Abstract
To investigate the association of the myelomeningocele (MMC) volume with prenatal and postnatal motor function (MF) in cases who underwent a prenatal repair. Retrospective cohort study (11/2011 to 03/2019) of 63 patients who underwent a prenatal MMC repair (37 fetoscopic, 26 open-hysterotomy). At referral, measurements of the volume of MMC was performed based on ultrasound scans. A large MMC was defined as greater than the optimal volume threshold (ROC analysis) for the prediction of intact MF at referral (2.7 cc). Prenatal or postnatal intact motor function (S1) was defined as the observation of plantar flexion of the ankle based on ultrasound scan or postnatal examination. 23/63 participants presented a large MMC. Large MMC lesions was associated with an increased risk of having clubfeet by 9.5 times (CI%95[2.1–41.8], p < 0.01), and reduces the chances of having an intact MF at referral by 0.19 times (CI%95[0.1–0.6], p < 0.01). At birth, a large MMC reduces the chance of having an intact MF by 0.09 times (CI%95[0.01–0.49], p < 0.01), and increases the risk of having clubfeet by 3.7 times (CI%95[0.8–18.3], p = 0.11). A lower proportion of intact MF and a higher proportion of clubfeet pre- or postnatally were observed in cases with a large MMC sac who underwent a prenatal repair.
Trial registration: Clinicaltrials.gov NCT02230072 and NCT03794011 registered on September 3rd, 2014 and January 4th, 2019.
Similar content being viewed by others
Introduction
In the United States, myelomeningocele (MMC), a type of open neural tube defect (NTD), is seen in 1 in 3000 live-births1. Myelomeningocele occurs when the neural placode extends through the spinal defect with accompanying herniation of the meninges. Exposure of the neural elements to amniotic fluid causes progressive injury, which leads to changes in the cytoarchitecture of the spinal cord from irritation of the primary lesion2,3,4,5,6,7. Because of this, motor and sensory deficits accompany the diagnosis of MMC2,3,4,5, 8. The Management of Myelomeningocele Study (MOMS), a multicenter randomized clinical trial, reported improved motor function and a decrease in the need for ventriculo-peritoneal shunting during the first 12 months of life in those patients who underwent prenatal MMC repair as compared to the postnatal repair patients9.
Oliver et al. demonstrated that MMCs prenatally or postnatally repaired were associated with a higher rate of clubfeet and lower extremity impairment when compared to myeloschisis lesions, another type of open NTD where the spinal cord remains within the spinal canal10. They hypothesized that additional neurologic injury may result from cerebrospinal fluid hydrostatic forces within the MMC sac with secondary stretching of the neural placode and spinal nerves, which would represent another acquired mechanism of injury in cases of open spinal dysraphism.
In large MMCs, the excessive posterior herniation of the neural placode outside of the level of the wide open osseous spinal canal results in more significant stretching and thinning of the neural placode as well as the spinal nerves that are located along the anterior surface of the neural placode. We hypothesized that the overstretching of the neural placode in cases with large MMC volumes will result in higher motor function impairment.
The objectives of our study were: (1) to compare brain findings between large and non-large MMC sac volumes at the time of referral and 6 weeks after the prenatal surgery; (2) to compare prenatal motor function from mid-gestation to delivery between large and non-large MMC sac volume; and (3) to determine the relationship between the volume of the lesions and postnatal neurological short-term outcomes.
Results
Characteristics of the population
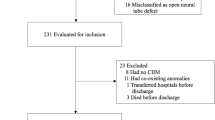
Sixty-three MMC cases who underwent prenatal NTD repair were included in this study (37 fetoscopic and 26 open-hysterotomy). The median volume of the sac at the time of referral was 1.6 (0.05–44.5) cc. Twenty-three cases presented with a “large” sac and 40 with a “non-large” sac.
Volume of the lesion and the neuroanatomic findings by MRI at the time of referral and 6 weeks after the prenatal surgery
At the time of referral, the anatomical level of the lesion and ventricle size were similar between large and non-large MMC lesions (Table 1). Six weeks after the surgery, there was no difference between large and non-large MMC sacs for the size of the ventricles and the proportion of fetuses that showed hindbrain herniation reversal (Table 1).
