Abstract
Suspension made of Fe2(SO4)3(aq), polyvinyl alcohol and carbon nanotubes is placed in electric field to separate charges. Charges remain separated as suspension solidifies, forming composite films with cations and anions enriched at opposite sides. Polarized films behave as junction diodes with forward current and threshold voltage found to be 10–4–10–5 A and 2.4–2.6 V at ± 5 V. Rectification is preserved in strained composite films.
Similar content being viewed by others
Introduction
Diodes consist of two different semiconductor materials and produce one-direction current (e.g. p–n junctions). Attributed to equalization of Fermi level, the band bends and a built-in-potential (ϕ) forms at interface where density of free carriers is as low as an insulator1. This insulating region, also known as depletion layer (DL), becomes widened in reverse bias voltage. In this case, the ϕ rises and current can barely pass before breakdown. Upon forward bias, interfacial barrier is reduced and current flows at low voltage. However, production of junction diodes is lengthy and involves use of organic solvents and toxic elements2. Conductive polymers (CPs) represent a new type of electronic materials and can be classified into intrinsic (type-I) and extrinsic (type-II); the former is a type of thermoset and transports carriers through conjugated networks (e.g. polyacetylene)3. The latter requires conductive fillers that form networked paths at percolation threshold (η). Accordingly, the type-II can use both thermoplastic and thermoset polymers as matrix and has become the subject of great interest in materials science community4,5. This work demonstrates for the first time the generation of type-II based p–n junctions where DL is created by applying elecric field (E) to suspension made of electrolytes, polymer and carbon nanotubes (CNTs). Composite based thin film diodes are highly flexible and can be used as sensing devices, e.g. blood pressure detection and humidity controlled sensor1.
Experimental
Suspension of Fe2(SO4)3(aq)/polyvinyl alcohol/multi-walled carbon nanotubes
Polyvinyl alcohol (PVA) does not thermally decompose before 120 °C and, attrubuted to hydrophilic and hydrophobic moieties from alkyl and hydroxyl, exhibits affinity to both CNTs and electrolytes. CNTs, on the other hand, are one-dimension conductor made of rounded graphite sheets. Accordingly, tube strength and conductivity display anisotropy and are mainly contributed by C–C bonds along tube axis6. Owing to their high aspect ratio and formation of percolation paths at low mass filling fraction (fCNT), CNTs are now widely used as conductive fillers to make type-II CPs7. Similar to PVA, CNTs also exhibit an amphiphilicity and can strongly interact with ions in aqueous solution8. In this work, multi-walled CNTs (MWCNTs) are introduced into Fe2(SO4)3-PVA composites to improve electrical conductivity and ion adsorption. PVA powders (2.5 g, MW = 120,000, CCP, LTD) and Fe2(SO4)3(aq) (SHOWA) are dissolved in deionized water (50 ml) at 80 °C. MWCNTs made by catalytic pyrolysis of hydrocarbons (> 95% purity, tube diameter and length = 10–50 nm and 15–30 μm, Integrated Bio, LTD) are added into polymer-electrolyte solution, followed by ball milling for 10 h at room temperature (Fig. 1a). Previous workers claim that ball milling may damage tube structure and broadens tube length distribution accordingly9. Here SEM and raman reveal structural change to be minor; the ID/IG being 1.38 before and 1.41 after treatments (supplementary information 1a-d). Suspensions made of different electrolyte concentrations (el-Conc) and fCNT are prepared where el-Conc = 0.1, 0.3, 0.5 and 1 M and fCNT = 2.5, 5, 7.5, 10, 12.5 and 13 wt%.
