Abstract
NEU1 sialidase hydrolyzes sialic acids from glycoconjugates in lysosomes. Deficiency of NEU1 causes sialidosis with symptoms including facial dysmorphism, bone dysplasia, and neurodegeneration. However, the effects of NEU1 deficiency on emotional activity have not been explored. Here, we conducted the behavioral analysis using Neu1-knockout zebrafish (Neu1-KO). Neu1-KO zebrafish showed normal swimming similar to wild-type zebrafish (WT), whereas shoaling was decreased and accompanied by greater inter-fish distance than WT zebrafish. The aggression test showed a reduced aggressive behavior in Neu1-KO zebrafish than in WT zebrafish. In the mirror and 3-chambers test, Neu1-KO zebrafish showed more interest toward the opponent in the mirror and multiple unfamiliar zebrafish, respectively, than WT zebrafish. Furthermore, Neu1-KO zebrafish also showed increased interaction with different fish species, whereas WT zebrafish avoided them. In the black–white preference test, Neu1-KO zebrafish showed an abnormal preference for the white region, whereas WT zebrafish preferred the black region. Neu1-KO zebrafish were characterized by a downregulation of the anxiety-related genes of the hypothalamic–pituitary–adrenal axis and upregulation of lamp1a, an activator of lysosomal exocytosis, with their brains accumulating several sphingoglycolipids. This study revealed that Neu1 deficiency caused abnormal emotional behavior in zebrafish, possibly due to neuronal dysfunction induced by lysosomal exocytosis.
Similar content being viewed by others
Introduction
Lysosomal disorders are identified as incurable diseases, affecting a very small number of patients, namely 1 in 50001. Approximately 70 types of lysosomal diseases have been reported and shown to be genetically induced by gene mutations in lysosomal-related proteins, mostly catabolic enzymes and lysosome membrane proteins1. Dysfunction of lysosomal enzymes in patients is known to result in the accumulation of non-degraded substrates, thus inducing a reduction in lysosomal functions.
Sialidosis (Online Mendelian Inheritance in Man (OMIM) #256550) is a lysosomal disease with low incidence (1:250,000 to 1:2,000,000 live births), classified into type I (milder symptoms) and type II (severe symptoms), which can be subdivided into 3 forms: congenital, infantile, and juvenile2,3. Symptoms of patients with sialidosis include myoclonus, spinal deformities, cherry red spots, inner ear hearing loss, hepatosplenomegaly, and visual failure4,5. In addition, patients with sialidosis have been reported to show psychiatric abnormalities, such as mental decline, low IQ, and intellectual disability2,6.
Sialidosis is caused by a genomic DNA mutation in neuraminidase 1 (NEU1) (chromosome location: 6p 21.3). The NEU1 protein is a sialidase (EC 3.2.1.18), a representative of the lysosomal glycosidases that cleaves sialic acids from the nonreducing ends of glycoconjugates, mainly from oligosaccharides and glycoproteins7. In lysosomes, desialylation of oligosaccharides by NEU1 is known to be crucial in regulating the initial step in the degradation of sugar chains. A part of NEU1 localizes to the plasma membrane, where NEU1 regulates cell attachment, insulin signaling, and elastin binding8,9,10. The enzymatic activity of NEU1 is activated/stabilized by cathepsin A. Cathepsin A dysfunctions due to mutations in CTSA (cathepsin A gene) causes galactosialidosis (OMIM # 256540), which is characterized by similar symptoms to those of sialidosis. Several DNA mutations in the NEU1 gene in patients with sialidosis have been reported2,11, with these mutations inducing alterations in the activity of sialidase, lysosomal localization, complex formation, structure, and half-life of NEU1 polypeptides12,13,14, thus leading to a significant impact on the symptoms of the disease.
So far, both SM/J and Neu1-KO mice have been used for sialidosis studies. SM/J mice are known to possess a single substitution mutation (L209I) in the NEU1 protein, leading to a decrease in the activity of lysosomal sialidase, smaller body size, and possible alterations of immune responses15. Likewise, Neu1-KO mice exhibit a smaller body, the curvature of the cervical spine, induction of vacuolation in the kidneys, and an enlarged spleen16. Using rodent models, the molecular mechanism of the incidence of sialidosis symptoms has been partially identified. For instance, excessive exocytosis of serine proteases in the bone niche has been reported to lead to loss of bone marrow retention17. The activated components of the transforming growth factor β (TGF-β) and wingless-related integration site (WNT) signaling pathways in the exosome are involved in the abnormal fibrotic process in muscle connective tissue fibroblasts18.
While the mechanism of the dysfunction of the NEU1 deficiency-induced bone and muscle formation has been sufficiently studied, little is known about the involvement of NEU1 in emotional regulation, unlike other lysosomal diseases. For instance, patients with Fabry disease are known to show mood disorders, including anxiety and depression, whereas patients with Niemann-Pick disease type C show psychiatric symptoms, including hallucinations, delusions, cognitive decline, dementia, depression, bipolar disorder, as well as disruptive and aggressive behavior19. In addition, patients with α-mannosidosis, mucopolysaccharidosis, and Tay–Sachs disease have also been reported to exhibit mood disorders20. As lysosomal diseases have been shown to share many common symptoms and sialic acid is known to be present in high levels in central nerves21, we hypothesized that a NEU1 deficiency might affect emotional and social interactions.
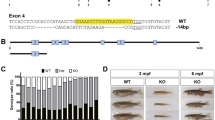
Here, we focused on teleost to examine the involvement of NEU1 in emotional behavior. Fish are excellent experimental animals for the analysis of social interaction, including group formation, aggressiveness, social preference, and boldness. Most emotional related-genes are known to be conserved across vertebrates, and several methodologies of behavioral analysis have been established in fish22,23. In addition, recent studies have revealed that neu1 genes are conserved in fish species, with their enzymatic properties24,25,26,27. Fish Neu1 is known to be localized at the lysosome and its enzymatic properties, such as optimal pH and substrate specificity, have been reported to be shared among fish species. Recently, we established a Neu1 knockout zebrafish (Neu1-KO) strain using the CRISPR/Cas9 genome editing method26. The Neu1-KO zebrafish was shown to possess 5 nucleotide deletions in the first exon, which caused a frameshift, resulting in the interruption of the translation of the Neu1 polypeptide. The Neu1-KO zebrafish was characterized by a smaller body size with the bending spine similar to patients with sialidosis and Neu1-KO mice. This study aimed to reveal the effects of a Neu1-deficiency on the behavior of Neu1-KO zebrafish.
Results
Neu1-KO zebrafish exhibited lower social interaction and aggression
A previous study reported that older Neu1-KO zebrafish (> 11 months old) exhibited spinal bending, inducing a suppression in their swimming ability26. Thus, this study employed younger Neu1-KO zebrafish that had not developed any such symptoms and were considered suitable for behavioral analysis. To confirm the swimming ability of young adult Neu1-KO zebrafish, 6-months fish were transferred to the normal tank and their swimming behavior was observed after 5 min of acclimation. We found that Neu1-KO zebrafish did not exhibit any abnormal behavior, such as freezing and limited swimming along the tank wall, and their swimming distance and average swimming speed did not differ from those of WT (Fig. 1a–c).