Comparisons between large and non-large MMC lesions in the fetoscopic or the open-repair groups are presented in Supplementary Table 1 and Supplementary Table 2. There was no difference between large and non-large MMC lesions for neuroanatomic findings by MRI at the time of referral and 6 weeks after the prenatal surgery in both subgroups.
At referral, there was no correlation between the volume of the lesion and ventricular size (r = 0.03, p = 0.78), or anatomical level of the lesion (r = 0.18, p = 0.16).
At six post-operative weeks, there was no correlation between the volume of the lesion and hindbrain herniation (HBH) grade (r = 0.04, p = 0.78) or ventricular size (r = 0.1, p = 0.99).
Impact of the MMC volume on prenatal motor function
Fetuses with large MMC sac volumes showed a higher rate of clubfeet and a lower proportion of intact motor function at the time of referral (Table 1). At the time of referral, a higher MMC volume increases the risk of having clubfeet by 9.5 times (95% CI [2.1–41.8]) and reduces the chances of having an intact motor function by 0.19 times (95% CI [0.1–0.6]) (Table 2).
During the last US scan before delivery, the proportion of cases with an intact motor function was higher in cases of non-large lesions compared to large lesions (Table 1).
In the fetoscopic subgroup, there was no difference between large and non-large MMC lesions for the proportion of clubfeet or intact motor function at the time of referral, 6 weeks after the surgery or during the last US before delivery (Supplementary Table 1).
In the open-hysterotomy subgroup, there was a higher proportion of cases with clubfeet and a lower proportion of cases with intact motor function at the time of the referral in the large MMC group compared to the non-large MMC group (Supplementary Table 2).
Impact of the MMC volume on postnatal neurological short outcomes
At birth, cases with larger MMCs showed a higher rate of clubfeet and a lower proportion of intact motor function compared to non-large MMCs (Table 1). There was a trend for a higher proportion of intact motor function at 12 months of life.
Higher MMC volume reduces the chances of having an intact motor function at birth (OR = 0.11 CI95[0.01–0.49], p < 0.01) after adjusting for the presence of an intact motor function at initial evaluation, anatomical level of the lesion, type of surgery, gestational age at delivery, and learning curve.
Dehiscence or leakage of CSF, need for postnatal repair of the defect at birth, need for hydrocephalus treatment in the first year of life, and independent ambulation at 30 months of age was similar between large and non-large MMC (Table 1).
In the fetoscopic subgroup, there were significantly fewer cases with an intact motor function at birth in the large MMC group compared to the non-large MMC group, and a trend for a higher proportion of cases with clubfeet in the large MMC group (Supplementary Table 1). In this subgroup, having a large MMC reduces the chances of having an intact motor function at birth (OR = 0.1 CI95[0.01–0.6], p = 0.02) after adjusting for the learning curve, anatomical level of the lesion, presence of an intact motor function at referral, and gestational age at delivery.
In the open-hysterotomy subgroup, there were significantly more cases with an intact motor function, and a lower proportion of cases with clubfeet in the non-large MMC group compared to the large MMC group (Supplementary Table 2).
Discussion
Results from this study suggest that cases considered to be candidates for in-utero MMC repair with large MMC lesions are expected to have an increased odds for having clubfeet at birth (3.7-fold) and are less likely to have an intact motor function (sacral one motor function) at birth (0.09-fold) after a prenatal repair compared to non-large MMC lesions.
Oliver et al. reported their experience with 182 NTD cases prenatally repaired (142 MMC and 40 MS), using the same methodology we used in this study to assess the volume of the lesion with similar volumes (4.7 cc in cases of MMC with clubfeet or 3 cc in cases of MMC without clubfeet)10. Their findings are consistent with previous reports of improved postnatal lower extremity function in prenatally repaired cases of myeloschisis compared to MMC11,12,13. A recent study exploring prenatal neurologic findings in cases of open spinal dysraphism found no association between MMC sac volume and prenatal clubfeet14. However, comparison with our cohort may be difficult because they did not report the anatomical level of the lesion, which is a well-known predictive factor for clubfeet12.
