Abstract
Indications of extracorporeal cardiopulmonary resuscitation (ECPR) are still debatable, particularly in patients with cancer. Prediction of the prognosis of in-hospital cardiac arrest (IHCA) in patients with cancer receiving ECPR is important given the increasing prevalence and survival rate of cancer. We compared the neurologic outcomes and survival rates of IHCA patients with and without cancer receiving ECPR. Data from the extracorporeal membrane oxygenation registry between 2015 and 2019 were used in a retrospective manner. The primary outcome was 6-month good neurologic outcome, defined as a Cerebral performance category score of 1 or 2. The secondary outcomes were 1- and 3-month good neurologic outcome, and 6-month survival. Among 247 IHCA patients with ECPR, 43 had active cancer. The 6-month good neurologic outcome rate was 27.9% and 32.4% in patients with and without active cancer, respectively (P > 0.05). Good neurologic outcomes at 1-month (30.2% vs. 20.6%) and 3-month (30.2% vs. 28.4%), and the survival rate at 6-month (39.5% vs. 36.5%) were not significantly different (all P > 0.05) Active cancer was not associated with 6-month good neurologic outcome by logistic regression analyses. Therefore, patients with IHCA should not be excluded from ECPR solely for the presence of cancer itself.
Similar content being viewed by others
Introduction
With the implementation of extracorporeal cardiopulmonary resuscitation (ECPR) as a rescue therapy for patients with refractory cardiac arrest becoming widespread, both the survival rate and neurologic outcome have steadily increased over the last decades1,2,3,4. In patients who undergo refractory cardiac arrest, ECPR assists with rapid restoration of perfusion, leading to improved survival5,6. However, ECPR has only been adopted for selected patients as recommended by the American Heart Association guidelines due to its high cost and need for many trained personnel7,8.
Meanwhile, as the survival rate of patients with cancer has increased, so has the rate of admission due to the intensive and continued care of patients, and some eventually suffer from in-hospital cardiac arrest (IHCA)9,10,11. Despite the fact that patients with cancer account for 14% of patients with IHCA, according to the study12, they receive less intensive treatment because of the risk of acquired coagulopathy and the uncertainty of the long-term outcomes; furthermore, their prognosis is poor compared to those without cancer13,14,15.
Although cancer is traditionally considered as one of the contraindications for extracorporeal membrane oxygenation (ECMO)16, considering the increasing survival rate and the fact that patients with cancer represent a considerable portion of IHCA cases, it is reasonable to consider implementing ECPR in these cases, particularly based on advance directives and anticipated life expectancy. While some studies have shown increased survival of patients with cancer using ECMO or extracorporeal life support (ECLS), the majority are targeted to paediatric patients, hematologic malignancies, or acute respiratory failure without cardiac arrest17,18,19,20,21; therefore, it remains unclear whether ECPR could benefit patients with cancer who suffer IHCA.
In the current study, we investigated the neurologic outcomes and survival rates of adult patients with cancer who underwent IHCA and who received ECPR; the outcomes of these patients were compared with those of adults without cancer who suffered IHCA and received ECPR.
Results
Baseline characteristics
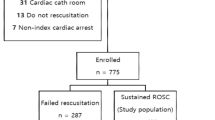
Using data from the ECMO registry, 977 patients from January 2015 to December 2019 were reviewed. Among these patients, we excluded 462 who only received ECMO and not ECPR, 229 patients who received veno-veno ECMO, and 39 patients who received ECPR in an OHCA setting. Finally, 247 patients were included for final analysis; among them, 43 patients had active cancer at the time of arrest, and the remaining 204 patients had no cancer. And the details in patients with active cancer are demonstrated in supplementary Fig. 1.
The characteristics of the subjects, including age, sex, obesity, comorbidities, and laboratory data on the day of arrest, are listed by group (active cancer and cancer-free) in Table 1. The prearrest CPC score, proportion of male sex, obesity, laboratory findings on the day of arrest (except for creatinine), and medical history, including cardiac arrest, acute myocardial infarction, hypertension, diabetes, cerebrovascular accident, liver cirrhosis, and transplantation, were not significantly different between the two groups. The patients with active cancer were significantly older, and the proportion of angina, arrhythmia, heart failure, percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), and chronic kidney disease (CKD) was higher in cancer-free patients (P < 0.05).