DL creation by E application to suspensions
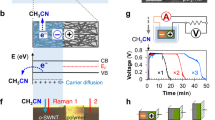
The DL is created according to following procedures. First, suspension is transferred to a petri dish placed between cupper plate electrodes at room temperature (i-ii, Fig. 1b). Second, electrodes are electrified so E forms and Fe3+ and SO42− are separated to opposite sides of suspension (ii, Fig. 1b). Since spacing between plates is fixed (d = 1 cm) the intensity of E is simply expressed as V/cm according to uniform field theory E = V/d. Third, E is applied until suspension solidifies and becomes composite films (iii-iv, Fig. 1b). Fourth, composites films made with and without E treatments are compared. Note all resultant composite films have been kept in oven (60 °C, 24 h) and weighed before and after to calculate the residual water content (~ 0.1 wt%).
Structural and electrical characterizations of composite films
As made composite films are inspected by optical and scan electron microscope (OM equipped with precision measurement s ystem & SEM, Hitachi-SU8010) which give information regarding to film thickness, surface texture and CNT distribution. The current–voltage (I–V) profiles, electrical conductivity (σ) and resistivity (ρ), normalized ρ at 25 °C (ρ/ρ25°C), forward current (IF), threshold voltage (Vth), revere and forward device resistance (RR and RF) are measured using a semiconductor device analyzer equipped with mobile-probe system (± 5 V, Agilent B1500A). It is worth mentioning for conventional devices that DL suffers greatly from heating according to Joule’s laws expressed as P = VI and I = Q/t where P, V. I, Q and t denote power, voltage, current, thermal energy and time. Accordingly, the IF is defined as the maximum forward current before onset of thermal damage1. The RR/RF ratio, on the other hand, is a key parameter often used to characterize electrical rectification, namely, RR/RF ≈ 1 indicates ohmic conductors (i.e. symmetric I-V profile) and RR/RF > 1 for polarized conductors1. All electrical measurements are carried out at a low relative humidity (RH = 25%) to evade H2O doping since both PVA and CNTs are electronically sensitive to moisture10.
Elemental analyses
X-ray photoelectron emission spectroscopy (XPS, ULVAC-PHI PHI 5000 Versaprobe II) is employed to probe distribution and content of S and Fe at film surfaces where P and N denote positively and negatively charged sides (iv, Fig. 1b). XPS peak intensity (It) is calculated according to full width at half maximum (FWHM) and signal intensity (Cx) of S2p and Fe2p spectra expressed as Cx = nx/∑ni = (Ix/Xx)/∑(Ii/Xi) where Xx is corrected relative sensitivity factor and Ix is signal intensity11. The P/N ratio which is based on Cx indicate the level of E induced film polarization, i.e. P/N ≅ 1 for non-polar and, P/N > 1 or < 1 for polar.
Work function measurements
The work function (Φ) of composite films is calculated according to Φ = hv—Eo—EF where excitation energy (hv), cut-off energy (Eo) and Fermi energy (EF) are determined by tangential equation and ultraviolet photoelectron spectroscopy (UPS) at hv = 21.22 eV12. Supplementary information 2a-c shows UPS spectra where Eo, EF and Φ are found to be 13.2 and -3.2 and 4.91 eV. For the sake of clarity, the Φ and UPS are only calculated for samples made of el-Conc = 0.1 and 0.5 M at E = 0 and 600 V/cm (supplementary information 2d-h). The obtained Φ is then used as reference to evade formation of Schottky potential at lead-sample interface, namely, the Φ of metal leads must be lowered and greater respectively than P and N of samples13. Study here selects Ag (Φ = 4.26 eV) and Pt (Φ = 5.65 eV) paste as metal leads in connection with P and N.