An inverse relationship between sac volume and degree of HBH severity was reported by Nagaraj et al., and it was hypothesized that MMC sac volume may have a protective effect on the degree of HBH by maintaining some hydrostatic pressure within the spinal canal14. We only included cases that underwent prenatal repair who had HBH, so we did not compare cases with or without HBH.
Experimental and clinical studies suggest that the neurological deficits associated with NTD occur in a step-wise fashion6, 15, 16. Our study confirms previous reports that concluded that larger MMC sizes are associated with lower extremity impairment10, an observation that could be described as a “third-hit” hypothesis.
First, NTD results in a closure defect in the posterior portion of the vertebral spine in the third week of gestation6. The leak of cerebro spinal fluid (CSF) through the defect exerts a siphoning effect on the cerebellum and medulla. As the gestation proceeds, HBH impairs the path and dynamics of CSF flow resulting in hydrocephalus, which exacerbates the herniation through the foramen magnum, worsening the problem.
Second, the segmental neurological damage at the NTD site can be secondary to both chemical toxicity and recurrent physical trauma of the neural placode in the intra-uterine environment, as reported in animal studies4, 17, 18.
Third, the lower extremity impairment that we described in cases of large MMC, may be due to increased traction of the neural placode and nerve roots due to mechanical expansion of the larger sacs. It is possible that the additive effects of all these mechanisms may lead to a more permanent damage of the placode nerve root even after prenatal repair or large MMC.
Our results indicate that the fetal spinal cord and nerves are sensitive to stretch injury which could impact fetal surgical planning for in-utero treatment. When deciding technique and closure strategies for repair, additional care should be shown to minimize traction to the MMC lesion and nerves. Based on our results, measuring the volume of the lesion prior to surgery would be worth considering to assist in intraoperative decision making.
This study included fetuses from two different operative approaches undergoing prenatal repair from a single center. Standard protocols for evaluation and management of care were used for all cases. We acknowledge that the retrospective design is a major limitation of our study. Due to the outcomes seen in NTD cases being multifactorial in nature, it is possible the assumed effect secondary to nerve root overstretching in large lesions could be due to accumulating effects of the volume of the lesion or intrauterine insults that are unknown.
In our cohort, fetuses who present with a large MMC (> 2.7 cc in our cohort) at referral for the evaluation of a prenatal MMC repair are less likely to have an intact motor function at birth and more likely to present clubfeet at birth. These results suggest that spinal cord and nerves are sensitive to stretch injury, a “third-hit” hypothesis.
Methods
This is a retrospective study of 103 fetuses who underwent a prenatal MMC repair (fetoscopic or open-hysterotomy technique) performed at a single institution between November 2011 and March 2019. All patients who underwent prenatal NTD repair during this period were included in this study. Myeloschisis cases (n = 34), cases where surgery was aborted due to fetal intolerance (n = 3), the lesion was not confirmed to be a myelomeningocele (MMC) by direct visualization (n = 2), or when the mother had a contraindication for an MRI study (n = 1) were excluded from the analysis. All fetuses who underwent prenatal repair and satisfied all MOMS trial inclusion criteria and none of the exclusion criteria were included9.
Initial evaluation
At the time of referral, all patients underwent fetal MRI on a 1.5-T Philips Ingenia superconducting magnet (Philips Medical Systems, Best, The Netherlands). Details of imaging protocol are presented in Supplementary Material. Ventricular width was measured bilaterally at the level of the posterior horns of the lateral ventricles determined by the location of the parieto-occipital fissure on coronal images. Mean ventricular width was calculated, and ventriculomegaly was defined as a mean ventricular width > 10 mm. Severe ventriculomegaly was defined as ≥ 15 mm.
