Abstract
Wheezing diseases are one of the major chronic respiratory diseases in children. To explore the effects of meteorological and environmental factors on the prevalence of children wheezing diseases, clinical data of children hospitalized with wheezing diseases in Suzhou, China from 2013 to 2017 were collected. Meteorological and environmental factors from 2013 to 2017 were obtained from the local Meteorological Bureau and Environmental Protection Bureau. Relationships between wheezing diseases and meteorological and environmental factors were evaluated using Pearson’s correlation and multivariate regression analysis. An autoregressive integrated moving average (ARIMA) model was used to estimate the effects of meteorological and environmental variables on children wheezing diseases. Children wheezing diseases were frequently presented in infants less than 12 months old (1897/2655, 58.28%), and the hospitalization rate was highest in winter (1024/3255, 31.46%). In pathogen-positive specimens, the top three pathogens were respiratory syncytial virus (21.35%), human rhinovirus (16.28%) and mycoplasma pneumoniae (10.47%). The seasonality of wheezing children number showed a distinctive winter peak. Children wheezing diseases were negatively correlated with average temperature (P < 0.001, r = − 0.598). The ARIMA (1,0,0)(0,0,0)12 model could be used to predict temperature changes associated wheezing diseases. Meteorological and environmental factors were associated with the number of hospitalized children with wheezing diseases and can be used as early warning indicators for the occurrence of wheezing diseases and prevalence of virus.
Similar content being viewed by others
Introduction
Wheezing is the most common and specific symptom associated with asthma in young children1. It has been reported that one-third of children have had at least one wheezing episode before the age of 2 years and 50% of children have it before the age of 6 years. Moreover, 40% of children with wheezing diseases will continue to wheeze after childhood2, and the prevalence of repeated wheezing in infants less than 1 year is more than 10%3,4,5. The global prevalence associated with childhood asthma has increased significantly over the past 40 years, which is a crucial cause of children's medical treatment and hospitalization, affecting children’s health and causing a large economic burden on the family and society.
Children wheezing diseases include low respiratory tract infections (LRTIs) with wheezing and acute exacerbation of asthma caused by infection6. Wheezing is a lower respiratory tract symptom induced by various viral respiratory infections7. Related study shows that respiratory virus infection is the main cause of wheezing in children, approximately 80% of children with acute wheezing had respiratory viral infection8. It mainly includes respiratory syncytial virus (RSV), human rhinovirus (HRV), adenovirus (ADV), influenza virus types A and B (Inf-A and Inf-B), and parainfluenza virus I/II/III (Pinf I–III), human metapneumovirus (hMPV), and human bocavirus (HBoV)7,9,10,11. Recurrent infections of the lower respiratory tract can present as recurrent wheezing12, causing LRTIs with wheezing including bronchiolitis, bronchitis with wheezing, pneumonia with wheezing, and acute exacerbation of asthma caused by infection13,14,15,16.
Previous studies have confirmed that age, climate, air pollution, obesity, and breastfeeding of children are inextricably linked with the occurrence of wheezing diseases in children17,18,19. An increasing number of studies have reported that meteorological and environmental factors were associated with wheezing diseases, and found that air pollution has become the ninth risk factor for death in human heart and lung diseases20.
Several epidemiological studies showed that prolonged exposure to air pollution or a terrible environment could causally contribute to the exacerbation of asthma symptoms. Pollution from traffic and industry such as PM10, PM2.5, NO, and NO2 could cause higher asthma prevalence after the school-age during childhood21. Indoor allergen exposure and environmental tobacco smoke were predisposing wheezing factors to onset and persistence22. Literature reported that air pollutants other than CO were positively associated with hospitalizations for asthma and acute upper and lower respiratory infection23,24. A study in Brazil showed that CO and O3 but not PM10 and SO2 were observed to be associated with hospitalizations25. A multicenter study summarized that PM2.5 played a crucial role in wheezing diseases in preschool children26. In addition, pneumonia in children associated with air pollutants, including PM10, PM2.5, SO2, O3, NO2, and CO, was well analyzed in a meta-analysis27. However, the meteorological and environmental factors vary from different countries and regions. Although China has conducted air pollution-related health research since the 1990s, it is limited to a few large cities and the health effects assessed are relatively one-sided. Therefore, it is necessary to provide a theoretical basis for the relationship between the occurrence and development of children wheezing diseases and meteorological and environmental factors, particularly in a typical subtropical area such as Suzhou.
In this study, we retrospectively analyzed the clinical and etiological characteristics of wheezing children in Suzhou from 2013 to 2017. The patients included were LRTIs with wheezing and acute exacerbation of asthma caused by infection. Several studies have reported that epidemic trends of common respiratory viruses were associated with meteorological and environmental factors28,29,30. Our previous study has found that meteorological factors could affect seasonality of certain respiratory virus31. Thus, we evaluated the association between children’s wheezing diseases and meteorological and environmental factors. The autoregressive integrated moving average (ARIMA) model was used to investigate the effects of meteorological and environmental factors on children wheezing diseases. The purpose of this study was to evaluate the effects of meteorological and environmental factors on children wheezing diseases, which may be useful for predicting the pattern of children wheezing diseases and the prevalence of virus.