Analysis of shoaling in Neu1-KO and WT zebrafish. (a–c) Analysis of the behavior of Neu1-KO zebrafish (6 months) in the normal tank for 5 min. (a) Tracking. (b) Swimming distance. (c) Average swimming speed. WT, wild-type and KO, Neu1-KO zebrafish. n = 7 for each group. (d–h) Shoaling of Neu1-KO zebrafish. Five fish were set in the experimental tank, and their average inter-fish distance was immediately estimated (d, without acclimation) . After 30 min acclimation in the test tank, inter-fish distances were evaluated again (f, with acclimation). Closed blue triangles and red squares with single line indicate WT and KO, respectively. (e) Average fish number in the top area for 5 min. (g) Average distance between nearest and (h) farthest neighbors analyzed for 5 min in WT and Neu1-KO zebrafish during the shoaling test without acclimation. Results are shown as means ± standard deviation. All values were compared using Mann–Whitney U test. *p < 0.05, **p < 0.01. n.s., not significant.
We conducted the shoaling test with Neu1-KO zebrafish using the novel tank apparatus. Shoaling is thought to provide each fish with multiple benefits, including access to mates, efficient foraging, and defense against predators28. First, 5 zebrafish were transferred to a novel tank, and then the estimation of their inter-fish distance was performed every 30 s immediately after the start of the test without acclimation. The inter-fish distance is known to represent the shoaling formation. We observed that both WT and Neu1-KO zebrafish formed shoaling after their transfer to the tank, probably due to anxiety. However, after 240 s, the inter-fish distance in Neu1-KO zebrafish was demonstrated to be significantly increased compared with that of WT (p < 0.01, Fig. 1d). Next, to remove the effect of anxiety induced by the novel tank, fish were acclimated for 30 min and then used for behavioral assays. In general, the number of fish in the top reflects the place preference of the shoal, which is likely to be near the bottom for more anxious fish. In Neu1-KO zebrafish, the place preference of shoal did not differ from WT (Fig. 1e). We found that under acclimation conditions, the increased fish intervals were sustained in Neu1-KO zebrafish compared with those of WT zebrafish (p < 0.01, Fig. 1f). The distance between the farthest neighbors was significantly longer in the Neu1-KO zebrafish relative to that in WT (3.5-fold increase, p < 0.0001, Fig. 1h), whereas the distance between the nearest neighbors was not (Fig. 1g). These results indicated low shoaling in Neu1-KO zebrafish.
Next, we assessed the aggressiveness of Neu1-KO zebrafish. We noted that WT zebrafish started continuous chasing accompanied by the rapid acceleration of swimming (Fig. 2a,b). In contrast, Neu1-KO zebrafish seemed indifferent to each other and swam independently exhibiting slight chasing behavior. According to the induction of rapid acceleration of swimming, we found that WT zebrafish tended to have longer swimming distance and faster swimming speed than Neu1-KO zebrafish. (Fig. 2c,d). During a 10 min observation, WT zebrafish exhibited a significantly longer aggressive behavior (total average: 255.5 s) than Neu1-KO zebrafish (average 34.0 s, p < 0.01, Fig. 2e). These results suggested a low aggressiveness in Neu1-KO zebrafish.
Analysis of aggressive behavior in Neu1-KO and WT zebrafish. Two unfamiliar male zebrafish were set in the transparent aquarium and their aggressive behavior was observed for 10 min. (a) Tracking of 2 males in WT and Neu1-KO. Red and blue lines indicate the swimming tracking of 2 males. (b) Swimming acceleration. (c) Swimming distance. (d) Swimming speed. (e) Aggressive behavior. n = 6 for each group. Results are shown as means ± standard deviation. All values were compared using Mann–Whitney U test. n.s., not significant.
Neu1 knockout zebrafish showed abnormal social preferences
The mirror test is generally used for studying the sociality, aggressiveness, and boldness of fish (Fig. 3a). In the mirror test, test fish are known to recognize the opponent in the mirror as an unfamiliar zebrafish. Here, we employed the mirror test on Neu1-KO zebrafish to evaluate their emotional responses. As a result, we found that the swimming behaviors of Neu1-KO zebrafish were drastically different from those of WT zebrafish. Both WT and Neu1-KO zebrafish showed an interaction with the mirror (towards the opponent in the mirror), but Neu1-KO zebrafish were noted to approach the mirror more than WT zebrafish (Fig. 3b), despite exhibiting a similar total swimming distance to that of WT (Fig. 3c). This result indicated that the increased interaction observed in Neu1-KO zebrafish might not be due to hyperactivity or impulsive behavior. We also observed that Neu1-KO zebrafish exhibited a significant increase in the total time in the approach area (2.4-times longer than WT, p < 0.01, Fig. 3d). However, the total entry into the approach area in Neu1-KO zebrafish was shown to be less than that in WT (p < 0.01, Fig. 3e), indicating that Neu1-KO zebrafish stayed for a longer period in the approach area in a single invasion compared with WT zebrafish. After approaching the mirror, the zebrafish showed a chasing behavior towards the opponent (itself in the mirror). We found that the chasing time in Neu1-KO zebrafish was significantly longer than that in WT zebrafish during the first 10 min after the beginning of analysis (p < 0.01), whereas dropped to the WT level after 10 min (Fig. 3f). Taken together with the results of Figures 1 and 2, Neu1-KO zebrafish exhibited increased interest toward the opponent in the mirror, but not due to shoaling and aggressiveness.
Analysis of Neu1-KO and WT zebrafish behavior in a mirror test. (a) Apparatus used in the mirror test. (b) Tracking of WT and Neu1-KO zebrafish. (c) Swimming distance. (d) Time spent in the approach area. (e) Frequency of entry into the approach area. (f) Time of chasing the opponent in the mirror. n = 10 for each group. Results are shown as means ± standard deviations. All values were compared using Mann–Whitney U test. n.s., not significant.
To better understand the interaction of Neu1-KO zebrafish with unfamiliar zebrafish, we conducted a 3-chambers test using 2 chambers: an empty chamber and a chamber containing 4 unfamiliar zebrafish that had been kept in a different aquarium from test fish (Fig. 4a). During the 5 min trial, we found that WT zebrafish swam throughout the tank, showing interest in both chambers, and especially in the zebrafish tank (Fig. 4b). We observed that WT zebrafish swam along the front wall of the chambers after their entry into the fish chamber area. In contrast, we found that the swimming trajectory in Neu1-KO zebrafish showed limited locomotion in the fish chamber area accompanied by a remarkable swimming along the chamber wall (Fig. 4b). Whereas both WT and Neu1-KO zebrafish covered a similar swimming distance (Fig. 4c), the time spent in the chamber area was 4.0-fold longer in Neu1-KO zebrafish than in WT (p < 0.01, Fig. 4d). However, we noted that the frequency of entry into the fish chamber area did not differ between WT and Neu1-KO zebrafish (Fig. 4e). Unlike the differences noted regarding the fish chamber, both WT and Neu1-KO zebrafish showed similar responses toward the empty chamber (Fig. 4f,g).