At the time of referral, detailed transabdominal fetal US examinations (General Electric Voluson E8 or E10, GE Healthcare, Milwaukee, WI, USA) were performed in all patients referred to our Fetal Center in order to evaluate patient eligibility for prenatal NTD repair. The anatomical level of the lesion was defined as the upper bony spinal defect on US scans. A high level of the lesion was defined as an upper bony spinal defect at or above the second vertebral body (L2)9.
Measurements of the volume of the lesions were performed using US scans to calculate the volume of the prolate ellipsoid (volume = length × width × depth × 0.52)10 (Fig. 1).
Volume of the myelomeningocele: ultrasound measurements. (A) Sagittal view of a myelomeningocele at 20 weeks of gestation: length. (B) Transversal view of a myelomeningocele at 20 weeks of gestation: width and depth. (C) Volume of the myelomeningocele: prolate ellipsoid volume: length × width × depth × 0.52.
ROC curve analysis and Youden index were performed to determine the best cut-off to predict an intact motor function at referral based on the volume of the lesion. A 2.7 cc cut-off was found to have the best sensitivity (0.63) and best specificity (0.75) to predict an intact motor function at referral (Supplementary Material). Therefore, a large lesion was defined as greater than 2.7 cc and a non-large lesion as less than or equal to 2.7 cc.
Prenatal NTD repair
The surgical approach for NTD repair was determined based on maternal choice. During the pre-surgical evaluation, patients were not informed specifically about the volume of the lesion; however, the anatomical level of the lesion was discussed with them. Patients who underwent a prenatal NTD repair were managed per our standard protocol of steroid administration, prophylactic tocolysis (preoperative indomethacin and post-operative nifedipine, and intra- and post-operative magnesium sulfate), and prophylactic antibiotics (cefazolin or if allergic, clindamycin and gentamicin). Open repair was considered the standard of care for prenatal NTD repair and was performed through an open-hysterotomy approach using the same methodology as reported by the MOMS trial9. From 2014, fetoscopic repair was offered under our fetoscopic repair protocol provided by the U.S. Food and Drug Administration (FDA) IDE #G140201, the Baylor College of Medicine Institutional Review Board (IRB) protocols H-34680 (7/1/2014) and H-43359 (11/15/2018), our Fetal Therapy Board, and a Data Safety Monitoring Board. These protocols are registered on ClinicalTrials.gov (NCT02230072 and NCT03794011)19. The fetoscopic technique was performed as described elsewhere20. A single layer closure was performed in the first 23 cases of our MMC cohort. In all subsequent cases, a three layer closure was performed using a collagen patch on top of the dura (Durepair, Medtronic, USA), with muscle flaps and skin closure21. During both fetoscopic and open-hysterotomy surgeries, if a complete skin closure was not expected to be possible due to excessive tension on the skin edges, relaxing incisions were performed. These are bilateral vertically oriented incisions performed 2–3 cm from the edge of the defect to facilitate a tension-free midline closure.
Data was retrospectively obtained under an IRB (Baylor College of Medicine) approved protocol H-38479 (2/11/2016). All patients signed an informed consent prior to inclusion in the study. All methods were performed in accordance with the relevant guidelines and regulations. The first 12 cases in both the fetoscopic and the open-hysterotomy techniques, including prenatal repair of myeloschisis, were considered to be part of our learning curve20.
Post-repair evaluation
Six weeks after the repair, the posterior fossa was carefully evaluated by MRI, and the degree of Chiari II malformation was evaluated using Sutton et al.22 (Supplementary Material). Reversal of HBH was identified when the most caudal portion of the cerebellum was seen above the foramen magnum. Ventricular width was measured bilaterally at the level of the posterior horns of the lateral ventricles determined by the location of the parieto-occipital fissure on coronal MRI images. Mean ventricular width was calculated, and ventriculomegaly was defined as a mean ventricular width > 10 mm. Severe ventriculomegaly was defined as ≥ 15 mm.