Methods
Climate and geography of Suzhou City
Suzhou City is located in the southeast of the Yangtze River Delta (120°E, 31°N) and is a special economic zone belonging to the northern subtropical monsoon maritime climate zone. It has a warm, humid and rainy climate, with the obvious monsoon, distinct seasons, long winter and summer, and short spring and autumn. Its annual average temperature is 15–17 °C, and annual average precipitation is nearly 1076.2 mm. The population of Suzhou City grew from 6.539 million in 2013 to 10.684 million in 2017.
Patients
The clinical data of admission patients diagnosed with LRTIs with wheezing, including bronchiolitis, bronchitis with wheezing, pneumonia with wheezing, and acute exacerbation of asthma caused by infection in the respiratory inpatient department at Children’s Hospital of Soochow University, were identified and reviewed from January 2013 to December 2017 in Suzhou. Basic information, including the child’s name, gender, age, admission date, chief complaint, radiological tests, laboratory tests diagnosis, allergic sensitization, and therapy, was collected, and a retrospective analysis was performed. This study was approved by the Institutional Human Ethical Committee of Children's Hospital of Soochow University (number: 2020CS001). All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all the guardians who participated in this study.
The wheezing diseases were defined according to the Global Initiative for Asthma1 and as per bronchiolitis32 and community-acquired pneumonia guidelines33. Exclusion criteria included primary immunodeficiency, cystic fibrosis, bronchopulmonary dysplasia, chronic cardio-lung diseases, bronchiectasis, tuberculosis, and active tobacco consumption as exclusion criteria.
Meteorological and environmental data collection
Meteorological data for Suzhou, including monthly average temperature (°C), monthly average humidity (%), total monthly rainfall (mm), total monthly sunshine (h), and monthly average wind speed (m/s), were provided by the Suzhou Meteorological Bureau located at 120°E, 31°N. Environmental data, including PM2.5 (µg/m3), PM10 (µg/m3), NO2 (µg/m3), SO2 (µg/m3), CO (mg/m3), and O3 (µg/m3), were provided by the Suzhou Environmental Protection Bureau at 120°E, 31°N.
Nasopharyngeal aspirates collection and detection
Samples of nasopharyngeal aspirates from children hospitalized with wheezing diseases were obtained according to a standard protocol. These samples were obtained from each patient within 24 h of admission by introducing a sterile plastic catheter into the lower pharynx via the nasal cavity. Furthermore, 3–5 mL of nasopharyngeal secretion was taken and divided into two parts after full shaking. One part of nasopharyngeal secretion was analyzed for seven common respiratory viruses, including RSV, ADV, Inf-A, Inf-B, and Pinf I–III, by using commercial slide-based assays with virus-specific fluorescence-labeled monoclonal antibodies (Light Diagnostics Respiratory Viral Screen DFA, Chemicon International, USA, from 2001 to 2005 and D3 UltraTM DFA Respiratory Virus Screening & ID Kit, Diagnostic Hybrids Inc., USA, from 2006 to 2011). HRV, hMPV and HBoV were detected using polymerase chain reaction, according to a standard protocol described previously31.
Statistical analysis
The measurement data were expressed as mean ± standard deviation (\({\overline{\text{x}}}\) ± s), all of which were tested for linear trend, normal distribution and homogeneity of variance. One-way analysis of variance was used to analyze the comparison between multiple groups. Associations between the number of children wheezing diseases and meteorological and environmental factors were evaluated using Pearson’s correlation analysis. Moreover, because of collinearity among meteorological and environmental factors, the associations were analyzed using multiple regression analysis; the standardized coefficients eliminate the influence of the dependent variable and the unit of the independent variable, and its absolute value directly reflects the degree of the independent variable’s influence on the dependent variable. Before the multiple linear regression analysis, the collinearity between the independent variables was diagnosed. As multicollinearity is present when the variance inflation factor (VIF) is higher than 534, we used VIF of the independent variables to determine if a multicollinearity problem exists among the independent variables. Independent variables with VIF values less than 5 could be included in multiple regression. After selecting the variables, we used stepwise regression to analyze the associations between meteorological and environmental factors and the number of wheezing diseases.
At present, the ARIMA model is the most commonly used model in time series analysis, which can be used to predict diverse diseases and analyze multiple relationships between independent variables and diseases35. The ARIMA model contains three parameters: P, D and Q. It is displayed in the classical notation form of (p, q, d) (P, Q, D)m. The parameters p and P represents the lag number of time series data used in the prediction model (autoregressive lags), q and Q represent the lag number of prediction error adopted in the prediction model (moving average lags), d and D represent orders of differencing, and m indicates the cycle of month. ARIMA is expressed in mathematical form as: yt = µ + ϕ1 * yt-1 + ⋯ + ϕp * yt-p + θ1 * et-1 + ⋯ + θq * et-q, where yt is the predicted number of wheezing children at time t, ϕ is the coefficient of AR and θ is the coefficient of MA. From the investigation of the correlation between meteorological and environmental factors and the number of wheezing children, we hope to study the predictive effect of meteorological and environmental factors on the number of wheezing children. In this case, multiple time series analysis based on ARIMA models was performed using the data from 2013 to 2016 (estimation period) to analyze the effect of meteorological and environmental factors on wheezing diseases of children. We then predicted the incidence of wheezing diseases in 2017(evaluation period) to evaluate the predictive effect of the model. Dependent variables (the number of wheezing children) were modeled as ARIMA processes with continuous predictors using IBM Statistical Package for the Social Sciences (SPSS) Expert Modeler (automatic model selection) and custom ARIMA models; this could automatically select the most suitable ARIMA model for studying the influence of meteorological and environmental factors on wheezing diseases of children. An optimal ARIMA model would be mainly diagnosed by normalized Bayesian information criterion (BIC) value, determination coefficient (R2), Root Mean Square Error (RMSE) and Mean Absolute Percentage Error (MAPE). The Ljung-Box Q test was used to test whether the residual series was white noise. To examine the temporal association of temperature with asthmatic diseases, we fitted the models with different lag structures from the current month (lag 0) to ≤ 2 lag months (lag 2) using the distributed lag model by SPSS Expert Modeler (automatic model selection) and custom ARIMA models. The parameters such as Estimate, Standard Error (S.E.), P-value, stationary R2 value, and normalized BIC were automatically selected by the expert modeling procedure.
