Abstract
Plant genetic resources constitute the most valuable assets of countries. It is of great importance to determine the genetic variation among these resources and to use the data in breeding studies. To determine the genetic diversity among genotypes of Cucurbita pepo L. species of pumpkin, which is widely grown in Erzincan, 29 different pumpkin genotypes collected were examined based on the morphological parameters and molecular characteristics. SSR (Simple Sequence Repeat) markers were used to determine genetic diversity at the molecular level. The analysis of morphological characterization within genotypes showed a wide variability in morphological traits of plant, flower, fruit, and leaf. In the evaluation performed using SSR markers, all primers exhibited polymorphism rate of %100. Seven SSR markers yielded a total of 15 polymorphic bands, the number of alleles per marker ranged from 2 to 3, and the mean number of alleles was 2.14. Polymorphic information content (PIC) ranged from 0.06 (GMT-M61) to 0.247 (GMT-P41), and the mean PIC value per marker was 0.152. Cluster analysis using Nei's genetic distance determined that 29 genotypes were divided into 4 major groups. The present findings have revealed the genetic diversity among pumpkin genotypes collected from Erzincan province and may form the basis for further breeding studies in pumpkin.
Similar content being viewed by others
Introduction
The family Cucurbitaceae comprises about 118 genera and 825 species1. The genus Cucurbita belonging to this family are among the leading ones that show great diversity in morphological characteristics. This genus consists of 22 wild and 5 cultivated species2. C. maxima Duch. (winter squash), C. moschata Duch. ex Lam. (butternut squash), C. pepo L. (pumpkin/summer squash), C. argyrosperma Hubersyn. C. mixta Pang and C. ficifolia Bouche are important cultivars3. Cucurbita pepo L. is an important species of Cucurbitaceae family with high economic value and genetic diversity4 and shows a wide variation in fruit characteristics such as fruit size, shape and color. Although Turkey is outside the area of primary genetic diversity for Cucurbita species, its geographical location and favorable ecological conditions have allowed Cucurbita species with significant genetic diversity over the years5. However, despite the agricultural and biological importance of squash/pumpkin (Cucurbita spp.) species, molecular studies have been very limited so far. Today, the widespread use of biotechnological methods has provided many advantages in crop breeding. Different DNA markers have been used successfully in diversity studies evaluating inter- and intra-species genetic relationships. Many studies have been conducted to examine genetic diversity among Cucurbita species using various molecular markers such as Amplified fragment length polymorphism (AFLP)6, Random amplification of polymorphic DNA (RAPD)7, Inter Simple Sequence Repeat (ISSR)8, Sequence related amplified polymorphism (SRAP)9, and Simple sequence repeat (SSR)10. Allozymes and different DNA marker systems (RFLP, AFLP, ISSR) were used to detemine genetic variability within Cucurbita pepo L. species8,11,12. Most marker systems used to date have limitations associated with their dominant and/or unreliable nature. Simple sequence repeats (SSRs) are suitable to detect variation within varieties since they are reliable, co-dominant and highly polymorphic as well as detect high levels of allelic diversity13. After these markers were first found in humans14, they began to be used in other living organisms as well. SSRs are repetitive DNA sequences of 1–6 base pair units15,16, with abundance abundant in the genome. Certain SSR markers have functional significance in chromatin organization, regulation of gene activity, and recombination17, but they are more often apparently randomly distributed in the nonfunctional genomic regions. SSR markers can be used effectively in population genetics and gene mapping studies because of their advantages as an informative marker system including requiring small amounts of DNA, being codominant and stable, being abundant and scattered throughout the genome, being reproducible and suitable for automation, and having a high level of polymorphism18. The SSR technique has successfully been used in the assessment of genetic diversity in cucurbit species such as pumpkin/squash19,20,21,22, bowler23, snake melon24 watermelon25,26, bitter melon27, cucumber28. The rate of foreign fertilization in pumpkin is very high. Due to foreign pollination, lines different from the original seed may occur, leading an increased genetic variation. Over time, pumpkin cultivars have spread to the regions of our country with both natural and artificial selections and have been formed from different populations in these regions. This type of plant genetic resources in our country establishes the basis of genetic materials of breeding studies. However, it is important to prevent the disappearance of such local genetic resources to be used in breeding studies. A comprehensive characterization study consisting of morphological and molecular parameters has not yet been carried out in Erzincan province. In this study, it was aimed to determine the degree of genetic relationship at the molecular level by using SSR markers as well as the morphological characteristics of certain pumpkin genotypes grown in Erzincan province.