Analysis of social preference in Neu1-KO and WT zebrafish using the 3-chambers test. (a–g) Analysis of the 3-chambers test with 4 unfamiliar zebrafish in Neu1-KO and WT zebrafish. (a) Apparatus of the 3-chambers test using 4 zebrafish. (b) Tracking of WT and Neu1-KO zebrafish. (c) Total swimming distance. (d) Time spent in the fish chamber area. (e) Total entry into the fish chamber area. (f) Time spent in the empty chamber area. (g) Total entry into the empty chamber area. n = 8 for each group. (h–o) Analysis of the 3-chambers test with 4 cavefish in Neu1-KO and WT zebrafish. (h) Apparatus of the 3-chambers test with 4 cavefish. (i) Tracking of WT and Neu1-KO zebrafish. (j) Total swimming distance. (k) Swimming speed. (l) Time spent in the fish chamber area. (m) Total entry into fish chamber area. (n) Time spent in the empty chamber area. (o) Total entry into empty chamber area. n = 10 for each group. Results are shown as means ± standard deviation. All values were compared using Mann–Whitney U test. n.s., not significant.
Next, we estimated the social preference for different fish species using cavefish (Mexican tetra, Fig. 4h). Cavefish is an unfamiliar fish for zebrafish, with a red body color distinctive from zebrafish. In addition, as cavefish are blinded due to degeneration of their eyes, the effects of the behavior of zebrafish on cavefish could be ignored. We observed that WT zebrafish showed vigilance and anxiety toward cavefish and swam far from the chamber, whereas sometimes invaded the fish chamber area probably due to interest/exploration, which was however followed by rapid escape from the chamber area (Fig. 4i). In contrast, Neu1-KO zebrafish showed a vigilant behavior only at the beginning of the trial and often invaded the fish chamber area (Fig. 4i). Due to their vigilant action, we recorded longer swimming distance and higher average velocity in WT compared with Neu1-KO zebrafish (p < 0.05, Fig. 4j,k). Based on their increased interest toward cavefish, the time spent in the fish chamber was shown to be drastically increased in Neu1-KO zebrafish (15-folds increase, p < 0.01, Fig. 4l), although the total entry into the fish chamber area was not different from that of WT (Fig. 4m). Both WT and Neu1-KO zebrafish exhibited similar responses to the empty chamber (Fig. 4n,o). Taken together, Neu1-KO zebrafish exhibited an abnormal social preference toward unfamiliar fish, which was seemingly attributed to their excessive high boldness or abnormal recognition.
For further analysis of the boldness/recognition behavior of Neu1-KO zebrafish, we carried out the black–white preference test (Fig. 5a). In general, adult zebrafish are known to instinctively prefer black to white areas because fish have a low risk of being detected by predators in a black background23. As expected, we noted that WT zebrafish stayed in the black area longer than in the white area during the 5 min analysis (Fig. 5b). In contrast, Neu1-KO zebrafish were found to obviously prefer the white to the black areas (Fig. 5b). The time spent by Neu1-KO zebrafish in the white area was significantly increased compared with that of WT (4.6-fold increase, p < 0.05, Fig. 5c), with the total entry of Neu1-KO zebrafish into the white area being less than that observed in WT (2.8-fold decrease, p < 0.05, Fig. 5d), indicating that Neu1-KO zebrafish stayed on the white side for a longer time per invasion. In addition, zebrafish are known to feel anxious toward the white color23. As shown in Fig. 5b, WT zebrafish showed anxiety accompanied by swimming along the wall and erratic movements/zig-zagging, which was not observed in Neu1-KO zebrafish. We further observed that the anxiety of WT fish induced a higher swimming speed compared with that exhibited by Neu1-KO zebrafish in both the black and white areas. (Fig. 5e,f).
Analysis of the black–white preference test in Neu1-KO and WT zebrafish. (a) Apparatus in the black–white preference test. (b) Tracking of WT (upper panel) and Neu1-KO zebrafish behavior (lower panel). (c) Time spent in the white area. (d) Total entry into the white area. (e,f) Swimming speed in white (e) and black area (f). n = 5 for each group. Results are shown as means ± standard deviation. All values were compared using Mann–Whitney U test.
To examine the alteration of emotions in Neu1-KO zebrafish, we estimated the mRNA levels of anxiety-related genes in WT and Neu1-KO zebrafish brains. The mineralocorticoid receptor (Mr), which is also conserved in zebrafish23, is known to regulate the activity of the hypothalamic–pituitary–adrenal (HPA) axis during the stress response29. We found that the mr mRNA level was significantly downregulated in Neu1-KO zebrafish compared with that in WT (2.6-fold decrease, p < 0.05, Fig. 6a). Neuropeptide Y is a downstream molecule of the Mr signaling through negative feedback30 and is known to be expressed at the locus coeruleus (LC) in zebrafish23. We noted that the mRNA level of npy was also significantly reduced in Neu1-KO zebrafish (p < 0.05, Fig. 6b). While both orexin (Orx) and melanin-concentrating hormone (Mch) are known as anxiogenic factors through the stimulation of the HPA axis, only the Mch mRNA levels were shown to be downregulated in Neu1-KO zebrafish brains compared with WT (p < 0.05, Fig. 6c,d). The sympathetic–adrenal–medullary (SAM) axis has also been reported to regulate anxiety behaviors through the elevation of the levels of catecholamine in vertebrates. The present study evaluated the gene expression of tyrosine hydroxylase (th), which converts tyrosine to dihydroxyphenylalanine, a precursor of dopamine and noradrenaline, for which zebrafish are known to possess 2 isoforms (Th1 and Th2)31. As a result, the levels of th1 and th2 in Neu1-KO zebrafish were not altered compared with WT (Fig. 6e,f). Taken together, Neu1-KO zebrafish showed an excess increased interest/exploratory activity, possibly due to decreased anxiety accompanied by the downregulation of the HPA axis signaling. Isotocin (Ist, teleost homologues of oxytocin) is a factor known to positively regulates societal and negative aggressiveness32, whereas vasotocin (Avt, teleost homologues of vasopressin) accelerates aggressiveness in zebrafish33. We found that the expression of ist in the brain of Neu1-KO zebrafish was significantly higher than that in WT (p < 0.05), whereas the levels of avt mRNA did not differ between Neu1-KO and WT zebrafish (Fig. 6g,h), confirming that Neu1 deficiency caused low shoaling and aggressiveness in zebrafish.
Changes in gene expression in Neu1-KO zebrafish. mRNA levels of mr (a), npy (b), orx (c), mch (d), th1 (e), th2 (f), ist (g), and avt (h) in WT and Neu1-KO zebrafish. Each level of gene expression in Neu1-KO zebrafish is relative to the value in WT fish. n = 5 for each group. Results are shown as means ± standard deviation. All values were compared using Mann–Whitney U test. n.s., not significant.