Statistical analysis was performed using SPSS version 26.0 (IBM, New York). All statistical tests were two-tailed; a p-value of < 0.05 was considered statistically significant.
Ethics declarations
This study was approved by the Institutional Human Ethical Committee of Children’s Hospital of Soochow University (number: 2020CS001). A written consent was obtained from all the guardians who participated in this study.
Results
Patient characteristics
The demographic characteristics of the children included in this study are shown in Table 1. There were 2286 males (70.23%) and 969 females (29.77%) with male/female sex ratio of 2.36:1. The age of 3255 children with wheezing disease ranged from 1 month to 14 years, with an average age of 2.17 ± 1.85 years. Children of 0–12 months old had the highest proportion of wheezing diseases. Among 3255 children with the wheezing disease, most cases were pneumonia with wheezing (83.2%, 2708/3255). Moreover, the wheezing children number varied by season (Supplementary Table S1), the highest rate of wheezing diseases was reported in winter (31.46%) and the lowest in summer (17.79%).
Clinically, wheezing children mainly manifested wheezing, cough, fever, and lung rales, with an average white blood cell count of 10.72 ± 5.04 × 109 cells/mL and average C-reactive protein of 7.14 ± 16.58 mg/mL. Some children had shortness of breath and dyspnea. Among the 3255 patients selected, 2079 were examined for pulmonary function tests. By 5 years of age, several children were capable of performing reproducible spirometry if coached by an experienced technician. The pulmonary function of wheezing children younger than 5 years old was tested using the tidal breathing analysis. An ECO Medics V’max 26 Pediatric pulmonary instrument (ECO Medics AG, Switzerland) was used to obtain TBFVLs and flow-time curves. 86.39% of examined patients showed obstructive ventilation dysfunction of the lower respiratory tract confirmed by tidal breathing function test, of which 565 cases were mild (27.18%), 581 cases were moderate (27.95%), and 650 cases were severe (31.27%). We performed radiological tests on 2884 children; patchy shadows were most observed among these children (Table 1). A total of 1948 patients were examined for allergic sensitization tests, showing that 21.87% of children were allergic to food and 10.27% to dust mites. For therapy, inhaled drugs are the mainstay of treatment for these wheezing children. After symptomatic treatment, these children had a good prognosis.
Pathogen characteristics
Of the 3255 children selected, 2740 were tested for nasopharyngeal secretions, among which 795 (29.01%) were detected with one or more pathogens. The top three pathogens were RSV (21.35%), HRV (16.28%), and MP (10.47%) (Fig. 1).
Distribution of pathogens in children with wheezing disease. RSV, respiratory syncytial virus; HRV, human rhinovirus; MP, mycoplasma pneumoniae; HBoV, human bocavirus; Pinf, parainfluenza virus; Inf, influenza virus; ADV, adenovirus; HMPV, human metapneumovirus. Figures were generated using Adobe Illustrator version CC 2018 (https://www.adobe.com/cn/products/illustrator.html).
Description of meteorological and environmental data
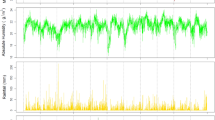
The monthly average concentrations of meteorological and environmental factors in Suzhou from 2013 to 2017 are shown in Fig. 2. The monthly average temperature was 17.7 ± 8.4 °C, average humidity was 72.3 ± 6.2%, total rainfall was 118.7 ± 90.7 mm, total sunshine was 140.9 ± 51.4 h, and the wind speed was 2.61 ± 0.37 m/s (Fig. 2A). The average monthly concentration of PM2.5, PM10, NO2, SO2 and CO showed a similar pattern with the number of wheezing children, which showed a larger peak in December (Fig. 2B). All meteorological factors were lower in winter, and temperature, humidity, and rainfall were higher in summer (Table 2). PM2.5, PM10, SO2, NO2, and CO were all higher in winter and lower in summer. However, the change of O3 concentration was larger in summer (Table 3).
Monthly distribution of meteorological (A) and environmental (B) factors among children hospitalized with wheezing diseases. (A) Mean temperature, relative humidity, total rainfall, total sunshine, wind velocity and wheezing children number from 2013 to 2017 in Suzhou. (B) PM2.5, PM10, O3, NO2, SO2, CO and wheezing children number from 2013 to 2017 in Suzhou. Figures were generated using Adobe Illustrator version CC 2018 (https://www.adobe.com/cn/products/illustrator.html).