Material and method
Plant material
In this study, the 29 pumpkin genotypes were collected from different regions of Erzincan province (Table 1). Seedlings of 29 different genotypes were produced in the unheated greenhouse of the Erzincan Horticultural Research Institute. Morphological and molecular identification studies of 29 local pumpkin genotypes collected were performed. Experimental research and field studies on plants, including the collection of plant material, complies with relevant institutional, national, and international guidelines and legislation. This study was carried out within the scope of the project. Therefore, all permissions for the collection of plant material and field studies were obtained through the Coordinator of Scientific Research Projects of Erzincan Binali Yıldırım University.
Determination of morphological properties
Morphological identification studies were carried out in the fields and laboratories of the Erzincan Horticultural Research Institute. Genotypes were evaluated in terms of different phenotypic characteristics including plant (growth habit, branching, degree of branching), leaf (leaf blade: size, incisions, density of green color of upper surface, marbling, mottling), petiole (attitude of petiole, green color, length, thickness, degree of prickles) and fruit (shape, major color, intensity of major color, number of colors, diameter, length, indices) traits.
SSR analysis
For SSR analysis, plant genomic DNA was isolated with minor modifications to the protocol defined by Saghai-Maroof29. 50 ml isolation buffer was prepared and heated to 70 °C in a water bath and 100 μl of β-mercaptoethanol [Merck®] was added into it. The samples were weighed on a precision balance to 0.3 g and grinded with liquid nitrogen. The ground samples were taken into 2.0 ml eppendorf tubes, 1000 μl of isolation buffer solution was added, and incubated in a 70 °C water bath for 60 min by turning upside down every 10 min. 750 μl of chloroform: isoamyl alcohol (24:1) was added to the samples and slightly turned upside down. Mixed samples were centrifuged at 14,000 rpm for 20 min at 4 °C. At the top layer (supernatant) of the three layers formed was removed using a pipette and transferred to new eppendorf tubes. The same proportion of chloroform:isoamyl alcohol was added again to the supernatant and centrifuged at 14,000 rpm for 20 min at 4 °C. The upper phase was transferred to new eppendorf tubes and 100 μl of 10 M ammonium acetate and 100 μl of 3 M sodium acetate were added. 2.5 times of isopropanol (− 20 °C) was added to the resulting mixture and slightly turned upside down. When the DNA pellet was seen, the eppendorf tubes were centrifuged at 14,000 rpm for 20 min at 4 °C. The supernatant was obtained by removing the liquid part from the tubes. The tubes were centrifuged at 14,000 rpm for 1 min at 4 °C and then left to dry in the incubator at 37 °C for 15 min. 100 µl of TE buffer was added to the genomic DNAs obtained from the samples and stored at + 4 °C. To measure the purity of DNA samples, 4 µl of DNA + 996 µl of TE buffer was added, and absorbance (A) values were read in the spectrophotometer at 260 nm and 280 nm wavelengths. DNA samples with a 260/280 value between 1.1 and 1.8 were labeled as pure DNA. Using the formula 50 (multiplication coefficient for DNA) × 250 (dilution coefficient) × OD 260 (read value at 260 nm), the amount of DNA in the stock was calculated and working solutions containing 50 ng/l DNA were prepared from the stock DNA. Information about the SSR primers used in our study is given in Table 2.
Data analysis
The PIC values of each SSR markers were calculated using the formulas given below. Allelic data were used to compute PIC value of SSRs, the codominant molecular marker system, using the Power Marker30 program31. Genetic variation within genotypes was determined by Nei's gene diversity index32, Shannon information index33, and the Popgen program34. NTSYS-pc version 2.11 f35 was used for the clustering analysis of the data set obtained from the SSR markers. The clustering was performed with the SAHN subprogram using the unweighted pair group method with arithmetic Mean (UPGMA) method. The STRUCTURE 2.2 program was used to determine the genetic structures of the genotypes36. In many genetic diversity studies with pumpkin, genotypes are successfully separated into groups using the STRUCTURE program37,38. The F-statistic (FST) value reflects the variation between sub-populations39. By using the GenAlex program, principal coordinate analysis was performed to better understand the diversity among genotypes.