Evaluation of the prenatal motor function
Ultrasound video clips of the lower extremity movements were reviewed by an expert OB/GYN examiner to score the level of neurologic function based on metameric nerve distribution (Supplementary material) at three different time points: (1) At the time of referral, (2) 6 weeks after prenatal surgical repair and (3) during the last US scan before delivery. Since sacral one (S1) motor function reflects an “intact” motor function, the proportion of cases with S1 motor function was calculated at each of the time points of motor function assessment.
Management and indications for delivery and labor
Further details about the management of cases who underwent open or fetoscopic NTD repair are included in the Supplementary Material.
Evaluation at birth
The repair site was clinically examined by a pediatric neurosurgeon within the first 24 h of life to identify any area of dehiscence or CSF leakage at the repair site. The functional segmental motor function was determined by a detailed neurological examination performed by a pediatric neurosurgeon within the first 48 h of life, as reported elsewhere23. Details of the neurological examination are included in the Supplementary Material section. Plantar flexion of the ankle was attributed to the first sacral metameric level (S1), which is considered an “intact” motor function.
After discharge, infants were followed at our institution’s multidisciplinary Spina Bifida Clinic, or at their referral centers, every 3 months during their first 12 months of life, and biannually afterward. The need for hydrocephalus treatment was determined using standard clinical and imaging criteria. Ventriculo-peritoneal shunt or endoscopic third ventriculostomy with choroid plexus cauterization was performed if indicated.
Ambulatory skills were evaluated at 30 months of life, and classified as (1) walking independently (with or without orthotics), (2) walking with an assistive device (crutches or walker), or (3) non-ambulatory.
Statistical analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS), version 24.0 (IBM Corporation, Armonk, NY, USA). Quantitative variables were expressed as mean ± standard deviation if they had a normal distribution (by Kolmorov-Smirnov test) and compared with Student’s t-test. Quantitatives variables with non-normal distribution were expressed as median [range] and compared with Mann–Whitney U test. Pearson’s Chi square or Fisher’s exact tests were used to compare qualitative data. Correlations between the volume of the lesion and other quantitative variables such as the anatomical level of the lesion (using a numerical score, Th5 = 1 to S1 = 14), ventricular size, and the HBH grading classification, were performed using Spearman correlation analyses. Linear and logistic regression analyses were performed, controlling for potential confounding variables (anatomical level of lesion, learning curve, type of repair, presence of clubfeet, intact motor function at referral, gestational age at delivery) when indicated. Predictive values of large NTD for neurosurgical, post-operative and neonatal outcomes were expressed as odds ratios (OR) with a 95% confidence interval (95% CI).
Data availability
AS (corresponding report) and RC (primary author) have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Data could be available on request to the corresponding author.
References
Parker, S. E. et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res. Part A Clin. Mol. Teratol. 88, 1008–1016 (2010).
Bowman, R. M., McLone, D. G., Grant, J. A., Tomita, T. & Ito, J. A. Spina bifida outcome: A 25-year prospective. Pediatr. Neurosurg. 34, 114–120 (2001).
Shin, M. et al. Improved survival among children with spina bifida in the United States. J. Pediatr. 161, 1132-1137.e3 (2012).
Heffez, D. S., Aryanpur, J., Hutchins, G. M. & Freeman, J. M. The paralysis associated with myelomeningocele: Clinical and experimental data implicating a preventable spinal cord injury. Neurosurgery 26, 987–992 (1990).
Wasserman, R. M. & Holmbeck, G. N. Profiles of neuropsychological functioning in children and adolescents with spina bifida: Associations with biopsychosocial predictors and functional outcomes. J. Int. Neuropsychol. Soc. 22, 804–815 (2016).
Dias, M. S. Neurosurgical management of myelomeningocele (spina bifida). Pediatr. Rev. 26, 50–60 (2005) (discussion 50–60).
Al-Shanafey, S. N. et al. Reduction in neural injury with earlier delivery in a mouse model of congenital myelomeningocele: Laboratory investigation. J. Neurosurg. Pediatr. 12, 390–394 (2013).
Hunt, G. M. & Oakeshott, P. Outcome in people with open spina bifida at age 35: Prospective community based cohort study. BMJ 326, 1365–1366 (2003).