Bivariate relationship of meteorological and environmental factors with wheezing diseases
Using Pearson’s correlation analysis, a significant negative correlation was found between the average monthly temperature and wheezing diseases in hospitalized children (P < 0.001, r = − 0.598) and the total monthly rainfall was also found to be negatively associated with wheezing diseases (P = 0.007, r = − 0.348). In terms of environmental factors, PM2.5 (P = 0.007, r = 0.347), PM10 (P = 0.001, r = 0.402), NO2 (P < 0.001, r = 0.464), and CO (P = 0.002, r = 0.387) were positively correlated with the number of wheezing diseases, whereas O3 was negatively associated with the number of wheezing diseases (P = 0.002, r = − 0.384), as shown in Fig. 3.
Correlation between meteorological, environmental factors and wheezing diseases in children (Pearson correlation analysis) (A) mean temperature; (B) total rainfall; (C) PM2.5; (D) PM10; (E) NO2; (F) CO; (G) O3. Figures were generated using GraphPad Prism version 5 (https://www.graphpad.com/scientific-software/prism/) and Adobe Illustrator version CC 2018 (https://www.adobe.com/cn/products/illustrator.html).
Multiple regression analysis of the association between meteorological and environmental factors and wheezing diseases
Considering the correlation between various meteorological and environmental factors, multiple regression analysis was used to identify the most crucial meteorological and environmental factors. On the basis of VIF, we excluded PM2.5 and PM10 and continued to perform stepwise regression of the remaining variables. It showed a negative correlation between wheezing diseases and the monthly average temperature (Table 4). Table 5 shows no statistical difference between environmental factors and wheezing diseases. Moreover, no statistical difference was observed between sex and age range in the multiple regression of meteorological and environmental factors. Consistently, there was a negative correlation between seasonal wheezing diseases and the seasonal average temperature (Supplementary Table S2).
Construction of autoregressive mean sliding model
According to the above analysis results, the temperature changes were associated with the occurrence and development of wheezing diseases in children. Therefore, we established an ARIMA model on the basis of temperature changes to predict the number of wheezing diseases in hospitalized children. Figure 4 shows the results of ARIMA using an expert modeler. The ARIMA (1,0,0)(0,0,0)12 was the optimal model with the most suitable normalized BIC (5.575), at 15.088 of RMSE value and 25.042 of MAPE value. The stationary R2 value was 0.556 for the ARIMA model, which was automatically selected by the expert modeling procedure. Temperature with 0-month lag (β = − 0.037, t = − 5.743, P < 0.001) had a significant negative effect on the number of wheezing diseases in the ARIMA analysis. The series of residuals was white noise based on the Ljung-Box test (P = 0.599), which meets the model evaluation criteria. The actual values matched well and fell within the 95% confidence interval of the predicted values from 2013 to 2016. Figure 4 shows the predicted values of ARIMA model from January to December in 2017; each predicted value was very close to each actual value, suggesting that a predictive model for wheezing diseases in children could be established on the basis of temperature.
ARIMA (1,0,0)(1,1,0)12 model with mean temperature as the covariate. Good agreement was found between observed and predicted wheezing diseases incidence. LCL, lower confidence interval; UCL, upper confidence interval. Figures were generated using Adobe Illustrator version CC 2018 (https://www.adobe.com/cn/products/illustrator.html).
Consider the possibility of delayed effects that may cause some predictors omitted at this stage. The effect of temperature, rainfall, PM2.5, PM10, NO2, CO, O3 was estimated at the lag periods of 1 and 2 months using the distributed lag model. The effect of rainfall, PM2.5, PM10, NO2, CO, O3 was significant at the lag periods of 0, 1 or 2 months, whereas these independent covariants showed lower stationary R2 than temperature (Supplementary Table S3). Therefore, the fitting effect of temperature is the best among these independent covariants to predict the number of wheezing children.
Discussion
Gene, environment, infection, and the body’s immune function play a crucial role in the occurrence of wheezing diseases. A close relationship has been found between changes in atmospheric pollutant concentrations and children’s wheezing diseases36,37. According to the World Health Organization statistics, among the deaths of children below five years old, at least one in ten child’s death is related to climate and environmental factors38. Therefore, it is crucial to analyze the relationship between meteorological and environmental factors and children’s respiratory diseases.
Our data demonstrated a complex correlation between children’s wheezing diseases and meteorological factors including temperature, mean relative humidity, total rain, total hours of sunshine, and mean wind velocity and environmental factors including PM2.5, PM10, NO2, SO2, CO, and O3. Monthly and seasonal average temperature showed more close association with wheezing diseases. The ARIMA model that incorporated temperature performed well in the prediction of wheezing diseases.
Wheezing often occurs in infants and young children whose trachea and bronchus lack elastic tissue and have a poor supporting effect. Because of poor cilia movement and clearance, their airway is typically not smooth. Moreover, they have incomplete helper T cell function, leading to scarce secretion of IgA and IgG. We included hospitalized patients with wheezing symptoms, who were mostly children below 2 years (Table 1). These truly reflected the age distribution of wheezing children. In this study, the children were LRTIs with wheezing and acute exacerbation of asthma caused by infection, and they were approximately 2 years old. Overall, these wheezing diseases were mainly caused by respiratory viral infections in infants7. Our previous study had reported that the prevalence of respiratory virus causing LRTIs, such as RSV, IV-A and IV-B, were affected by temperature31. This may influence our outcome that temperature was a crucial factor in wheezing children. However, bias may exist as outpatients were not considered. In addition, outpatients would be included in our future studies.
In this study, the temperature was identified to be the most crucial factor associated with wheezing diseases. Multiple regression analysis was used to study multicollinearity, and VIF was used to include or exclude multicollinearity at various steps. The temperature was the final factor that was associated with the wheezing disease, reflecting that temperature was a comprehensive factor that could represent other meteorological and environmental factors.