Results
Morphological properties of pumpkin genotypes
In this study, 29 pumpkin genotypes belonging to Cucurbita pepo were collected from different locations in Erzincan province. This pumpkin population has been characterized according to morphological and molecular traits. Since changes in morphological traits occurred in response to external conditions, it is important to support these morphological variations with molecular studies. Morphological features of genotypes are given in Tables 3, 4 and 5. It was observed that there were significant morphological differences in plant phenotype, leaf, flower and fruit characteristics among the collected Cucurbita pepo genotypes. The plant growth habit was considered as creeping in 14 genotypes, semi-creeping in 10 genotypes and shrub in 5 genotypes. Branching was determined in 24 genotypes, while other 5 genotypes did not have branching characteristics. Leaf attitude of petiole was identified as erect in 16 genotypes and semi-erect in 13 genotypes. In addition, pumpkin genotypes showed high variation in terms of leaf characteristics such as leaf blade size, incisions of leaf blade, green color of leaf blade and green color of petiole. Incisions of leaf blade was weak in 11 genotypes, medium in 9 genotypes, strong in 1 genotype and very strong in 1 genotype, whereas in 7 genotypes incisions of leaf blade were absent (Table 3). As with other morphological features, it was observed that there was variation among genotypes in terms of flowers (male and female). It was determined that approximately 10 of the genotypes had ring at inner side of corolla and that there were no rings in the female flowers of 19 genotypes. In terms of pistil color in female flowers, genotypes are divided into 2 groups as yellow and orange. It was observed that in vast majority (approximately 76%) of the genotypes pistil colour was yellow. Based on the expression of colored ring at inner side of corolla of male flowers, genotypes are divided into 5 groups as absent, weak, medium, strong and very strong. It was observed that the majority of the genotypes (11 genotypes) had strong expression of colored ring at inner side of corolla. Genotypes were divided into 3 groups as yellow, yellow-green and green according to color of pedicel of male flower. It was determined that 12 genotypes had yellow, 9 genotypes had yellow-green and 8 genotypes had green color. Differences were determined between genotypes according to the hairiness of pedicel of male flower. Genotypes were divided into 3 groups based on this trait. 9 genotypes were classified as weak, 11 genotypes as medium and 9 genotypes as strong (Table 4). In addition, pumpkin genotypes showed high variation in fruit shapes and skin colours. It was determined that fruit shape of 8 genotypes were transverse elliptical, 8 genotypes were wide elliptical, 6 genotypes were elliptical, 4 genotypes were transverse wide elliptical, 2 genotypes were cylindrical and 1 genotype was ovoid. Four different colors were determined as the major colour of skins of the pumpkin genotypes: cream (6 genotypes), yellow (2 genotypes), orange (1 genotype) and green (20 genotypes) (Table 5).
SSR analysis
The 7 SSR markers used in our study produced a total of 15 polymorphic bands, the number of alleles per marker ranged from 2 (GMT-P41, GMT-M61, GMT-M259, GMT-P18, GMT-P25 and GMT-M30 markers) to 3 (GMT-P68 marker) and the mean number of alleles was f 2.14 (Table 6). The PIC value ranges from 0.06 (GMT-M61) to 0.247 (GMT-P41), with a mean of 0.152. The markers GMT-P41, GMT-P25 and GMT-P68 were found to be the best among the markers used to discriminate between genotypes due to their higher PIC values. (Table 6).
Cluster analyzes and principal component analyzes for SSR markers
Comparative analysis of molecular sequence data enables the determination of proximity or distance between genotypes as well as the construction of a phylogenetic tree for clustering genotypes. For this purpose, cluster analysis was performed between pumpkin genotypes using UPGMA based on Nei's genetic distance. According to the results of this analysis, four major clusters were formed. Dice genetic similarity coefficient was used to estimate genetic diversity. This coefficient is often used to estimate genetic distance. The highest genetic difference (0.63) was found between genotypes ≠ 36 and ≠ 46 genotypes. As a result of the analysis, pumpkin genotypes were divided into four major groups. In the first cluster, mostly genotypes of Bahçeliköy (60%), Cevizli (90%), Çatalarmut (100%), Çayırlı (100%), Üzümlü (100%) and Ortayurt (50%) locations were included. In the second group, only single genotype of Bahçelikoy location (≠ 3) was determined. In the third group, single genotype was found for each of Bahçeliköy (≠ 2) and Ortayurt (≠ 51) locations. In the fourth group, there were 4 genotypes collected from Cevizli (≠ 46) and Ortayurt (≠ 49, ≠ 50 and ≠ 53) locations (Fig. 1).