Neu1-KO zebrafish exhibited an accumulation of sphingoglycolipids in the brain
We assessed the alterations of sialoglycoconjugates in Neu1-KO zebrafish brain by lectin blotting using MAM (Sia α2-3 linkage specific) and SSA lectin (Sia α2-6 linkage specific). Although the recombinant zebrafish Neu1 has been reported to recognize both Sia α2-3 and α2-6 linkages in oligosaccharides and glycoproteins26, we could not detect a clear difference in the glycosylation pattern between Neu1-KO and WT zebrafish brains in our MAM and SSA lectin blot analysis (Fig. 7a,b). In mammals, Neu1 is known to desialyze polysialic acids (PSA) accompanied by a polysialic acid turnover in the regulation of neurotrophin34 and lamination of newly generated hippocampal granule cells35. However, we observed that the PSA pattern in Neu1-KO zebrafish brains did not differ from that of WT (Fig. 7c and Supplementary Fig. 1). Interestingly, the glycosphingolipid (GSL) pattern in the Neu1-KO zebrafish brain was shown to drastically differ from that in the WT brain, despite gangliosides not being substrates for zebrafish Neu126. TLC analysis of the neutral lipid fraction showed an accumulation of lactosylceramide (LacCer) and globoside Gb4 in Neu1-KO zebrafish, whereas these two GSLs were almost undetectable in WT (p < 0.05, Fig. 7d). We further noted that the GSL pattern in the acid fraction exhibited an increase in GM3 and GM1 in the Neu1-KO zebrafish brain (p < 0.05), but not in the WT, whereas the levels of GD1b and GT1b were almost the same between Neu1-KO and WT zebrafish brains (Fig. 7e).
Alteration of glycoconjugates patterns in Neu1-KO zebrafish. (a–c) Lysates of zebrafish brains were applied for lectin and western blotting. (a) MAM lectin. (b) SSA lectin. (c) Anti-PSA immunoblotting. Ma, Molecular marker. (d,e) GSLs were extracted from zebrafish brains and fractionated into neutral and acid fractions. Two fractions were applied for thin layer chromatography and GSLs were detected using orcinol-H2SO4. Neutral (d) and acid fraction (e) of GSLs. Results are shown as means ± standard deviation. All values were compared using Mann–Whitney U test.
Furthermore, we focused on the lysosomal conditions in Neu1-KO zebrafish. Neu1 is known to form a multienzyme complex with cathepsin A, β-galactosidase, and N-acetylgalactosamine-6-sulfatase36. Due to the deficiency of Neu1, the ctsa and galns mRNA levels were shown to be significantly increased in Neu1-KO zebrafish brains compared with WT (p < 0.01 for ctsa, and p < 0.05 for galns); however, that was not the case for glb1 (Fig. 8a–c). Transcription factor EB (Tfeb) is known as a master regulator of mammalian lysosomal genes, including Neu1 and Ctsa37. As expected, the Neu1-KO zebrafish brain showed higher tfeb mRNA levels compared with the WT brain (p < 0.05, Fig. 8d) accompanied by an upregulation of lamp1a (p < 0.05), but not lamp1b (Fig. 8e,f), showing the same pattern as Neu1-KO zebrafish muscle26. In mammals, TFEB is known to activate the translocalization of lysosomal enzymes to the plasma membrane38, with NEU1 being a negative regulator of lysosomal exocytosis through the desialylation of LAMP117. Immunoblot analysis revealed that the level of the Lamp1 protein was upregulated in Neu1-KO zebrafish brain compared with WT, coinciding with the lamp1a mRNA level (p < 0.05, Fig. 8g and Supplementary Fig. 2). Moreover, the observed molecular weight of Lamp1 in the Neu1-KO brain was shown to be higher than that in the WT brain, possibly due to glycosylation similar to that observed in Neu1-KO mice (Fig. 8g). These results suggested the possible induction of lysosome exocytosis and neuronal dysfunction in Neu1-KO zebrafish. To confirm the involvement of lysosomal exocytosis in zebrafish behavior, we exposed WT zebrafish to alarm substances, which is known to evoke an anxiety behavior and suppress bold behaviors, and evaluated the levels of the Lamp1 protein in the brain. We accordingly found that zebrafish exposed to alarm substances showed a significant upregulation of neu1 in the brain compared with non-exposed fish (p < 0.05, Fig. 8h). Moreover, the npy mRNA level in the treated zebrafish brain confirmed the induction of anxiety via the HPA axis (p < 0.05, Fig. 8i). In addition, we noted that the level of the Lamp1 protein in alarm substance-exposed zebrafish was significantly decreased compared with that in non-exposed zebrafish (42% decrease relative to non-exposed, p < 0.05, Fig. 8j and Supplementary Fig. 2). These results suggested that upregulation of the neu1 gene during zebrafish anxiety suppressed lysosomal exocytosis, leading to a reduction in exploratory/boldness behavior.
Changes in the expression of lysosomal genes in Neu1-KO zebrafish. mRNA levels of ctsa (a), glb1 (b), galns (c), tfeb (d), lamp1a (e), and lamp1b (f) in WT and Neu1-KO zebrafish. Each level of gene expression in Neu1-KO zebrafish is relative to the value in WT fish. n = 7 for each group. (g) Lysates of zebrafish brains were applied for western blotting using anti-lamp1 and anti-β actin antibodies. Quantitative analyses of the intensities of Lamp1 and β-actin bands were carried out and results are presented as Lamp1/β-actin. (h–j) WT zebrafish were exposed to alarm substances and then the neu1 (h) and npy mRNA levels (i) were estimated by real-time PCR. Lysates of zebrafish brains were applied for western blotting using anti-lamp1 and anti-β actin antibodies (j). Quantitative analyses of the intensities of Lamp1 and β-actin bands were carried out and results are presented as Lamp1/β-actin. n = 6 for each group. Results are shown as means ± standard deviation. All values were compared using Mann–Whitney U test. n.s., not significant.
Discussion
The deficiency in NEU1 sialidase is responsible for human sialidosis7. Although the mechanisms of muscle and bone dysfunctions in sialidosis have been clarified, the role of NEU1 in the regulation of emotional activity has not been explored to this day. We accordingly found that Neu1-KO zebrafish exhibited excess boldness/exploratory behavior in various tests, accompanied by a decrease in the expression of anxiety-related genes. In addition, we observed that lysosomal exocytosis was enhanced in Neu1-KO zebrafish.
First, the present study revealed the low shoaling of Neu1-KO zebrafish. The low shoaling behavior in Neu1-KO fish indicated an anxiety reduction. Moreover, Neu1-KO zebrafish exhibited a drastic alteration in the expression of emotional genes, especially in the HPA axis. In general, a decreased HPA axis has been considered as a low anxiety condition in zebrafish39. In addition, we noted that Neu1-KO zebrafish showed high interaction with the "unfamiliar" opponent in the mirror. As Neu1-KO zebrafish showed low aggression toward the unfamiliar zebrafish, the induction of a high interaction in the mirror test in Neu1-KO zebrafish was assumed to be caused by their increased interest toward the unfamiliar zebrafish. Neu1-KO zebrafish exhibited high interaction not only toward multiple unfamiliar zebrafish but also toward an unfamiliar fish species, cavefish. Interestingly, zebrafish are known to show strong avoidance behavior toward unfamiliar fish species40. Taken together, Neu1-KO zebrafish were determined to be bold, but not impulsive, because we did not observe any hyperactivity (enhancement of locomotion) in Neu1-KO zebrafish, as in the case of patients with sialidosis41. Chronic stress and dopamine/noradrenalin neurons are also known to be involved in boldness23; however, Neu1-KO zebrafish did not exhibit any alterations in the expression of tyrosine hydroxylase genes. Considering the instincts characterizing all living animals, excess boldness/exploratory behavior must be avoided to protect life. As such, anxiety-related genes play a role in interrupting the excessive activity under situations associated with potential danger. In this study, we found that Neu1-KO zebrafish showed increased interactions with unfamiliar zebrafish, different fish species, and white areas, without exhibiting aggression or anxiety, although these were factors that should have been handled with caution. Thus, we concluded that Neu1-KO zebrafish show abnormal behavior. Furthermore, induction of anxiety evoked the upregulation of neu1 in the zebrafish brain accompanied by reduced levels of Lamp1, suggesting that the significance of enhanced Neu1 and reduced lysosomal exocytosis might be to inhibit the boldness/exploratory activity under situations that require caution.