Molecular dynamic calculations
Molecular dynamic (MD) calculation is performed to characterize E induced charge separation in suspension and, single-walled CNTs (SWCNTs) are used as modelling matrix for the following reasons. First, less carbon atoms are invovled so calcualtion time is shortened. Second, SWCNTs resemble MWCNTs in terms of adsorption, transport, chemical reactivity and optical properties14. Two (5,0) SWCNTs (2 × 240 = 480 atoms), 3 PVA molecules [(CH2–CHOH)n, n = 20], 50 H2O, 40 Fe3+ and 60 SO42− are built in a 3 × 3 nm cell with ionic ratio of 40:60 according to net equation Fe2(SO4)3 → 2Fe3+ + 3SO42−. Calculations are carried out on three different structures defined as complex-I (50 H2O + 40 Fe3+ + 60 SO42−), complex-II (50 H2O + 40 Fe3+ + 60 SO42− + 3 PVA) and complex-III (50 H2O + 40 Fe3+ + 60 SO42− + 3 PVA + 2 CNTs). Each is geometrically optimized using NVT ensemble for 500 ps at 300 K and E = 600 V/m. The COMPASS force field is used for MD simulations and, the Non-Bond Task apply settings are based on Waals & Coulomb force. The cutoff distance, spline and buffer width are set at 12 Å, 1 Å and 0.5 Å.
Results and discussion
Conventional devices are made by chemical doping to create DL where the number of uncompensated carriers is dependent of doping level. Even doping of p and n impurities gives step junctions with ϕ = Emaxw/2 and NA = ND where Emax, w, NA and ND denote the maximum E at interface, width of DL, the number of acceptor and donor carriers at DL. Abrupt junction however forms if doping is uneven. In this case, NA ≠ ND and w = [2εs/e·(NA + ND)/(NA·ND)·ϕ]1/2 where εs and e are permittivity and electron charge. Instead of chemical doping, the DL here is created by separating Fe3+ and SO42− in composite films (Fig. 2) so formation of abrupt junction is unlikely and all electrical parameters are studied on the basis of step junction. Figure 3a displays a typical example of composite films with thickness measured to be 1 mm. Clearly, CNTs are well-dispersed and mostly embedded according to SEM images obtained from different regions of cross-section (Fig. 3b–c). The log(σ)-fCNT plot indicates fCNT = η = 10–12 wt% at which the σ is 2–3 orders of magnitude greater than value obtained at fCNT = 2.5 wt% (Fig. 3d). The small fluctuation of ρ/ρ25°C with rising temperature at RH = 25%, however, excludes H2O doping (insert, Fig. 3d)10.
Figure 4a-d shows XPS profiles where P-Fe2p, P-S2p, N-Fe2p and N-S2p denote Fe2p and S2p spectra recorded at P and N of composite films made from el-Conc = 1 M and E = 30 V/cm. The Fe2p1/2 and Fe2p3/2 are present in P-Fe2p; the former consists of Fe3+multiplets (singlet, 722–730 eV) and Fe2+ satellite for the latter (triplet, 707–717.5 eV, Fig. 4a)15. We find that CNT addition truly promotes ion absorption thus giving a greater It compared with CNTs-free samples (top and lower, Fig. 4a). Detection of Fe2+, on the other hand, indicates Fe3+ → Fe2+ reduction through CNTs acting as reductant16. Similar profiles also appear at N with (i) It(P) < It(N) for CNTs-free (lower, Fig. 4b) and (ii) It(P) ≈ It(N) for CNTs loaded films where It(P) and It(N) denote XPS peak intensity at P and N (top, Fig. 4b). The (i) implies that cations move with E and become enriched at N. The (ii) is due to ion-tube physisorption thus reducing mobility of ions toward electrodes8. In S2p spectra, sulfates (SO42−) appear in the form of doublet [167.9 eV (S2p3/2) and 169 eV (S2p1/2)], attributable to spin–orbit interaction (Fig. 4c–d)15. Again, It(P) ≈ It(N) and It(P) < It(N) are present respectively in films with and without CNT addition (top and lower, Fig. 4c and d). Table 1 lists P/N ratio and content of Fe and S for composites made at different E. First, samples made at E = 0 exhibit fluctuations at 0.97–1.15 for S and 0.55–1.09 for Fe, attributable to random diffusions of ions in solution17. The P/N then stabilizes at 1 for samples made at E = 30 V/m. Second, P/N = 1.14 (S) and 0.75 (Fe) appears at samples produced at E = 600 V/m, verifying charge separation to opposite sides of composite films (i.e. P/N ∝ E for S and P/N ∝ E−1 for Fe). It is worth mentioning in wafer technology that junction is created by doping thus producing a concentration gradient of dopants along DL. Here micro-analyses along film thickness do not verify a concentration gradient of cations (supplementary information 3), again supporting DL creation through film polarization (i.e. P/N > 1 or P/N < 1 at surfaces).