This study found that wheezing diseases peaked mainly in winter. The number of wheezing diseases in hospitalized children had a highly negative correlation with average temperature, which was consistent with the results of studies conducted in different regions39,40,41,42. One study conducted in Shanghai, China, found that a lower temperature was associated with a higher risk of asthma attack40, indicating the dangers of cold weather to wheezing children. Apart from the cold temperature, a panel study designed in six Australian cities had investigated that fluctuation in temperature also corresponded to a higher risk of an asthma attack. They found that a large intra-day temperature variation induced the risks of children’s wheezing symptoms39.
Children’s respiratory tracts are more sensitive to climate change; thus, a long-term temperature drop or a lower temperature level has an effect on wheezing diseases. Several pathways through which low temperature affected the occurrence of childhood asthma had been proposed in the existing literature. The low temperature had significant effects on suppressing the immune system of humans43, reduced the corresponding antiviral and bacterial capabilities, and increased the adrenaline secretion in the cold environment, which could also reduce the immune function. Studies have shown that an increase in temperature was associated with a decrease in lung function in children with asthma44. In addition, in the cold environment, the parasympathetic nervous system was stimulated to increase inflammation by generating mediators such as cysteinyl leukotrienes; thus, the contraction of the bronchial smooth muscle was increased45. Mechanistically, transient receptor potential melastatin 8 (TRPM8) is involved in a mechanism triggered by cold temperature. It was investigated as a mediator to trigger exacerbations of childhood asthma. TRPM8 receptor is involved in cold-induced mucus hypersecretion through the Ca(2+)-PLC-PIP2-MARCKS signaling pathway46. In addition, Fisher JT found that TRPM8 is a key molecule leading to respiratory sensations such as dyspnea and cold-induced asthma and cough47.
The statistical results of this study showed that the concentration of five pollutants had a positive correlation with the number of wheezing diseases in hospitalized children. Particulate matters, in which PM2.5 and PM10 are the most common, harbor a complex effect on wheezing airways. Several PM components are redox-active and capable of inducing cellular oxidative stress and injuries including inflammation and cell death. The convergence of regulatory signals generated by particulate matter-induced oxidative stress in dendritic cells and their interactions may also be responsible for asthma exacerbations48. Exposure to PM may cause acute pulmonary injury and inflammation, increasing airway responsiveness and airway remodeling, either alone or in combination with allergic sensitization49. Consistent with this, a study conducted by Samoli et al. found an association between exposure to PM10 and the occurrence of wheezing diseases50.
SO2 and NO2 can act as irritating gases in the respiratory tract of children. The former is known as “the culprit of atmospheric pollution” and is the main substance that induces acid rain. Several studies in recent years had found that reduced childhood pulmonary function was closely associated with NO2 and SO2 exposure51,52. A population-based mother–child cohort study followed 620 Spanish pregnant women, who were exposed to NO2 during pregnancy, from pregnancy to 4.5 years after the birth of the baby. They found that for every 10 μg/m3 increase in NO2 level, the infant’s forced expiratory volume in one second (FEV1) decreased by nearly 17.4 mL at the age of 4.5 years51. At the same time, the reactivity of the airway was higher with an increase in SO2, which led to the occurrence of wheezing53.
This study identified a negative association with exposure to O3. However, in a study conducted in São José dos Campos, a decrease in O3 concentrations could lead to fewer hospitalizations, although the outcome was hospitalization because of pneumonia among children25. Similarly, some researchers found that prolonged exposure to O3 could reduce lung function and increase the incidence of wheezing diseases, particularly in children54, which is inconsistent with our study results. This may be because of the relatively lower O3 concentrations in Suzhou. Furthermore, there is a close relationship between O3 and solar radiation. In summer, the total sunshine is longer, and the photochemical reaction is enhanced, which is conducive to O3 formation. In other words, the concentration of O3 is low in winter. The number of wheezing children rose under the influence of low temperatures, which may mask the effect of O3, and the associations were not obvious in this study.