The level of Lamp1 was shown to be upregulated in the Neu1-KO zebrafish brain, and its molecular weight was found to be higher than that of WT, possibly due to glycosylation. Similarly, Neu1-KO mice have been reported to show excessive lysosomal exocytosis in neural cells accompanied by increased levels of Lamp117. Lysosomal exocytosis observed in NEU1 deficiency has been suggested to be deeply involved in the symptoms of patients with sialidosis and Neu1-KO mice, in contrast to other lysosomal diseases accompanied by lysosome expansion. Patients with sialidosis have been reported to show muscle hypotonia, similar to Neu1-KO mice with skeletal muscle atrophy accompanied by an expansion of the epimysial and perimysial spaces42. Neu1-deficiency has been shown to cause the production of excessive lysosomes that contain activated TGF-β and WNT/β-catenin signaling molecules, leading to the conversion of fibroblasts into myofibroblasts18. A previous study reported that upregulation of lamp1a occurred also in the muscles of Neu1-KO zebrafish26, suggesting the induction of exocytosis in the whole Neu1-KO zebrafish body. However, the involvement of lysosomal exocytosis in emotional dysregulation in Neu1-KO zebrafish remains unclear. Recently, the long-term structural plasticity of dendritic spines was reported to be regulated in the hippocampus by lysosomal exocytosis, accompanied by increased activity of cathepsin B43. One of the possible reasons behind the abnormal behavior of Neu1-KO zebrafish might be the dysfunction of short-term memory. More specifically, the behavior of animals expressing low exploratory activity in a novel tank exploration test and increased swimming speed in the mirror test, and not showing any fear response to unfamiliar fish species are thought to be related to the dysfunction of short-term memory44. Furthermore, the involvement of sialidase in hippocampal neuronal cells has also been explored. For instance, the activity of sialidase was shown to be increased in response to neuronal excitation in the rat hippocampus45. Moreover, increased release of sialic acid has been detected during in vivo microdialysis during fear conditioning. Furthermore, Neu1-KO mice were reported to accumulate amyloid-beta (Aβ) in the CA3 region of the hippocampus, induced by enhanced lysosomal exocytosis46. The accumulation of Aβ has been suggested to be involved in the development of Alzheimer’s disease, with impaired cognition being one of the major symptoms of the disease. In addition, the present study showed the abnormal accumulation of GM1 ganglioside in the brain of Neu1-KO zebrafish. In particular, GM1 is known to promote the accumulation of Aβ47. These findings suggested that Neu1-KO zebrafish might exhibit Alzheimer’s disease-like symptoms, inducing this abnormal behavior. Further studies are needed to clarify whether dysfunctions in short-term memory and memory maintenance are involved in the behavior of Neu1-KO zebrafish.
We did not observe any significant accumulation of sialoglycoproteins in Neu1-KO zebrafish brains, unlike Neu1-KO zebrafish larvae26. It should be mentioned that PSA is known to regulate neurogenesis, memory, and abnormal PSA profiles have been reported to induce autism spectrum and bipolar disorders21. We found that the levels of PSA in Neu1-KO zebrafish were not different from those in WT, although mammalian Neu1 is known to desialyze PSA. In contrast, Neu1-KO zebrafish were shown to accumulate some GSLs in the brain, such as GM1, GM3, LacCer, and Gb4. However, these accumulations in Neu1-KO zebrafish could not be caused by the deficiency of the activity of the Neu1 enzyme because gangliosides are not substrates for zebrafish Neu126. A similar accumulation of gangliosides has been found in the brains of mice in several lysosomal diseases48. For example, GM1, GM2, GM3, and GD3 were reported to be accumulated in Gaucher disease. Despite this finding, the relationship between gangliosides and abnormal animal behavior has not been fully clarified. Recently, the involvement of Neu3 and Neu4 in short-term memory was assessed using Neu3−/−/Neu4−/− double knockout mice49. The double-knockout mice showed impaired short-time memory accompanied by decreased accumulation of GM1 and GM3 in the brain. Furthermore, immunocytochemistry revealed that GM3 accumulated in the brains of double-knockout mice, mainly in the lysosomes of phagocytic cells, microglia, and pericytes in the brain. We observed here that the levels of neu3s (neu3.1, neu3.2, neu3.3, neu3.4, and neu3.5) and neu4 in the brain of Neu1-KO zebrafish were not changed compared with those in WT26; however, the accumulation of GM3 might have affected the behavior of Neu1-KO zebrafish, similar to Neu3−/−/Neu4−/− double-knockout mice. Interestingly, gangliosides have been found to not accumulate in the brain of Neu1-KO mice and patients with sialidosis50,51. A comparison of the composition of gangliosides in the brain between normal mice and zebrafish revealed that rich GM1 and little LacCer and GM3 were detected in the mouse brain, but not in zebrafish51,52. Such a difference might affect the accumulation pattern of gangliosides under a Neu1-deficiency.
In this study, we investigated the effects of Neu1 deficiency on emotional behavior in zebrafish. Since Neu1 sialidase is conserved in vertebrates, the phenotype observed in Neu1-KO zebrafish may be common in humans. However, several limitations need to be noted. First, the extent to which brain function is conserved between fish and mammals is controversial. For example, anxiety-related peptide hormones are highly conserved in vertebrates, but their expression patterns and the number of paralogous genes may not match between fish and mammals. Secondly, the distribution and structure of sialoglycans and their related enzymes in fish differ in part from that in humans. As mentioned above, the pattern of glycolipids in zebrafish also differs from that in humans. Humans possess four sialidase (Neu1, Neu2, Neu3, and Neu4), while zebrafish have six sialidases (Neu1, five Neu3 paralogs, and Neu4). To solve these limitations, it is highly desirable to elucidate the mechanism of abnormal behavior in Neu1-KO. Functional changes in central neurons caused by Neu1 deficiency should be investigated in zebrafish and mammals in comparison.
In summary, the present study revealed that Neu1 deficiency altered emotional behaviors, such as boldness and social preference, suggesting that Neu1-KO zebrafish could be used as a potential new emotional dysregulation model for the study of lysosomal desialylation. Sialoglycoconjugates involved in neuronal functions have attracted much attention, and Neu1 is the dominant sialidase in vertebrates. In addition, Neu1 is a negative regulator of lysosomal exocytosis. Therefore, Neu1-KO zebrafish might be a useful model for the study of diseases characterized by the involvement of lysosomal exocytosis.