Figure 5a shows I–V profiles of composite films made from PVA loaded respectively with electrolyte (el-Conc = 1 M, sample 1) and CNTs (fCNT = 12wt%, sample 2) at E = 30 V/cm. Addition of both electrolyte and CNTs into PVA yields sample 3 (i.e. samples 1 + 2). Clearly, samples 1 and 2 exhibit a linear I–V character; the latter due to networking of CNTs at η exhibits a lower R (insert, Fig. 5a)18. Sample 3, on the other hand, resembles a diode (insert, Fig. 5a). Diode character is enhanced as el-Conc of sample 3 is reduced to 0.5 M (defined as sample 4, insert, Fig. 5b). Data above indicates following, first, CNTs provide samples with σ (i.e. sample 2), second, diode character is only present in samples made from electrolyte, CNTs and E treatment (i.e. samples 3 and 4). Third, diode profiles vanish when CNTs or electrolytes are removed from sample 3 (i.e. samples 1 and 2). Fourth, the optimal el-Conc for diode production is 0.5 M (i.e. sample 4). I-V measurements are then carried out on samples 5 and 6 to probe optimal E for diode production where 5 and 6 denote composite films made by addition of el-Conc = 0.1 and 0.3 M respectively into sample 2 (supplementary information 4a–b). Clearly, diode character reoccurs in samples 5 and 6 with IF measured to be 310 μA and 75 μA at 5 V (E = 600 V/cm). Highlighted I–V profiles of sample 6 further reveal an enhanced diode character where Vth lies 2.4–2.6 V at E = 600 V/cm (supplementary information 5a-c). Note that DL is a polarized region with field vector against external E the IF is therefore expected to be lower than a conductor, accounting for IF(E = 0V) = 120 μA > IF(E = 30V) = 85 μA > IF(E = 600V) = 75 μA at 5 V (supplementary information 5a–c). Additional evidence in support of film polarity comes from the Φ measurements (Table 2). First, ΦP(600 V) > ΦN(600V) is present where ΦP and ΦN denote Φ obtained at P and N. Second, Φ rises as el-Conc increases from 0.1 to 0.5 M, indicative of enriched ions at surfaces. However, the Φ varies significantly from region to region as el-Conc reaches 1 M (not shown), attributed to excessive ions that diffuse randomly thus compromising DL formation17. Supplementary information 6 shows stability of diode characteristic over 12 months kept in the oven.
The I–V profiles of samples 1, 2 and 3 at ± 6 V and highlighted profile at ± 4 V (a). The I–V profiles of samples 2, 3 and 4 at ± 6 V and highlighted profile at ± 4 V (b). Highlighted I–V profiles of sample 4 made at E = 30 V/cm (c) and 600 V/cm (d). Highlighted I–V profiles of (c) and (d) at ± 5 V and I = ± 2 μA (e).