According to present findings, the genotypes Bahçeliköy (≠ 1, ≠ 2), Çatalarmut (≠ 7, ≠ 9), Çayırlı (≠ 23), Üzümlü (≠ 26, ≠ 29, ≠ 32, ≠ 34) were placed on upper left section of the Principle Axis-1. The genotypes Bahçeliköy (≠ 4), Çatalarmut (≠ 8, ≠ 10, ≠ 13), Çayırlı (≠ 14), Üzümlü (≠ 25, ≠ 27, ≠ 30, ≠ 36) and Cevizli (≠ 38, ≠ 40, ≠ 41 ≠ 42) were gathered on lower left section of Axis-1. The genotypes Bahçeliköy (≠ 3) and Ortayurt (≠ 50, ≠ 53) were placed on lower right section of Axis -1. The genotypes Çatalarmut (≠ 6), Cevizli (≠ 46) and Ortayurt (≠ 49, ≠ 51) were gathered on upper right section of Axis-1 (Fig. 2).
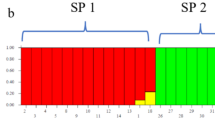
Genetic structure analysis of SSR markers
ΔK is used to determine optimal values of K. The highest value in our study was obtained as K = 4 (Fig. 3). The low population size (K value) in our study is thought to be due to the high gene flow between the sample collection regions. Similar results have been reported for the population structure of pumpkin genotypes in other studies21. In our study, 22 genotypes were found in the first subpopulation, 1 genotype in the second subpopulation, 2 genotypes in the third subpopulation, and 4 genotypes in the fourth subpopulation (Fig. 4; Table 7). The FST (F-statistics) values in the first, second, third and fourth subpopulations were determined as 0.0399, 0.0217, 0.072 and 0.000, respectively (Table 8).
Genetic structure of genotypes according to SSR data (Cucurbita pepo) genotypes given in K = 4 are presented in Table 4).
Discussion
Examination of morphological characterization within genotypes showed a wide variation of genotypes in terms of morphological characteristics (plant, flower, fruit, leaf). In many studies of Cucurbitaceae family, it has been emphasized that diversity is high in terms of morphologic characteristics40,41,42,43. In a similar study by8, it has been determined that pumpkin genotypes showed high diversity in terms of fruit characteristics44 have showed that major color of the skin was yellow in 21 (24%) pumpkin genotypes green in 2 (2%), green-yellow grayish in 15 (18%), dark yellow -green grayish in 22 (27%), light yellow in 17 (21%) and dark yellow in 4 (5%). It was observed that 7 SSR markers used in pumpkin genotypes yielded a total of 15 bands and the number of alleles per locus was 2.14. The SSR method has been successfully applied to various species to identify genetic relationships21,45,46,47,48. These markers have proven to effectively improve genetic diversity analysis and are very effective tools in genetic diversity and association studies due to their high polymorphic nature and transferability49,50,51. In similar studies of Cucurbita pepo species, researchers have found the mean number of alleles amplified per SSR marker primers as 321,52. The results are similar to the results in our study. In many studies using SSR markers, it has been stated that SSR markers are successful to detect polymorphism and diversity in species belonging to the genus Cucurbita11,52,53. Polymorphic information content (PIC) is an important value that evaluates the efficiency of polymorphic loci and determines the discrimination ability of markers. In some studies, the PIC value changed according to the number of SSR markers used and the number of genotype and analysis method. In other studies, with SSR markers, the PIC value was found between 0.49 and 0.75 for melon and between 0.18 and 0.64 for cucumber. Of the markers, PKCT111 was considered the most informative as it showed the greatest genetic variation54. In a study conducted in Kenya with 96 pumpkin samples using SSR markers, the mean PIC value was determined as 0.49, and cluster analysis showed that the level of similarity between genotypes was high55. Based on genetic structure analysis and UPGMA analysis, 4 groups were identified. Principle component analysis (PCA) presents spatial distribution of relative genetic distance between the populations56. In present study, PCA analysis was performed for better and more detailed visualization of the variation within and between the populations. With the aid this method, a 2-D diagram is generated based on closeness or distance matrix between the genotypes and the distances between the resultant groups put forth the actual distances57. Expanding our knowledge about genetic variation of genotypes is crucial for crossbreeding studies used to obtain lines resistant to various stress conditions or more productive varieties. Therefore, the assessment of genetic variability in the gene source is the first step, called pre-breeding, to improve and develop superior varieties. SSRs with high polymorphism information content successfully assisted in the differentiation of genotypes in this study. The results of this study suggest that SSR analysis can be used successfully in the estimation of genetic diversity among pumpkin genotypes and potentially be included in future studies examining diversity in a larger collection of pumpkin genotypes from various regions. It is thought that the results of this study will contribute to the existing pumpkin cultivation and conservation of genetic resources in Turkey. The outcomes obtained in this study provide significant findings for the future in marker selection, characterization of genetic source, cultivation and selection of pumpkin genetic source.