Materials and methods
Fish
Neu1-KO zebrafish were established in our previous study26. Zebrafish larvae were kept in a 0.5 L tank and fed the paramecium, while juvenile and adult zebrafish were reared in a 3 L water aquarium and fed brine shrimp and a commercial diet (Otohime B2, Marubeni Nisshin Feed Co., Ltd, Tokyo, Japan) twice a day under a 14/10 h light and dark cycle at 28 °C until the analysis. Water quality conditions were kept as follows: pH 6.8–7.5, nitrate < 12.5 mg L−1, nitrite < 0.3 mg L−1, and chlorine < 0.1 mg L−1. As old Neu1-KO zebrafish (> 11 months) were shown to exhibit a decline in swimming ability due to spine curvature26, the present study employed young adult zebrafish (< 6 months) without symptoms of spine curvature. Mexican tetra (cavefish) were commercially obtained from a local market and kept under the same light/dark and feeding conditions as zebrafish.
All protocols for this study were approved by the Kagoshima University Committee for Animal Experiments and performed at the Faculty of Fisheries, Kagoshima University, in accordance with relevant guidelines and regulations. This study was carried out in compliance with the ARRIVE guidelines (http://www.nc3rs.org.uk/page.asp?id=1357). To minimize the effects of environmental changes to zebrafish emotional behaviors, the behavioral tests were conducted in the same room where the zebrafish breeding tank was set up.
Fish behavioral analysis
Normal swimming behavior
A fish was placed at the center of the tank, measuring 20 × 18.5 × 6 cm (length × width × height). After 5 min of acclimation, their behavior was recorded for 5 min using a digital video camera (HDR-CX430, Sony, Tokyo, Japan). The camera was positioned directly above the tank. The moving locus, speed, and total distance traveled were analyzed using the Move-tr/2D software (Library, Tokyo, Japan).
Shoaling behavior
The shoaling behavior of zebrafish was estimated with or without acclimation, as described elsewhere53, with slight modifications. Briefly, 5 fish were released in the center of the transparent tank (28 × 17.5 × 14 cm), and their behaviors were immediately recorded for 5 min (without acclimation). After the test, fish were kept in the same tank. Then, 30 min after the test, the behavior of fish was recorded again for 5 min (with acclimation). The camera was fixed at the side views of the swimming tanks. The fish-internal distances between 5 fish were estimated every 30 s. The horizontal line in the middle of the tank divided the top and the bottom of the tank. Fish number in the top was counted every 5 s during the shoaling test, and the averages were calculated.
Aggressive behavior
Aggressive behavior was estimated as described elsewhere54, with slight modifications. Briefly, 2 male zebrafish were released into a transparent tank (24.5 × 7.9 × 10 cm) and their behaviors were recorded with a digital camera for 10 min. Aggressive behaviors were defined as chasing with an increase in swimming speed directed towards the opponent, circling, and biting. Swimming acceleration, swimming distance, swimming speed, and moving locus were obtained from the first 1-min behavioral data. Aggressive behavior was estimated using a 10 min analysis. The camera was fixed at the side views of the swimming tanks.
Mirror test
These experiments were conducted according to a previous study23, with slight modifications. The tank was 5 cm high, 10 cm wide, and 24 cm long, and a mirror was placed on one side. A fish was placed at the center of the tank. The behavior of the fish was recorded for 15 min using a digital video camera and subsequently analyzed using the Move-tr/2D software. The camera was positioned directly above the tank. The area within 5 cm of the mirror was defined as the approach zone. The frequency and total time of invasion into the approach area, swimming distance, moving locus, and total time of chasing the opponent in the mirror were analyzed.
Three-chambers test
The experiment was conducted as described elsewhere55 with slight modifications. Two small chambers (9.1 × 5.3 × 5.5 cm) were placed on one side of the experimental tank (26.8 × 19.5 × 5.5 cm). These 2 small chambers were termed empty chamber and fish chamber, respectively. The area within 1 cm of the chamber was defined as the chamber area. Then, 2 male and 2 female zebrafish were placed in the fish chamber, whereas the other one was left empty. Each test fish was placed at the center of the experimental tank. After 5 min of acclimation, the swimming behaviors of the test fish were captured for 5 min using a video camera. The camera was positioned directly above the tank. The time spent in the chamber area, invasion into each area, total distance traveled, and moving locus were analyzed using the Move-tr/2D software. For the estimation of the behavior of zebrafish toward unfamiliar fish, 4 cavefish were set in the fish tank instead of 4 zebrafish, and the following steps were performed in the same manner as before.
Black–white preference test
The black–white preference test was conducted according to our previous study23 with slight modifications. The tank measured 23 × 13 × 6 cm was divided into 2 compartments (black and white background). A fish was placed in the black area of the tank, and the swimming behaviors of zebrafish were immediately captured using a video camera for 5 min. The camera was positioned directly above the tank. We measured the swimming time on the total time spent on the white side, the number of invasions into the white side, speed, and moving locus.
Real-time PCR
The mRNA expression levels of various genes were analyzed using cDNAs obtained from zebrafish brains. The brains of Neu1-KO and WT zebrafish were dissected after anesthesia with ice water. Total RNA was extracted from the zebrafish brain using Sepasol-RNA I Super G solution (Nacalai Tesque, Kyoto, Japan), followed by cDNA synthesis using ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan).
Real-time PCR was conducted by StepOne real-time PCR (Thermo Fisher Scientific, Waltham, MA) using KOD SYBR qPCR Master Mix (TOYOBO) and specific primers for genes of neu1, lysosomal-associated membrane proteins 1a and 1b (lamp1a and lamp1b, respectively), cathepsin A (ctsa), beta-galactosidase 1 (glb1), N-acetylgalactosamine-6-sulfatase (galns), transcription factor EB (tfeb), isotocin (ist), mineralocorticoid receptor (mr), melanin-concentrating hormone (mch), arginine vasotocin (avt), neuropeptide Y (npy), orexin (orx), and tyrosine hydroxylase 1 and 2 (th1 and th2, respectively; Supplementary Table 1). A standard curve for relative data quantification was derived from the serial dilutions of cDNA (Pffafl method). Normalization to the level of actb mRNA was done to compensate for the quality and quantity of mRNA in each sample.
Lectin and western blotting
Zebrafish were anesthetized in ice water, and brains were harvested. Brains were homogenized with 300 μL of 1% Triton X-100/phosphate-buffered saline (PBS) containing 10 μg mL−1 leupeptin, 1 mM ethylenediaminetetraacetic acid (EDTA), and 200 μM phenylmethanesulfonyl fluoride using a BioMasher (Nippi, Tokyo, Japan), followed by centrifugation at 13,200×g for 15 min. The obtained lysates were resolved by SDS-PAGE and then transferred onto PVDF membranes, followed by blocking with 1% bovine serum albumin (BSA). For glycoprotein detection, the membrane was incubated with biotin-labeled MAM (Maackia amurensis lectin, recognizing Siaα2-3Gal, 1/500 diluted, J-CHEMICAL, Tokyo, Japan) and SSA lectin (Sambucus Sieboldiana Lectin, recognizing Siaα2-6Gal, 1/500 diluted, J-CHEMICAL) and then reacted with HRP-streptavidin. Accordingly, polysialic acid, lamp1, and β-actin were detected by incubation with anti-polysialic acid monoclonal antibody (1/1000 diluted, clone 735, Abnova, Taipei, Taiwan), anti-lamp1 polyclonal antibody (1/1000 diluted, ab24170, Abcam, Cambridge, England), and anti-β-actin monoclonal antibody (1/1000 diluted, 66009-1-g, Proteintech, Rosemont, IL), respectively, followed by reaction with HRP-conjugated secondary antibodies. Detection of signals was carried out using ChemiDoc Touch (Bio-Rad, Hercules, CA) with an EzWestLumi plus chemiluminescence reagent (ATTO, Tokyo, Japan). Densitometric analysis was carried out using Image Lab Touch software (Bio-Rad).
