. Soil Agricultural Chemical Analysis (Chinese Agriculture Press, 2000).
Guan, S. Y. & Shen, G. Q. Enzyme activities in main soil in China. Tu Rang Xue Bao 21, 368–381 (1984).
Boldt-Burisch, K. & Naeth, M. A. Heterogeneous soil conditions influence fungal alkaline phosphatase activity in roots of lotus corniculatus. Appl. Soil Ecol 116, 55–63 (2017).
Richards, L. A. Diagnosis and Improvement of Saline and Alkali Soils. USDA Handbook Vol. 60 (USDA, 1954).
Li, L. et al. Exogenous spermidine improves drought tolerance in maize by enhancing the antioxidant defence system and regulating endogenous polyamine metabolism. Crop Pasture Sci. 69, 1076–1091 (2018).
Kamran, M. et al. Application of paclobutrazol affect maize grain yield by regulating root morphological and physiological characteristics under a semi-arid region. Sci. Rep. 8, 4818 (2018).
Vinale, F. et al. Trichoderma plant pathogen interactions. Soil Biol. Biochem. 40, 1–10 (2008).
Shaaban, M. & Abid, M. Amelioration of salt affected soils in rice paddy system by application of organic and inorganic amendments. Plant Soil Environ. 59, 227–233 (2013).
Wu, X.W. Improvement of Saline Land by Microorganism Fertilization in Weibei. Northwest University Master’s Dissertation (2015). (in Chinese)
Shim, D. J., Jithesh, B. E. A., Oh, C. T., Bang, I. S. & Shea, T. P. Trichoderma virens pdr-28: A heavy metal-tolerant and plant growth-promoting fungus for remediation and bioenergy crop production on mine tailing soil. J. Environ. Manage. 132, 129–134 (2014).
Hiradate, S., Morita, S., Furubayashi, A., Fujii, Y. & Harada, J. Plant growth inhibition bycis-cinnamoyl glucosides andcis-cinnamic acid. J. Chem. Ecol. 31, 591–601 (2005).
Altomare, C., Norvell, W. A., Björkman, T. & Harman, G. E. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum rifai 1295–22. Appl. Environ. Microbiol. 65, 2926–2933 (1999).
Nunzio, F. et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve n uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 9, 743 (2018).
Rawat, L., Singh, Y., Shukla, N. & Kumar, J. Alleviation of the adverse effects of salinity stress in wheat (Triticum aestivuml) by seed biopriming with salinity tolerant isolates of Trichoderma harzianum. Plant Soil 347, 387–400 (2011).
Parvaiz, A. et al. Role of Trichoderma harzianum in mitigating nacl stress in indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 6, 868 (2015).
Gessler, A. et al. Field and laboratory experiments on net uptake of nitrate and ammonium by the roots of spruce (Picea abies) and beech (Fagus sylvatica) trees. New Phytol. 138, 275–285 (1998).
Farhangiabriz, S. & Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 137, 64–70 (2017).
Mohan, M. M. Effect of Salt Stress on Growth Parameter, Lipid Peroxidation, Antioxidant Enzymes and Lignans of Sesame (Pondicherry University Department of Biotechnology School of Life Sciences, 2015).
Singh, S., Singh, A., Srivastava, P. K. & Prasad, S. M. Cadmium toxicity and its amelioration by kinetin in tomato seedlings vis-à-vis ascorbate-glutathione cycle. J. Photochem. Photobiol. B Biol. 178, 76–84 (2018).
Noctor, G., Gomez, L. A., Vanacker, H. & Foyer, C. H. Interactions between biosynthesis, comparmentation and transport in the control of glutathione homeostasis and signaling. J. Exp. Bot. 53, 1283–1304 (2002).
Rahman, A. et al. Manganese-induced salt stress tolerance in rice seedlings: Regulation of ion homeostasis, antioxidant defense and glyoxalase systems. Physiol. Mol. Biol. Plants 22, 291–306 (2016).
Acknowledgements
We thank the Trichoderma research team of the Forestry College of Shenyang Agricultural University.
Funding
This work was supported by Grants from the Key Laboratory of Saline-alkali Vegetation Ecology Restoration, Ministry of Education (Northeast Forestry University) (20200522-4); Initiation Foundation for Introduced Talent Person of Heilongjiang Bayi Agricultural University (XYB201901); Heilongjiang Bayi Agricultural University Support Program for San Heng San Zong (ZRCQC202002); Project funded by China Postdoctoral Science Foundation (2020M670930); Research and Development Program of Applied Technology in Heilongjiang Province (No. GA20B102), and Heilongjiang Bayi Agricultural University Postdoctoral Science Foundation.
Author information
Authors and Affiliations
Contributions
J.F.: conceptualization, methodology, formal analysis, writing-original draft, writing-review and editing. Y.X.: data curation, writing-review and editing. Z.-h.L., Y.-f.W.: formal analysis, writing-review and editing. K.Y.: conceptualization, methodology, formal analysis, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, J., Xiao, Y., Wang, Yf. et al. Saline–alkaline stress in growing maize seedlings is alleviated by Trichoderma asperellum through regulation of the soil environment. Sci Rep 11, 11152 (2021). https://doi.org/10.1038/s41598-021-90675-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90675-9
This article is cited by
-
Iron oxide nanoparticles enhance alkaline stress resilience in bell pepper by modulating photosynthetic capacity, membrane integrity, carbohydrate metabolism, and cellular antioxidant defense
BMC Plant Biology (2025)
-
Improving Alkaline Stress Tolerance in Maize through Seed Priming with Silicon Nanoparticles: A Comprehensive Investigation of Growth, Photosynthetic Pigments, Antioxidants, and Ion Balance
Silicon (2024)
-
The Combination Between Super Absorbent Polymers (SAPs) and Biofertilizers Could be an Ecofriendly Approach for Soil Chemical Properties Improving and Sustainable Wheat (Triticum Sativum) Production in Sandy Loam Soil
Journal of Soil Science and Plant Nutrition (2024)
-
Candidate genes controlling alkaline-saline tolerance in two different growing stages of wheat life cycle
Plant and Soil (2023)
-
With a little help from my friends: inoculation with Bacillus amyloliquefaciens and Trichoderma asperellum alleviates drought and salt stress in soybean
Theoretical and Experimental Plant Physiology (2023)