The ECPR-related variables, including the location of arrest, presumed cause of arrest, total resuscitation time, and post-ECPR management, are shown in Table 2. Among the patients with active cancer, the ward was the most common location of arrest, but the ICU was most common in cancer-free patients. The proportion of cardiovascular aetiologies was significantly higher in cancer-free patients, while patients with active cancer had a higher proportion of respiratory aetiologies (Fig. 1). The total resuscitation time, total epinephrine dose, and the proportion of initial shockable rhythm, TTM, PCI, CABG, valve surgery, embolectomy, and use of vasopressors were not significantly different between the two groups. However, the cancer-free patients had a longer ECMO duration and received more renal replacement therapy.
Good neurologic outcome at 6 months (primary outcome)
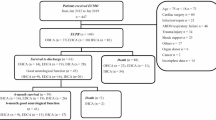
With regard to good neurologic outcome at 6 months from the day of arrest, 12 patients with active cancer (27.9%; 95% CI, 15.3%–43.7%) had a good neurologic status, , whereas 66 patients without cancer (32.4%; 95% CI, 26.0%–39.2%) were found to have a good neurologic status (P = 0.569) (Fig. 2a). Results after matching were in the same manner with the results without matching, in which patients with active cancer (28.2%; 95% CI, 15.0%–44.9%) showed a good neurologic status and patients without cancer (30.8%; 95% CI, 17.0%–47.6%) had a good neurologic status (P = 0.804) (Fig. 2b).
Good neurologic outcomes at 1 and 3 months, and the 6-month survival rate (secondary outcomes)
Likewise, regardless of the matching there was no significant difference between patients with active cancer and those without with regard to the rate of good neurologic outcomes at 1 month (without matching; 30.2% vs. 20.6%, P = 0.167, with matching; 30.8% vs. 17.9%, P = 0.187) and 3 months (without matching; 30.2% vs. 28.4%, P = 0.813, with matching; 30.8% vs. 23.1%, P = 0.444) (Fig. 2). However, by comparing the primary outcome, the rate of good neurologic outcomes in patients with active cancer was shown to decrease over time, whereas the rate in cancer-free patients steadily increased.
Seventeen patients with active cancer and 75 cancer-free patients survived until 6 months after the day of the arrest (39.5%; 95% CI, 24.0%–55.0% and 36.8%; 95% CI, 30.0%–43.0%, respectively); there was no significant difference between the two groups (P = 0.900 by log-rank test and hazard ratio 0.974; 95% CI, 0.639–1.485) (Figs. 2 and 3).
Good neurologic outcome at 6 months based on the cause of arrest
Acute coronary syndrome as a presumed cause of arrest showed the highest good neurologic outcomes at 6 months in both groups. Over 30% of cancer-free patients with presumed cardiac causes had good neurologic outcomes at 6 months; however, patients with active cancer having presumed cardiac aetiologies had variable outcomes, ranging from 14 to 50%. Other causes of arrest, such as acute aortic syndrome, bleeding, and pulmonary thromboembolism, had lower rates of good neurologic outcome compared to cardiac causes (Supplementary Fig. 2).
Association between active cancer and good neurologic outcome at 6 months
After dichotomizing patients into two groups, with and without good neurologic outcome at 6 months, univariable logistic regression analyses demonstrated that the presence of active cancer was not associated with good neurologic outcome at 6 months. Furthermore, multivariable logistic regression analysis, using the presence of active cancer and variables with P-values < 0.1, showed that the prearrest CPC score, initial shockable rhythm, total resuscitation time, and initial lactate were independently associated with 6-month good neurologic outcome, whereas the presence of cancer was also shown to have no association with 6-month good neurologic outcome (Table 3).
Methods
Data sources
We retrospectively reviewed the electronic medical records of all consecutive adult patients in the ECMO registry from January 2015 to December 2019 in Asan Medical Center, which serves as a tertiary referral centre. The ECMO registry is a database of adult patients who received ECMO regardless of the type. The current study protocol was approved by the Institutional Review Board of Asan Medical Center, and the requirement for informed consent was waived because of the retrospective nature of the analyses. All methods were carried out in accordance with relevant guidelines and regulations.
Study population
Among all consecutive adult patients from the ECMO registry from January 2015 to December 2019, we first excluded patients treated with ECMO in a non-arrest situation, those who received veno-veno ECMO, and those who received ECPR for out-of-hospital cardiac arrest (OHCA). The remaining patients with IHCA who were treated with ECPR were reviewed and divided into two groups based on the presence or absence of active cancer. Active cancer was defined according to the Haemostasis and Malignancy Scientific and Standardization Committee definition as follows: (1) Cancer diagnosed within the previous 6 months; (2) recurrent, regionally advanced, or metastatic cancer; (3) cancer for which treatment had been administered within the previous 6 months; and (4) haematological cancer that is not in complete remission22.