Thin layer chromatography
Total lipids were extracted from the zebrafish brain using chloroform:methanol (1:2, 1:1, 2:1, v/v). Total lipids were fractionated into acid and neutral fractions using DEAE-Sephadex A-25 (acetate form, Sigma-Aldrich, St. Louis, MO). Each fraction was saponified with 0.2 N NaOH in methanol followed by desalting using a Sep-Pak C18 cartridge (GL Sciences, Tokyo, Japan). The obtained lipids were subjected to thin layer chromatography (TLC) plates and separated in a solvent system of chloroform/methanol/0.2% aqueous CaCl2 (55:45:10, v/v/v) for acid glycosphingolipids (GSLs) and chloroform/methanol/H2O (60:25:4, v/v/v) for neutral GSLs. The separated GSLs were visualized using orcinol-H2SO4. Densitometric analysis was carried out using Quantity One software (Bio-Rad).
Exposure of alarm substances
Alarm substances were prepared according to Quadros et al.56. Briefly, the scales were removed from zebrafish by scalpel and then homogenized in cold PBS. After centrifugation at 12,000×g, the obtained supernatant was used as alarm substances in the study. A fish was exposed to a tank filled with 2 L of water containing alarm substances (equivalent to 0.06 fish origin/2 L) for 15 min.
Data analysis
Group size used in this study was estimated using IBM SPSS statistics software (Armonk, NY). Results were expressed as means ± standard deviation, and all values were compared using Mann–Whitney U test.
Abbreviations
- GSLs:
-
Glycosphingolipids
- HPA:
-
Hypothalamic–pituitary–adrenal
- PSA:
-
Polysialic acid
- SAM:
-
Sympathetic–adrenal–medullary
- TLC:
-
Thin layer chromatography
References
Platt, F. M., d’Azzo, A., Davidson, B. L., Neufeld, E. F. & Tifft, C. J. Lysosomal storage diseases. Nat. Rev. Dis. Primers. 4, 27 (2018).
Caciotti, A. et al. Type I sialidosis, a normosomatic lysosomal disease, in the differential diagnosis of late-onset ataxia and myoclonus: An overview. Mol. Genet. Metab. 129, 47–58 (2020).
Caciotti, A. et al. Type II sialidosis: review of the clinical spectrum and identification of a new splicing defect with chitotriosidase assessment in two patients. J. Neurol. 256, 1911–1915 (2009).
Spranger, J. & Cantz, M. Mucolipidosis I, the cherry red-spot myoclonus syndrome and neuraminidase deficiency. Birth Defects Orig. Artic. Ser. 14, 105–112 (1978).
Paschke, E. et al. Infantile type of sialic acid storage disease with sialuria. Clin. Genet. 29, 417–424 (1986).
Mitsiakos, G., Gialamprinou, D., Chouchou, P., Chatziioannidis, I. & Karagianni, P. Identification of a homozygous deletion of the NEU1 gene in a patient with type II sialidosis presenting isolated fetal ascites and central nervous system hypoplasia. Hippokratia 23, 169–171 (2019).
Miyagi, T., Takahashi, K., Hata, K., Shiozaki, K. & Yamaguchi, K. Sialidase significance for cancer progression. Glycoconj. J. 29, 567–577 (2012).
Uemura, T. et al. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene 28, 1218–1229 (2009).
Alghamdi, F. et al. A novel insulin receptor-signaling platform and its link to insulin resistance and type 2 diabetes. Cell. Signal. 26, 1355–1368 (2014).
Hinek, A., Bodnaruk, T. D., Bunda, S., Wang, Y. & Liu, K. Neuraminidase-1, a subunit of the cell surface elastin receptor, desialylates and functionally inactivates adjacent receptors interacting with the mitogenic growth factors PDGF-BB and IGF-2. Am. J. Pathol. 173, 1042–1056 (2008).
Ahn, J. H. et al. Type 1 sialidosis patient with a novel deletion mutation in the NEU1 gene: Case report and literature review. Cerebellum 18, 659–664 (2019).
Itoh, K. et al. Novel missense mutations in the human lysosomal sialidase gene in sialidosis patients and prediction of structural alterations of mutant enzymes. J. Hum. Genet. 47, 29–37 (2002).
Lukong, K. E. et al. Mutations in sialidosis impair sialidase binding to the lysosomal multienzyme complex. J. Biol. Chem. 276, 17286–17290 (2001).
Pattison, S., Pankarican, M., Rupar, C. A., Graham, F. L. & Igdoura, S. A. Five novel mutations in the lysosomal sialidase gene (NEU1) in type II sialidosis patients and assessment of their impact on enzyme activity and intracellular targeting using adenovirus-mediated expression. Hum. Mutat. 23, 32–39 (2004).
Rottier, R. J., Bonten, E. & d’Azzo, A. A point mutation in the neu-1 locus causes the neuraminidase defect in the SM/J mouse. Hum. Mol. Genet. 7, 313–321 (1998).
de Geest, N. et al. Systemic and neurologic abnormalities distinguish the lysosomal disorders sialidosis and galactosialidosis in mice. Hum. Mol. Genet. 11, 1455–1464 (2002).
Yogalingam, G. et al. Neuraminidase 1 is a negative regulator of lysosomal exocytosis. Dev. Cell 15, 74–86 (2008).
van de Vlekkert, D. et al. Excessive exosome release is the pathogenic pathway linking a lysosomal deficiency to generalized fibrosis. Sci. Adv. 5, eaav3270 (2019).
Sun, A. Lysosomal storage disease overview. Ann. Transl. Med. 6, 476 (2018).
Staretz-Chacham, O., Choi, J. H., Wakabayashi, K., Lopez, G. & Sidransky, E. Psychiatric and behavioral manifestations of lysosomal storage disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153, 1253–1265 (2010).
Schnaar, R. L., Gerardy-Schahn, R. & Hildebrandt, H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 94, 461–518 (2014).
Dreosti, E., Lopes, G., Kampff, A. R. & Wilson, S. W. Development of social behavior in young zebrafish. Front. Neural Circuits 9, 39 (2015).
Shiozaki, K. et al. Neuropeptide Y deficiency induces anxiety-like behaviours in zebrafish (Danio rerio). Sci. Rep. 10, 5913 (2020).
Honda, A. et al. Novel Nile tilapia Neu1 sialidases: Molecular cloning and biochemical characterization of the sialidases Neu1a and Neu1b. Gene 742, 144538 (2020).
Ryuzono, S. et al. Lysosomal localization of Japanese medaka (Oryzias latipes) Neu1 sialidase and its highly conserved enzymatic profiles with human. Gene 575, 513–523 (2016).
Okada, K. et al. Establishment and characterization of Neu1-knockout zebrafish and its abnormal clinical phenotypes. Biochem. J. 477, 2841–2857 (2020).
Shiozaki, K. et al. Identification of novel fish sialidase genes responsible for KDN-cleaving activity. Glycoconj. J. 37, 745–753 (2020).
Buske, C. & Gerlai, R. Shoaling develops with age in Zebrafish (Danio rerio). Prog. Neuro-Psychopharmacol. Biol. Psychiatry 35, 1409–1415 (2011).