Adzick, N. S. et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N. Engl. J. Med. 364, 993–1004 (2011).
Oliver, E. R. et al. Myelomeningocele sac associated with worse lower extremity neurologic sequela: Evidence for prenatal neural stretch injury?. Ultrasound Obstet. Gynecol. https://doi.org/10.1002/uog.21891 (2019).
Farmer, D. L. et al. The management of myelomeningocele study: Full cohort 30-month pediatric outcomes. Am. J. Obstet. Gynecol. 218(256), e1-256.e13 (2018).
Danzer, E. et al. Lower extremity neuromotor function and short-term ambulatory potential following in utero myelomeningocele surgery. Fetal Diagn. Ther. 25, 47–53 (2009).
Wilson, R. D. et al. Does a myelomeningocele sac compared to no sac result in decreased postnatal leg function following maternal fetal surgery for spina bifida aperta?. Fetal Diagn. Ther. 22, 348–351 (2007).
Nagaraj, U. D. et al. Myelomeningocele versus myelocele on fetal MR images: Are there differences in brain findings? In American Journal of Roentgenology, vol. 211, 1376–1380 (American Roentgen Ray Society, 2018).
Sival, D. A. et al. Neonatal loss of motor function in human spina bifida aperta. Pediatrics 114, 427–434 (2004).
Sival, D. A. et al. Spinal hemorrhages are associated with early neonatal motor function loss in human spina bifida aperta. Early Hum. Dev. 84, 423–431 (2008).
Michejda, M. Intrauterine treatment of spina bifida: Primate model. Z. Kinderchir. 39, 259–261 (1984).
Heffez, D. S., Aryanpur, J., Rotellini, N. A., Hutchins, G. M. & Freeman, J. M. Intrauterine repair of experimental surgically created dysraphism. Neurosurgery 32, 1005–1010 (1993).
Fetoscopic Meningomyelocele Repair Study (fMMC). ClinicalTrials.gov NCT02230072 https://clinicaltrials.gov/ct2/show/record/NCT02230072 (2014).
Belfort, M. A. et al. Fetoscopic open neural tube defect repair: Development and refinement of a two-port, carbon dioxide insufflation technique. Obstet. Gynecol. https://doi.org/10.1097/AOG.0000000000001941 (2017).
Belfort, M. A. et al. Comparison of two fetoscopic open neural tube defect (ONTD) repair techniques: Single-layer vs three-layer closure. Ultrasound Obstet. Gynecol. https://doi.org/10.1002/uog.21915 (2019).
Sutton, L. N. et al. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. J. Am. Med. Assoc. 282, 1826–1831 (1999).
Danzer, E. et al. Long-term neurofunctional outcome, executive functioning, and behavioral adaptive skills following fetal myelomeningocele surgery. Am. J. Obstet. Gynecol. 214(269), e1-269.e8 (2016).
Funding
Fetoscopic neural tube defect repair supported by grants from the Sterling Turner Foundation, O'Quinn Foundation, Fondren Foundation, and Tramuto Foundation.
Author information
Authors and Affiliations
Contributions
R.C.: conception, design, acquisition, analysis, interpretation and draft. A.M.R.: analysis, interpretation, draft and review. R.J.: analysis, interpretation, draft and review. W.W.: acquisition, interpretation and review. J.E.: review. J.C.: review. H.C.: review. G.O.: acquisition, review. R.D.: review. T.H.: review. A.N.: design, acquisition, review. M.B.: review. M.S.C review. A.S.: conception, design, interpretation, review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Corroenne, R., Mehollin-Ray, A.R., Johnson, R.M. et al. Impact of the volume of the myelomeningocele sac on imaging, prenatal neurosurgery and motor outcomes: a retrospective cohort study. Sci Rep 11, 13189 (2021). https://doi.org/10.1038/s41598-021-92739-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-92739-2
This article is cited by
-
Clinical impact of fetal sac size on closed neural tube defects
Child's Nervous System (2025)