There were several limitations in this study. First, this study was based on a single center in some areas of China, which may have potential biases because of the age structure and socioeconomic and production level55. Whether these models are suitable for other endemic areas and other infectious diseases may well require further study. We did not perform validity statistics on the ARIMA model, it is necessary to validate our conclusions with external cohorts from different countries, regions, ethnicities, and for longer periods of time. Second, in addition to meteorological and environmental factors, the factors that affected children wheezing diseases such as viral infection56, physical activity level57, and basic diseases such as rhinitis were not considered because of the availability of data. These factors might have an effect on the relationship between children’s wheezing diseases and meteorological and environmental factors. Third, the study used data at the monthly level, which in some respects did not allow for the detection of effects at the daily or weekly level, which are shorter term fluctuations. Finally, we provided limited evidence about the relationship between weather or environment and asthmatic diseases due to the limitations of statistical methods. More sophisticated models like Bayesian statistics, mathematical models and distributed lag non-linear models (DLNM) are needed to provide more evidence of environmental epidemiology and make better improvements in prediction. Despite these limitations, the source of wheezing diseases in our hospital accounted for at least 90% of the local area; therefore, we included a large number of patients to eliminate information bias, and used multiple linear regression to identify further the meteorological and environmental risk factors for wheezing children. Our study had a further understanding of the effect of meteorological and environmental factors on the prevalence of wheezing diseases, which could be useful and crucial in predicting the pattern of children wheezing diseases. Diverse seasons or regions may show different correlations between wheezing diseases and certain meteorological and environmental factors. Meteorological and environmental factors had a crucial influence on the prevalence of respiratory viruses such as RSV, HRV, and hMPV28,29,30, which were the main pathogens of wheezing diseases in children13,14,15,16. As there was an association among meteorological and environmental factors, viruses, and wheezing diseases, we included wheezing children with virus infection. The ARIMA model used in this study predicted the prevalence of wheezing diseases on the basis of temperature changes, which could be used as a reference for predicting the incidence of wheezing diseases and activity of virus in similar climatic and environmental conditions. The outcome was based on several types of diseases which were mainly caused by viral infections and inextricably linked; however, other potential factors such as characteristics of different diseases and age should also be considered. Although there were many studies focused on the relationship between daily meteorological factors and wheezing diseases, it is not practical to use daily climate to predict disease development clinically, and the climate in Suzhou changes little in the same month. Therefore, we predicted and evaluated the number of wheezing children by month at present. We also analyzed the effects of seasonal variation and wheezing to better understand the impact of meteorological and environmental factors on asthmatic disease cases, and the results of seasonal variation was consistent with that of month. This model further validated the results of this study on the correlation between temperature and wheezing in children. Therefore, it is of great significance to integrate meteorological and environmental research into public health thinking and prevention.
Conclusion
This study described the monthly and seasonal patterns of children wheezing disease and suggested that meteorological and environmental factors, particularly temperature, were associated with virus-induced wheezing diseases in children. On the basis of this, we further estimated the effect of temperature on wheezing diseases using ARIMA models. It could be effectively used as an early warning indicator for the occurrence of children wheezing diseases and for clinical application.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADV:
-
Adenovirus
- ARIMA:
-
Autoregressive integrated moving average models
- CO:
-
Carbon monoxide
- HBoV:
-
Human bocavirus
- HMPV:
-
Human metapneumovirus
- HRV:
-
Human rhinovirus
- Inf:
-
Influenza virus
- MP:
-
Mycoplasma pneumoniae
- NO2 :
-
Nitrogen dioxide
- O3 :
-
Ozone
- Pinf:
-
Parainfluenza virus
- PM:
-
Particulate matter
- RSV:
-
Respiratory syncytial virus
- SO2 :
-
Sulfur dioxide
- VIF:
-
Variance inflation factor
References
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2020. https://ginasthma.org/gina-reports/. (Accessed 14 June 2021).
Tenero, L., Tezza, G., Cattazzo, E. & Piacentini, G. Wheezing in preschool children. Early Hum. Dev. 89(Suppl 3), S13-17. https://doi.org/10.1016/j.earlhumdev.2013.07.017 (2013).
Smyth, R. L. & Openshaw, P. J. Bronchiolitis. Lancet 368, 312–322. https://doi.org/10.1016/s0140-6736(06)69077-6 (2006).
Bisgaard, H. & Szefler, S. Prevalence of asthma-like symptoms in young children. Pediatr. Pulmonol. 42, 723–728. https://doi.org/10.1002/ppul.20644 (2007).
Bouzas, M. L. et al. Wheezing in infants: Frequency, clinical characteristics and treatment. Jornal de pediatria 88, 361–365. https://doi.org/10.2223/jped.2187 (2012).
Keeley, D. & McKean, M. Asthma and other wheezing disorders in children. Clin. Evid. 14, 238–262 (2005).
Inoue, Y. & Shimojo, N. Epidemiology of virus-induced wheezing/asthma in children. Front. Microbiol. 4, 391. https://doi.org/10.3389/fmicb.2013.00391 (2013).
Velissariou, I. M. & Papadopoulos, N. G. The role of respiratory viruses in the pathogenesis of pediatric asthma. Pediatr. Ann. 35, 637–642. https://doi.org/10.3928/0090-4481-20060901-07 (2006).
Bierbaum, S. et al. Detection of respiratory viruses using a multiplex real-time PCR assay in Germany, 2009/10. Adv. Virol. 159, 669–676. https://doi.org/10.1007/s00705-013-1876-3 (2014).
Huijskens, E. G., Biesmans, R. C., Buiting, A. G., Obihara, C. C. & Rossen, J. W. Diagnostic value of respiratory virus detection in symptomatic children using real-time PCR. Virol. J. 9, 276. https://doi.org/10.1186/1743-422x-9-276 (2012).
Kim, J. K., Jeon, J. S., Kim, J. W. & Rheem, I. Epidemiology of respiratory viral infection using multiplex rt-PCR in Cheonan, Korea (2006–2010). J. Microbiol. Biotechnol. 23, 267–273. https://doi.org/10.4014/jmb.1212.12050 (2013).
Tenero, L., Piazza, M. & Piacentini, G. Recurrent wheezing in children. Transl. Pediatr. 5, 31–36. https://doi.org/10.3978/j.issn.2224-4336.2015.12.01 (2016).
Mansbach, J. M. et al. Detection of respiratory syncytial virus or rhinovirus weeks after hospitalization for bronchiolitis and the risk of recurrent wheezing. J. Infect. Dis. 223, 268–277. https://doi.org/10.1093/infdis/jiaa348 (2021).
Cho, H. K. Consideration in treatment decisions for refractory Mycoplasma pneumoniae pneumonia. Clin. Exp. Pediatr. https://doi.org/10.3345/cep.2020.01305 (2021).