Laryea, G., Schütz, G. & Muglia, L. J. Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Mol. Endocrinol. 27, 1655–1665 (2013).
Winkler, Z. et al. Hypoglycemia-activated hypothalamic microglia impairs glucose counterregulatory responses. Sci. Rep. 9, 6224 (2019).
Chen, Y. C., Priyadarshini, M. & Panula, P. Complementary developmental expression of the two tyrosine hydroxylase transcripts in zebrafish. Histochem. Cell Biol. 132, 375–381 (2009).
Zimmermann, F. F., Gaspary, K. V., Siebel, A. M. & Bonan, C. D. Oxytocin reversed MK-801-induced social interaction and aggression deficits in zebrafish. Behav. Brain Res. 311, 368–374 (2016).
Larson, E. T., O’Malley, D. M. & Melloni, R. H. Aggression and vasotocin are associated with dominant-subordinate relationships in zebrafish. Behav. Brain Res. 167, 94–102 (2006).
Sumida, M. et al. Rapid trimming of cell surface polysialic acid (PolySia) by exovesicular sialidase triggers release of preexisting surface neurotrophin. J. Biol. Chem. 290, 13202–13214 (2015).
Sajo, M. et al. Neuraminidase-dependent degradation of polysialic acid is required for the lamination of newly generated neurons. PLoS ONE 11, e0146398 (2016).
Bonten, E. J., Annunziata, I. & d’Azzo, A. Lysosomal multienzyme complex: pros and cons of working together. Cell. Mol. Life Sci. 71, 2017–2032 (2014).
Annunziata, I. et al. MYC competes with MiT/TFE in regulating lysosomal biogenesis and autophagy through an epigenetic rheostat. Nat. Commun. 10, 3623 (2019).
Magini, A. et al. TFEB activation promotes the recruitment of lysosomal glycohydrolases β-hexosaminidase and β-galactosidase to the plasma membrane. Biochem. Biophys. Res. Commun. 440, 251–257 (2013).
Du, W., Chen, X., Shi, M., Bian, F. & Zhao, Z., Ethanol affects behavior and HPA axis activity during development in zebrafish larvae. Sci. Rep. 10, 21402 (2020).
Stewart, W. J., Cardenas, G. S. & McHenry, M. J. Zebrafish larvae evade predators by sensing water flow. J. Exp. Biol. 216, 388–398 (2013).
Burton, B. K. & Giugliani, R. Diagnosing Hunter syndrome in pediatric practice: practical considerations and common pitfalls. Eur. J. Pediatr. 171, 631–639 (2012).
Zanoteli, E. et al. Muscle degeneration in neuraminidase 1-deficient mice results from infiltration of the muscle fibers by expanded connective tissue. Biochim. Biophys. Acta Mol. Basis Dis. 1802, 659–672 (2010).
Padamsey, Z. et al. Activity-dependent exocytosis of lysosomes regulates the structural plasticity of dendritic spines. Neuron 93, 132–146 (2017).
Lai, Y. H. et al. Duplicated dnmt3aa and dnmt3ab DNA methyltransferase genes play essential and non-overlapped functions on modulating behavioral control in zebrafish. Genes (Basel). 11, 1322 (2020).
Minami, A. et al. Rapid regulation of sialidase activity in response to neural activity and sialic acid removal during memory processing in rat hippocampus. J. Biol. Chem. 292, 5645–5654 (2017).
Annunziata, I. et al. Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid-β secretion via deregulated lysosomal exocytosis. Nat. Commun. 4, 2734 (2013).
Cebecauer, M., Hof, M. & Amaro, M. Impact of GM1 on membrane-mediated aggregation/oligomerization of β-amyloid: Unifying view. Biophys. J. 113, 1194–1199 (2017).
Walkley, S. U. Secondary accumulation of gangliosides in lysosomal storage disorders. Semin. Cell Dev. Biol. 15, 433–444 (2004).
Pan, X. et al. Neuraminidases 3 and 4 regulate neuronal function by catabolizing brain gangliosides. FASEB J. 31, 3467–3483 (2017).
Ulrich-Bott, B., Klem, B., Kaiser, R., Spanger, J. & Cantz, M. Lysosomal sialidase deficiency: increased ganglioside content in autopsy tissues of a sialidosis patient. Enzyme 38, 262–266 (1987).
Timur, Z. K., Akyildiz Demir, S., Marsching, C., Sandhoff, R. & Seyrantepe, V. Neuraminidase-1 contributes significantly to the degradation of neuronal B-series gangliosides but not to the bypass of the catabolic block in Tay-Sachs mouse models. Mol. Genet. Metab. Rep. 4, 72–82 (2015).
Viljetić, B., Labak, I., Majić, S., Štambuk, A. & Heffer, M. Distribution of mono-, di- and trisialo gangliosides in the brain of Actinopterygian fishes. Biochim. Biophys. Acta Gen. Subj. 1820, 1437–1443 (2012).
Buske, C. & Gerlai, R. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicol. Teratol. 33, 698–707 (2011).
Colman, J. R., Baldwin, D., Johnson, L. L. & Scholz, N. L. Effects of the synthetic estrogen, 17α-ethinylestradiol, on aggression and courtship behavior in male zebrafish (Danio rerio). Aquat. Toxicol. 91, 346–354 (2009).
Ribeiro, D. et al. Oxytocin receptor signalling modulates novelty recognition but not social preference in zebrafish. J. Neuroendocrinol. 32, e12834 (2020).
Quadros, V. A. et al. Strain- and context-dependent behavioural responses of acute alarm substance exposure in zebrafish. Behav. Process. 122, 1–11 (2016).
Acknowledgements
We appreciate the technical assistance of Daichi Sahashi, Yoichiro Hokazono, Akinobu Honda, Kazuki Oishi, Yurina Kubo, Hinako Takeno, Ryo Takase, Kazuhiro Tani, and Shoji Kodama in Kagoshima University.
Author information
Authors and Affiliations
Contributions
A.I. and K.O. estimated gene expressions; A.I. performed western blotting and lectin blotting; A.I., M.Komamizu, A.H., K.O., M.Kawabe and C.Y. estimated zebrafish behavior; AI, M.Komamizu and K.S. analyzed the data; A.I. and K.S. wrote the paper; M.Komatsu and K.S. designed the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ikeda, A., Komamizu, M., Hayashi, A. et al. Neu1 deficiency induces abnormal emotional behavior in zebrafish. Sci Rep 11, 13477 (2021). https://doi.org/10.1038/s41598-021-92778-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-021-92778-9
This article is cited by
-
Lactic acid-fermented by-product of Shochu distillery reduces anxiety behavior in Neuropeptide Y knockout zebrafish by the regulation of isotoin neuron
Journal of Material Cycles and Waste Management (2024)
-
Non-steroidal anti-inflammatory drug target gene associations with major depressive disorders: a Mendelian randomisation study integrating GWAS, eQTL and mQTL Data
The Pharmacogenomics Journal (2023)
-
Increased α-2,6 sialic acid on microglia in amyloid pathology is resistant to oseltamivir
GeroScience (2023)
-
Alteration of the neuronal and glial cell profiles in Neu1-deficient zebrafish
Glycoconjugate Journal (2022)