Outcomes
The primary outcome was a good neurologic status at 6 months from the day of IHCA, defined as 1 or 2 on the Cerebral Performance Category (CPC) score, at which stage patients are able to perform daily activities independently and are able to work in a sheltered environment. The secondary outcomes were a good neurologic status, defined in the same manner as in the primary outcome, at 1 and 3 months from the day of arrest. The survival rate at 6-month was also evaluated. In addition, by investigating the presumed cause of arrest, we compared the rate of 6-month good neurologic status between the two groups.
Statistical analysis
Data were first tested for normality. Continuous variables with normal distributions are presented as mean ± standard deviation, and those with non-normal distributions are expressed as median ± interquartile range (IQR). Categorical variables are presented as n (%). Continuous variables were compared using the Student’s t-test and Mann–Whitney U test as appropriate, and categorical variables were compared using the chi-square test and Fisher’s exact test accordingly.
With regard to good neurologic outcomes, the CPC score was dichotomized into two types, good (CPC 1 or 2) and poor (CPC 3–5) neurologic status, expressed as percentage with 95% confidence interval, and compared using a chi-square test. Propensity matching was also conducted with age, gender, comorbidities, presumed cause of arrest, and total resuscitation time, followed by comparing between those with cancer and those without. The cumulative survival rates of the two groups are presented with Kaplan–Meier curves and were compared by the log-rank test and the hazard ratio.
Univariable and multivariable logistic regression analyses about good neurologic outcome at 6 months were also performed to determine whether active cancer is independently associated with 6-month good neurologic outcome. All statistical analyses were performed using IBM SPSS Statistics V21.0 (SPSS Inc, Chicago, IL). P-values < 0.05 were considered statistically significant.
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Asan Medical Center (2021–0115) which waived the requirement for patient informed consent.
Discussion
In this study, we aimed to establish the short- and long-term good neurologic outcomes for patients who undergo IHCA and receive ECPR, specifically those for patients with active cancer, by comparing them with cancer-free patients. We aimed to show that patients with active cancer should not be excluded from receiving ECPR solely because of the presence of active cancer.
Of the 247 patients included in this study, 43 patients had active cancer, and the remaining 204 patients had no cancer. Respiratory causes of IHCA were higher in patients with active cancer, and cardiovascular causes were higher in cancer-free patients. Comparing patients with active cancer and patients without cancer, we found no significant difference in good neurologic outcomes at 1 month (30.2% vs. 20.6%), 3 months (30.2% vs. 28.4%), and 6 months (27.9% vs. 32.4%) from the day of arrest. Likewise, the survival rate at 6 months from the day of arrest was not significantly different between the two groups (P = 0.900).
No previous study has examined the good neurologic outcomes and survival rate of patients with active cancer who suffer IHCA and receive ECPR. Unlike traditional consensus, our study is considered to provide evidence for the implementation of ECPR to broaden its inclusion criteria, especially patients with active cancer who have been growing in number over the past few decades.
Previous studies have examined the survival to hospital discharge between patients with and without cancer14,23. Both of the two previous studies showed that patients with cancer had a lower survival rate than those without (31% vs. 46%), which is inconsistent with the results of our study, although Kang et al.23 investigated patients who underwent OHCA. Lower use of post-cardiac arrest management, such as angiography, PCI, TTM, and a smaller proportion of initial shockable rhythm in patients with cancer, was considered to be the major reason for the differences in survival rates; in support of this explanation, previous studies demonstrated that an initial shockable rhythm was associated with a higher rate of PCI due to its high likelihood of cardiac origin, which PCI could benefit24,25. However, our study showed no significant difference in the proportion of initial shockable rhythm and post-cardiac arrest management such as TTM, PCI, CABG, valve surgery, embolectomy, and use of vasopressors, except for renal replacement therapy and heart transplantation. Considering our results, it is reasonable to consider that offering proper post-cardiac arrest management, including ECPR, would increase the proportion of good neurologic outcomes and the survival rate of patients with active cancer to a level similar to that of patients without cancer. This is supported by the study of Champigneulle et al.26, who demonstrated that the 6-month survival rate was significantly different in the unmatched comparison of patients with and without malignancies who underwent cardiac arrest, but not in the matched comparison, although they did not focus on patients who received ECPR.