References
Jeffrey, C. A new system of Cucurbitaceae. Bot. Zhurn 90(3), 332–335 (2005).
Whitaker, T. W. & Bemis, W. P. Orijin and evolution of the cultivated Cucurbita. J. Torrey Bot. Soc. 102, 362–368 (1975).
Whitaker, T. W. & Bemis, W. P. Evolotion in genus Cucurbita. Evolution 18, 553–559 (1964).
Robinson, R. W., Mungerm, H. M., Whitaker, T. W. & Bohn, G. W. Genes of the Cucurbitaceae. HortScience 11, 554–568 (1976).
Dalda-Şekerci, A., Karaman, K. & Yetişir, H. Characterization of ornamental pumpkin (Cucurbita pepo L. var. ovifera (L.) Alef) genotypes: Molecular, morphological and nutritional properties. Genet. Resour. Crop Evol. 67(3), 533–547 (2020).
Ge, Y., Li, X., Yang, X. X., Cui, C. S. & Qu, S. P. Genetic linkage map of Cucurbita maxima with molecular and morphological markers. Genet. Mol Res. 14(2), 5480–5484 (2015).
Ntuli, N. R., Tongoona, P. B. & Zobolo, A. M. Genetic diversity in Cucurbita pepo landraces revealed by RAPD and SSR markers. Sci. Hortic. 189, 192–200 (2015).
Inan, N., Yildiz, M., Sensoy, S., Kafkas, S. & Abak, K. Efficacy of ISSR and SRAP techniques for molecular characterization of some Cucurbita genotypes including naked (hull-less) seed pumpkin. J. Anim. Plant Sci. 22(1), 126–136 (2012).
López-Anido, F. S. Cultivar-groups in Cucurbita maxima Duchesne: Diversity and possible domestication pathways. Diversity 13(8), 354 (2021).
Zhu, L. et al. Genome wide characterization, comparative and genetic diversity analysis of simple sequence repeats in Cucurbita species. Horticulturae 7(6), 143 (2021).
Katzir, N. et al. Further ISSR and preliminary SSR analysis of relationships among accessions of Cucurbita pepo. VII Eucarpia Meet. Cucurbit Genet. Breed. 510, 433–440 (2000).
Yildiz, M., Cuevas, H. E., Sensoy, S., Erdinc, C. & Baloch, F. S. Transferability of Cucurbita SSR markers for genetic diversity assessment of Turkish bottle gourd (Lagenaria siceraria) genetic resources. Biochem. Syst. Ecol. 59, 45–53 (2015).
Formisano, G. et al. Genetic diversity of Spanish Cucurbita pepo landraces: An unexploited resource for summer squash breeding. Genet. Resour. Crop Evol. 59(6), 1169–1184 (2012).
Litt, M. & Luty, J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am. J. Hum. Genet. 44(3), 397 (1989).
Queller, D. C., Strassmann, J. E. & Hughes, C. R. Microsatellites and kinship. Trends Ecol. Evol. 8, 285–288 (1993).
Kashi, Y., King, D. & Soller, M. Simple sequence repeats as a source of quantitative genetic variation. Trends Genet. 13(2), 74–78 (1997).
Li, Y., Korol, A., Fahima, T., Bailes, A. & Nevo, E. Microsatellites: Genomic distribution, putative functions and mutational mechanisms: A review. Mol. Ecol. 11, 2453–2465 (2002).
Powell, W., Machray, G. C. & Provan, J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1(7), 215–221 (1996).
Kaźmińska, K. et al. Genetic diversity assessment of a winter squash and pumpkin (Cucurbita maxima Duchesne) germplasm collection based on genomic Cucurbita-conserved SSR markers. Sci. Hortic. 219, 37–44 (2017).