Esposito, S., Argentiero, A., Gramegna, A. & Principi, N. Mycoplasma pneumoniae: A pathogen with unsolved therapeutic problems. Expert Opin. Pharmacother. https://doi.org/10.1080/14656566.2021.1882420 (2021).
Bianchini, S. et al. Role of respiratory syncytial virus in pediatric pneumonia. Microorganisms 8, 2048. https://doi.org/10.3390/microorganisms8122048 (2020).
Kim, H. S. et al. Effect of breastfeeding on lung function in asthmatic children. Allergy Asthma Proc. 36, 116–122. https://doi.org/10.2500/aap.2015.36.3818 (2015).
Peters, U., Dixon, A. E. & Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 141, 1169–1179. https://doi.org/10.1016/j.jaci.2018.02.004 (2018).
Vandini, S. et al. Respiratory syncytial virus infection in infants and correlation with meteorological factors and air pollutants. Ital. J. Pediatr. 39, 1. https://doi.org/10.1186/1824-7288-39-1 (2013).
Kurt, O. K., Zhang, J. & Pinkerton, K. E. Pulmonary health effects of air pollution. Curr. Opin. Pulm. Med. 22, 138–143. https://doi.org/10.1097/mcp.0000000000000248 (2016).
Eguiluz-Gracia, I. et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 75, 2170–2184. https://doi.org/10.1111/all.14177 (2020).
Fainardi, V., Santoro, A. & Caffarelli, C. Preschool wheezing: Trajectories and long-term treatment. Front. Pediatr. 8, 240. https://doi.org/10.3389/fped.2020.00240 (2020).
Nhung, N. T. T. et al. Acute effects of ambient air pollution on lower respiratory infections in Hanoi children: An eight-year time series study. Environ. Int. 110, 139–148. https://doi.org/10.1016/j.envint.2017.10.024 (2018).
Zheng, P. W. et al. Air pollution and hospital visits for acute upper and lower respiratory infections among children in Ningbo, China: A time-series analysis. Environ. Sci. Pollut. Res. Int. 24, 18860–18869. https://doi.org/10.1007/s11356-017-9279-8 (2017).
Tuan, T. S., Venâncio, T. S. & Nascimento, L. F. Air pollutants and hospitalization due to pneumonia among children. An ecological time series study. Sao Paulo Med. J. Revista paulista de medicina 133, 408–413. https://doi.org/10.1590/1516-3180.2014.00122601 (2015).
Chen, F. et al. The effects of PM(2.5) on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, Children, Homes and Health (CCHH) project. Environ. Pollut. 232, 329–337. https://doi.org/10.1016/j.envpol.2017.08.072 (2018).
Nhung, N. T. T. et al. Short-term association between ambient air pollution and pneumonia in children: A systematic review and meta-analysis of time-series and case-crossover studies. Environ. Pollut. 230, 1000–1008. https://doi.org/10.1016/j.envpol.2017.07.063 (2017).
Ali, S. T., Tam, C. C., Cowling, B. J., Yeo, K. T. & Yung, C. F. Meteorological drivers of respiratory syncytial virus infections in Singapore. Sci. Rep. 10, 20469. https://doi.org/10.1038/s41598-020-76888-4 (2020).
Lopes, G. P. et al. Identification and seasonality of rhinovirus and respiratory syncytial virus in asthmatic children in tropical climate. Biosci. Rep. https://doi.org/10.1042/bsr20200634 (2020).
Price, R. H. M., Graham, C. & Ramalingam, S. Association between viral seasonality and meteorological factors. Sci. Rep. 9, 929. https://doi.org/10.1038/s41598-018-37481-y (2019).
Chen, Z. et al. Association of meteorological factors with childhood viral acute respiratory infections in subtropical China: An analysis over 11 years. Adv. Virol. 159, 631–639. https://doi.org/10.1007/s00705-013-1863-8 (2014).
Ralston, S. L. et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 134(5), e1474–e1502. https://doi.org/10.1542/peds.2015-2862 (2014).
Bradley, J. S. et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 53, e25–e76. https://doi.org/10.1093/cid/cir531 (2011).
Kim, J. H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 72, 558–569. https://doi.org/10.4097/kja.19087 (2019).
Duan, Y. et al. Impact of meteorological changes on the incidence of scarlet fever in Hefei City, China. Int. J. Biometeorol. 60, 1543–1550. https://doi.org/10.1007/s00484-016-1145-8 (2016).
Virchow, J. C. & Barnes, P. J. Asthma. Semin. Respir. Crit. Care Med. 33, 577. https://doi.org/10.1055/s-0032-1326958 (2012).
Yamazaki, S. et al. Exposure to air pollution and meteorological factors associated with children’s primary care visits at night due to asthma attack: Case-crossover design for 3-year pooled patients. BMJ Open 5, e005736. https://doi.org/10.1136/bmjopen-2014-005736 (2015).
WHO. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease (2016).
Li, S. et al. Are children׳s asthmatic symptoms related to ambient temperature? A panel study in Australia. Environ. Res. 133, 239–245. https://doi.org/10.1016/j.envres.2014.05.032 (2014).
Guo, Y. et al. The association between cold spells and pediatric outpatient visits for asthma in Shanghai, China. PLoS ONE 7, e42232. https://doi.org/10.1371/journal.pone.0042232 (2012).