With regard to the rate of good neurologic outcomes over time, the rate in patients with active cancer decreased from 1 to 6 months following arrest, but cancer-free patients showed a steady increase in percentage. Although it seems natural that the rate of good neurologic outcome tend to decrease over follow-up, like the rate of patients with active cancer, as shown in a study by Meng-Rui et al.27, the result of cancer-free patients in our study contradicts previous studies5,28. This can be explained by the rate of heart transplantation that was performed in cancer-free patients. Generally, ECMO is considered as a bridge to heart transplantation for patients with decompensated heart failure29, which has a good long-term favourable outcome. As cancer-free patients had undergone more heart transplantation surgery than patients with active cancer (11.8% vs. 0.0%), this might have affected the long-term favourable neurologic outcomes. In addition, cardiovascular aetiologies that are found more frequently in cancer-free patients compared to patients with active cancer are also considered good prognostic factors.
The limitations of the current study mainly relate to its retrospective nature; with retrospective studies, there is always the possibility that selection bias may have influenced the results, particularly in the indication of ECPR in patients with active cancer. Secondly, the relatively small sample size led to the failure to demonstrate an association between the presence of active cancer and outcomes. A further non-inferiority trial will be needed to confirm our results. Thirdly, it was done by a single centre, which does not guarantee the adaptation of the generalized population. As a different environment could also make the result different, the results, therefore, should be interpreted with caution. Lastly, in terms of cost–benefit of ECPR in patients with active cancer, quality-adjusted life year (QALY) should be considered since it is not effective when malignancy itself which truncates patients’ life expectancy outweighs the benefit of ECPR which might increase QALY to some extent. However, we did not conduct cost–benefit analysis due to lack of data about the expenses for ECPR. Therefore, further investigation is needed in light of the total cost for ECPR and the benefit of QALY.
Conclusion
Patients with active cancer who suffer IHCA and undergo ECPR show similar 6-month good neurologic outcome and survival rate in comparison with patients without cancer. Therefore, ECPR should not be excluded as a treatment option solely on the basis of the presence of active cancer.
References
Richardson, A. S. et al. ECMO Cardio-Pulmonary Resuscitation (ECPR), trends in survival from an international multicentre cohort study over 12-years. Resuscitation 112, 34–40. https://doi.org/10.1016/j.resuscitation.2016.12.009 (2017).
Sakamoto, T. et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: A prospective observational study. Resuscitation 85, 762–768. https://doi.org/10.1016/j.resuscitation.2014.01.031 (2014).
Kagawa, E. et al. Assessment of outcomes and differences between in- and out-of-hospital cardiac arrest patients treated with cardiopulmonary resuscitation using extracorporeal life support. Resuscitation 81, 968–973. https://doi.org/10.1016/j.resuscitation.2010.03.037 (2010).
Lunz, D. et al. Extracorporeal membrane oxygenation for refractory cardiac arrest: A retrospective multicenter study. Intens. Care Med. 46, 973–982. https://doi.org/10.1007/s00134-020-05926-6 (2020).
Kim, S. J., Kim, H. J., Lee, H. Y., Ahn, H. S. & Lee, S. W. Comparing extracorporeal cardiopulmonary resuscitation with conventional cardiopulmonary resuscitation: A meta-analysis. Resuscitation 103, 106–116. https://doi.org/10.1016/j.resuscitation.2016.01.019 (2016).
Kehrl, T. & Kaczorowski, D. J. Extracorporeal life support for cardiopulmonary resuscitation for adults: Evolving evidence. Asaio J. 62, 364–369. https://doi.org/10.1097/mat.0000000000000358 (2016).
Panchal, A. R. et al. Part 3: Adult basic and advanced life support: 2020 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 142, S366-s468. https://doi.org/10.1161/cir.0000000000000916 (2020).
Gravesteijn, B. Y. et al. Cost-effectiveness of extracorporeal cardiopulmonary resuscitation after in-hospital cardiac arrest: A Markov decision model. Resuscitation 143, 150–157. https://doi.org/10.1016/j.resuscitation.2019.08.024 (2019).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30. https://doi.org/10.3322/caac.21442 (2018).
Lee, H. S. et al. Trends in receiving chemotherapy for advanced cancer patients at the end of life. BMC Palliat. Care 14, 4. https://doi.org/10.1186/s12904-015-0001-7 (2015).
Earle, C. C. et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue?. J. Clin. Oncol. 26, 3860–3866. https://doi.org/10.1200/jco.2007.15.8253 (2008).
Bruckel, J. T., Wong, S. L., Chan, P. S., Bradley, S. M. & Nallamothu, B. K. Patterns of Resuscitation Care and Survival After In-Hospital Cardiac Arrest in Patients With Advanced Cancer. J Oncol Pract 13, e821–e830. https://doi.org/10.1200/jop.2016.020404 (2017).
Miller, A. H., Sandoval, M., Wattana, M., Page, V. D. & Todd, K. H. Cardiopulmonary resuscitation outcomes in a cancer center emergency department. Springerplus 4, 106. https://doi.org/10.1186/s40064-015-0884-z (2015).