Kayak, N., Türkmen, Ö., Uncu, A. T. & Dal, Y. Characterization of edible seed pumpkin (Cucurbita pepo L.) lines by SSR (Simple Sequence Repeat) markers. Manas J. Agric. Vet. Life Sci. 8(2), 17–24 (2018).
Duman, ŞE., Uncu, A. T. & Kayraldız, A. Genetic diversity analysis with the development of new SSR markers in Cucurbita pepo L. population. TURJAF. 8(12), 2518–2527 (2020).
Yunli, W. et al. Genetic diversity of pumpkin based on morphological and SSR markers. Pak. J. Bot. 52(2), 477–487 (2020).
Wang, C. L., Zhu, Z.C. & Sheng, Y. Y. Study on the genetic relationship of cucurbitaceae crops by melon's SSR markers. Chinese Horti Abst. (2011).
Merheb, J. et al. Characterization of lebanese germplasm of snake melon (Cucumis melo subsp. melo var. flexuosus) using morphological traits and SSR markers. Agronomy 10(9), 1293 (2020).
Mujaju, C. et al. Genetic diversity in watermelon (Citrullus lanatus) landraces from Zimbabwe revealed by RAPD and SSR markers. Hereditas 147(4), 142–153 (2010).
Mashilo, J., Hussein, S., Odindo, A. & Amelework, B. Assessment of the genetic diversity of dessert watermelon (Citrullus lanatus var. lanatus) landrace collections of South Africa using SSR markers. Aust. J. Crop Sci. 11(11), 1392–1398 (2017).
Karaman, K., Dalda-Şekerci, A., Yetişir, H., Gülşen, O. & Coşkun, Ö. F. Molecular, morphological and biochemical characterization of some Turkish bitter melon (Momordica charantia L.) genotypes. Ind. Crops Prod. 123, 93–99 (2018).
Dar, A. A., Mahajan, R., Lay, P. & Sharma, S. Genetic diversity and population structure of Cucumis sativus L. by using SSR markers. 3 Biotech. 7(5), 1–12 (2017).
Saghai-Maroof, M. A., Soliman, K. M., Jorgensen, R. A. & Allard, R. W. L. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. 81(24), 8014–8018 (1984).
Liu, K. & Muse, S. V. PowerMarker: An integrated analysis environment for genetic marker analysis. J. Bioinform. 21(9), 2128–2129 (2005).
Anderson, J. A., Churchill, G. A., Autrique, J. E., Tanksley, S. D. & Sorrells, M. E. Optimizing parental selection for genetic linkage maps. Genome 36(1), 181–186 (1993).
Nei, M. Genetic distance between populations. Am. Nat. 106(949), 283–292 (1972).
Lewontin, R. C. The Apportionment of Human Diversity 381–398 (Springer, 1972).
Yeh, F. C., Yang, R. C. & Boyle, T. P. Version 1.31 Microsoft Windows-Based Freeware for Population Genetic Analysis (University of Alberta/CIFOR, 1999).
Rohlf, F. J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System (Applied Biostatistics, 1992).
Pritchard, J. K., Stephens, M. & Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 155(2), 945–959 (2000).
Blair, M. W., Soler, A. & Cortes, A. J. Diversification and population structure in common beans (Phaseolus vulgaris L.). PLoS ONE 7(11), e49488 (2012).
Hegay, S. et al. Comparing genetic diversity and population structure of common beans grown in Kyrgyzstan using microsatellites. Sci. J. Crop Sci. 1(4), 63–75 (2012).
Zargar, S. M., Farhat, S., Mahajan, R., Bhakhri, A. & Sharma, A. Unraveling the efficiency of RAPD and SSR markers in diversity analysis and population structure estimation in common bean. Saudi J. Biol. Sci. 23(1), 139–149 (2016).
Balkaya, A., Özbakir, M. & Kurtar, E. S. The phenotypic diversity and fruit characterization of winter squash (Cucurbita maxima) populations from the Black Sea Region of Turkey. Afr. J. Biotechnol. 9(2), 1–10 (2010).
Zhang, C. et al. Evaluation of morphological and molecular diversity among South Asian germplasms of Cucumis sativus and Cucumis melo. Int. Sch. Res. Notices. 134, 1–12 (2012).
Wimalasiri, D., Piva, T., Urban, S. & Huynh, T. Morphological and genetic diversity of Momordica cochinchinenesis (Cucurbitaceae) in Vietnam and Thailand. Genet. Resour. Crop Evol. 63(1), 19–33 (2016).