Stefaniak, J., Pac, A., Goryński, P. & Jedrychowski, W. Seasonal variation of hospital morbidity from asthma in Poland. Przegl. Epidemiol. 61, 567–575 (2007).
Xirasagar, S., Lin, H. C. & Liu, T. C. Seasonality in pediatric asthma admissions: The role of climate and environmental factors. Eur. J. Pediatr. 165, 747–752. https://doi.org/10.1007/s00431-006-0164-6 (2006).
LaVoy, E. C., McFarlin, B. K. & Simpson, R. J. Immune responses to exercising in a cold environment. Wilderness Environ. Med. 22, 343–351. https://doi.org/10.1016/j.wem.2011.08.005 (2011).
Li, S. et al. Ambient temperature and lung function in children with asthma in Australia. Eur. Respir. J. 43, 1059–1066. https://doi.org/10.1183/09031936.00079313 (2014).
Heir, T., Aanestad, G., Carlsen, K. H. & Larsen, S. Respiratory tract infection and bronchial responsiveness in elite athletes and sedentary control subjects. Scand. J. Med. Sci. Sports 5, 94–99. https://doi.org/10.1111/j.1600-0838.1995.tb00019.x (1995).
Li, M. et al. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism. J. Allergy Clin. Immunol. 128, 626-634.e621–625. https://doi.org/10.1016/j.jaci.2011.04.032 (2011).
Fisher, J. T. TRPM8 and dyspnea: From the frigid and fascinating past to the cool future?. Curr. Opin. Pharmacol. 11, 218–223. https://doi.org/10.1016/j.coph.2011.06.004 (2011).
Li, N. & Buglak, N. Convergence of air pollutant-induced redox-sensitive signals in the dendritic cells contributes to asthma pathogenesis. Toxicol. Lett. 237, 55–60. https://doi.org/10.1016/j.toxlet.2015.05.017 (2015).
Stanek, L. W., Brown, J. S., Stanek, J., Gift, J. & Costa, D. L. Air pollution toxicology—A brief review of the role of the science in shaping the current understanding of air pollution health risks. Toxicol. Sci. 120(Suppl 1), S8-27. https://doi.org/10.1093/toxsci/kfq367 (2011).
Samoli, E., Nastos, P. T., Paliatsos, A. G., Katsouyanni, K. & Priftis, K. N. Acute effects of air pollution on pediatric asthma exacerbation: Evidence of association and effect modification. Environ. Res. 111, 418–424. https://doi.org/10.1016/j.envres.2011.01.014 (2011).
Morales, E. et al. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax 70, 64–73. https://doi.org/10.1136/thoraxjnl-2014-205413 (2015).
Schultz, E. S., Hallberg, J., Pershagen, G. & Melén, E. Reply: Early-life exposure to traffic-related air pollution and lung function in adolescence. Am. J. Respir. Crit. Care Med. 194, 385–386. https://doi.org/10.1164/rccm.201604-0680LE (2016).
Cai, C. et al. Prior SO2 exposure promotes airway inflammation and subepithelial fibrosis following repeated ovalbumin challenge. Clin. Exp. Allergy. 38, 1680–1687. https://doi.org/10.1111/j.1365-2222.2008.03053.x (2008).
Lin, S., Liu, X., Le, L. H. & Hwang, S. A. Chronic exposure to ambient ozone and asthma hospital admissions among children. Environ. Health Perspect. 116, 1725–1730. https://doi.org/10.1289/ehp.11184 (2008).
Sanchez, J., Sánchez, A. & Cardona, R. Clinical differences between children with asthma and rhinitis in rural and urban areas. Colombia medica 49, 169–174. https://doi.org/10.25100/cm.v49i2.3015 (2018).
Jartti, T. & Gern, J. E. Role of viral infections in the development and exacerbation of asthma in children. J. Allergy Clin. Immunol. 140, 895–906. https://doi.org/10.1016/j.jaci.2017.08.003 (2017).
Westergren, T. et al. Relationship between physical activity level and psychosocial and socioeconomic factors and issues in children and adolescents with asthma: A scoping review. JBI Database System Rev. Implement. Rep. 15, 2182–2222. https://doi.org/10.11124/jbisrir-2016-003308 (2017).
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 82170012, 81970027, 81771676. Social Development Projects of Jiangsu Province, grant number BE2019671. Science and Technology Program of Suzhou, grant number SS201869. Jiangsu Provincial Medical Youth Talent, grant number QNRC2016766. Suzhou Medical Youth Talent, grant number GSWS2019047. Key Lab of Respiratory Disease of Suzhou, grant number SZS201714. Suzhou Medical Technology Projects of Clinical Key Diseases, grant number LCZX201809. The Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX20_ 2723).
Author information
Authors and Affiliations
Contributions
J.Q.H.: did statistical analysis, and was a major contributor in writing the manuscript. J.Z.: collected and analyzed clinical data. Z.R.C. and C.L.H.: took responsibility for study design. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Jq., Zhang, J., Hao, Cl. et al. Association of children wheezing diseases with meteorological and environmental factors in Suzhou, China. Sci Rep 12, 5018 (2022). https://doi.org/10.1038/s41598-022-08985-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-08985-5
This article is cited by
-
Study on the correlation between the number of mushroom poisoning cases and meteorological factors based on the generalized additive model in Guizhou Province, 2023
BMC Public Health (2024)
-
Predicting monthly hospital outpatient visits based on meteorological environmental factors using the ARIMA model
Scientific Reports (2023)