Guha, A. et al. Contemporary impacts of a cancer diagnosis on survival following in-hospital cardiac arrest. Resuscitation 142, 30–37. https://doi.org/10.1016/j.resuscitation.2019.07.005 (2019).
Fu, S. et al. Outcome analyses after the first admission to an intensive care unit in patients with advanced cancer referred to a phase I clinical trials program. J. Clin. Oncol. 29, 3547–3552. https://doi.org/10.1200/jco.2010.33.3823 (2011).
Kelly, B. & Carton, E. Extended indications for extracorporeal membrane oxygenation in the operating room. J. Intens. Care Med. 35, 24–33. https://doi.org/10.1177/0885066619842537 (2020).
Banfi, C. et al. Central extracorporeal life support in pheochromocytoma crisis. Ann. Thorac. Surg. 93, 1303–1305. https://doi.org/10.1016/j.athoracsur.2011.09.018 (2012).
Gow, K. W. et al. Extracorporeal life support for support of children with malignancy and respiratory or cardiac failure: The extracorporeal life support experience. Crit. Care Med. 37, 1308–1316. https://doi.org/10.1097/CCM.0b013e31819cf01a (2009).
Choi, K. B. et al. Extracorporeal life support in patients with Hematologic Malignancies: A single center experience. Korean J. Thorac Cardiovasc. Surg. 49, 280–286. https://doi.org/10.5090/kjtcs.2016.49.4.280 (2016).
Wohlfarth, P. et al. Extracorporeal membrane oxygenation in adult patients with hematologic malignancies and severe acute respiratory failure. Crit. Care 18, R20. https://doi.org/10.1186/cc13701 (2014).
Wu, M.Y. et al. The feasibility of venovenous extracorporeal life support to treat acute respiratory failure in adult cancer patients. Med. (Baltimore) 94, 893, https://doi.org/10.1097/md.0000000000000893 (2015).
Khorana, A. A. et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost 16, 1891–1894. https://doi.org/10.1111/jth.14219 (2018).
Kang, S. B. et al. Effect of cancer history on post-resuscitation treatments in out-of-hospital cardiac arrest. Resuscitation 137, 61–68. https://doi.org/10.1016/j.resuscitation.2019.02.005 (2019).
Dumas, F. et al. Emergency percutaneous coronary intervention in post-cardiac arrest patients without ST-Segment Elevation Pattern: Insights from the PROCAT II registry. JACC Cardiovasc. Interv. 9, 1011–1018. https://doi.org/10.1016/j.jcin.2016.02.001 (2016).
Noc, M. et al. Invasive coronary treatment strategies for out-of-hospital cardiac arrest: a consensus statement from the European association for percutaneous cardiovascular interventions (EAPCI)/stent for life (SFL) groups. EuroIntervention 10, 31–37. https://doi.org/10.4244/eijv10i1a7 (2014).
Champigneulle, B. et al. What is the outcome of cancer patients admitted to the ICU after cardiac arrest? Results Multicenter Study. Resuscitation 92, 38–44. https://doi.org/10.1016/j.resuscitation.2015.04.011 (2015).
Lee, M.-R. et al. Outcome of stage IV cancer patients receiving in-hospital cardiopulmonary resuscitation: A population-based cohort study. Sci. Rep. 9, 9478. https://doi.org/10.1038/s41598-019-45977-4 (2019).
Siao, F. Y. et al. Can we predict patient outcome before extracorporeal membrane oxygenation for refractory cardiac arrest?. Scand. J. Trauma Resusc. Emerg. Med. 28, 58. https://doi.org/10.1186/s13049-020-00753-6 (2020).
Poptsov, V., Spirina, E., Dogonasheva, A. & Zolotova, E. Five years’ experience with a peripheral veno-arterial ECMO for mechanical bridge to heart transplantation. J. Thorac. Dis. 11, S889-s901. https://doi.org/10.21037/jtd.2019.02.55 (2019).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Y.S.S and W.Y.K contributed to the idea and design of this study; P.J.K, Y.J.K, and S.M.R collected data and undertook the data analysis; Y.S.S wrote the draft; and S.H.J, S.B.H, and W.Y.K revised the manuscript; all authors have seen and approved the final version of the report.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shin, Y.S., Kang, PJ., Kim, YJ. et al. The feasibility of extracorporeal cardiopulmonary resuscitation for patients with active cancer who undergo in-hospital cardiac arrest. Sci Rep 12, 1653 (2022). https://doi.org/10.1038/s41598-022-05786-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-05786-8