Pratami, M. P., Chikmawati, T. & Rugayah, R. Further morphological evidence for separating Mukia Arn. from Cucumis L. Biodivers. J. 20(1), 211–217 (2019).
Turkmen, O., Seymen, M., Fidan, S. & Paksoy, M. Morphological parameters and selection of Turkish edible seed pumpkins (Cucurbita pepo L.) germplasm. Int. J. Agric. Biol. Eng. 10(5), 293–300 (2016).
Yu, K., Park, S. J., Poysa, V. & Gepts, P. Integration of simple sequence repeat (SSR) markers into a molecular linkage map of common bean (Phaseolus vulgaris L.). J. Hered. 91(6), 429–434 (2000).
Shirasawa, K. et al. An interspecific linkage map of SSR and intronic polymorphism markers in tomato. Theor. Appl. Genet. 121(4), 731–739 (2010).
Mimura, Y., Inoue, T., Minamiyama, Y. & Kubo, N. An SSR-based genetic map of pepper (Capsicum annuum L.) serves as an anchor for the alignment of major pepper maps. Breed Sci. 62(1), 93–98 (2012).
Miyatake, K., Shinmura, Y., Matsunaga, H., Fukuoka, H. & Saito, T. Construction of a core collection of eggplant (Solanum melongena L.) based on genome-wide SNP and SSR genotypes. Breed. Sci. 69(3), 498–502 (2019).
Ishii, T., Xu, Y. & McCouch, S. R. Nuclear-and chloroplast-microsatellite variation in A-genome species of rice. Genome 44(4), 658–666 (2001).
Ruizhen, H. F., Zhangying, X., Talukdar, A. & Guiquan, Z. Genetic diversity of differnt Waxy geneotypes in rice. Mol. Plant Breed. 2(2), 179–186 (2004).
Aljumaili, S. J. et al. Genetic diversity of aromatic rice germplasm revealed by SSR markers. Biomed. Res. Int. 1, 1–11 (2018).
Gong, L. et al. Genetic relationships and evolution in Cucurbita pepo (pumpkin, squash, gourd) as revealed by simple sequence repeat polymorphisms. Theor. Appl. Genet. 124(5), 875–891 (2012).
Katzir, N. et al. Relationships among accessions of Cucurbita pepo based on ISSR analysis. Cucurbitaceae 98, 331–335 (1998).
Paris, H. S. et al. Assessment of genetic relationships in Cucurbita pepo (Cucurbitaceae) using DNA markers. Theor. Appl. Genet. 106(6), 971–978 (2003).
Kiramana, J. K., Isutsa, D. K. & Nyende, A. B. Fluorescent SSR markers and capillary electrophoresis reveal significant genetic diversity in naturalized pumpkin accessions in Kenya. Glob. J. Biosci. Biotechnol. 6(1), 34–45 (2017).
Klaedtke, S. M. et al. Short-term local adaptation of historical common bean (Phaseolus vulgaris L.) varieties and implications for in situ management of bean diversity. Int. J. Mol. Sci. 18(3), 493 (2017).
Mohammadi, S. A. & Prasanna, B. M. Analysis of genetic diversity in crop plants-salient statistical tools and considerations. Crop Sci. 43, 1235–1248 (2003).
Acknowledgements
Some data in this study was presented at International Congress of Engineering and Natural Sciences Studies (ICENSS) Congress, held from 7 to 9 May 2021 in Ankara, Turkey. We would like to thank the Erzincan Binali Yıldırım University, Coordinator of Scientific Research Projects, which supported this study (Project No: FBA-2020-718). We would like to thank the Erzincan Horticultural Research Institute for providing invaluable contributions.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the conceptualization, methodology and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Öztürk, H., Dönderalp, V., Bulut, H. et al. Morphological and molecular characterization of some pumpkin (Cucurbita pepo L.) genotypes collected from Erzincan province of Turkey. Sci Rep 12, 6814 (2022). https://doi.org/10.1038/s41598-022-11005-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-11005-1
This article is cited by
-
Agromorphological characterization and molecular evaluation of a marker associated with Zym-1 allele resistance of local zucchini genotypes
Euphytica (2025)
-
Genetic diversity of wild Camellia oleifera in northern China revealed by simple sequence repeat markers
Genetic Resources and Crop Evolution (2024)