Diode performance is often justified according to quality factor and RR/RF ratio19; the former is used to characterize doped devices and is inapplicable to CPs. The latter concerns unidirectional conduction so Vth must low and high IF is required, for example, Vth = 0.7 V and IF = 10–3–10–5 A for Si-based diodes20. Experiments indicate that diode character becomes apparent at RR/RF > 3 and, the Vth and IF lie 2.4–2.6 V and 10–4–10–5 A for samples made at E = 30 and 600 V/cm (Table 3 and Fig. 5c–e). The RR/RF measurements also confirm optimal conditions for diode formation to be fCNT = 12 wt%, el-Conc = 0.5 M and E = 600 V/cm; the greatest value being RR/RF = 20.663 (Table 3). In CMOS technology, the Si substrates are intentionally stressed to break crystallographic symmetry thus altering band structure21. However, strains are confined at interfaces and can barely propagate to detriment conductive channels underneath. We find that film area and thickness increase and decrease respectively by 200% and 80% after hot-pressing (60 °C, 50 kg/cm2 and 30 min) and the RR/RF changes to 3.176 (E = 0), 2.358 (E = 30 V/m) and 4.03 (E = 600 V/m), indicating redistribution of ions and CNTs (Table 3 and supplementary information 7a–b).
Question however remains as to how charges separate in the presence of E, CNTs and PVA. Supplementary information 8 shows consecutive snapshots extracted from MD simulations of complex-I which contains three types of intermolecular forces arising from hydration (Fe3+⋅H2O/SO42−⋅H2O), H-bonds (H2O⋅H2O) and ion-ion attraction (Fe3+⋅SO42−). Calculation reveals that Fe3+ and SO42− are mostly paired in the absence of E (supplementary information 8a–d and 7a). As E is applied the paired Fe3+⋅SO42− rapidly move towards electrodes through hydration with H2O acting as carriers (blue arrow, supplementary information 8e–h). Pairing also occurs between adjacent S and O (SO42−·SO42−, supplementary information 9a–c) so excess of anions is dragged into Fe3+⋅SO42− clusters and produce unequal number of positive and negative charges at P and N, i.e. the number of cations (Fe3+) and anions (SO42−) is 22 (= 66 positive charges) and 36 (= 72 negative charges) (red arrow, supplementary information 8 h). In the absence of E, the displacing range of ion-ion clusters is 3–4 nm and decreases to 1–2 nm as E is applied, attributed to static attraction by electrodes (supplementary information 10a-b). PVA however acts as impermeable objects and prevents cations and anions from pairing, thus facilitating charge separation (complex-II, supplementary information 11a–c and 12a). Calculation indicates the number of cations and anions to be 3 and 2 at N and 4 and 7 at P, corresponding to 5 positive charges at N and 2 negative charges at P (t = 6 s, supplementary information 11d–f and 12b). H-bonds also exist between PVA and anions, accounting for simultaneous movements of both structures toward electrodes (i.e. CH2–CHOH⋅OSO32−, circle, supplementary information 9c). Owing to large mass, CNTs can barely move and therefore restrict movements of PVA and ion clusters (complex-III, Fig. 7a–b and supplementary information 13a). Aggregation of ions around tubes again verifies physisorption. CNTs however become polarized (white N and P) as E is applied and therefore attract more ions, resulting in It(tube) > It(tube-free) (top and lower, Figs. 4a, 6c,d and supplementary information 13b)22.
MD simulation is also carried out on water-free systems that give a large mean free path and reduced elastic scattering of ions, including 2CNTs + 3PVA + Fe3+ (type-a), 2CNTs + 3PVA + SO42− (type-b) and 2CNTs + 3PVA + Fe3+ + SO42− (type-c) (Fig. 7a–c). Upon E application, Fe3+ rapidly moves toward electrode (white arrow, Fig. 7a) while anion strongly interacts with C–C bonds of CNTs (Fig. 7b). Note that SO42− is a distorted tetrahedral structure where charges locate at O (insert, Fig. 7b). Accordingly, the C–C bonds may electronically correlate with anion and lie possibly between two O-S bonds (1) or O–S and O=S bonds (2) and two O=S bonds (3); the (2) however prevails according to MD data (inserts, Fig. 7b). Simultaneous addition of Fe3+ and SO42− into CNTs-PVA complexes results in (i) coupling of Fe3+ and SO42− and (ii) trapping of Fe3+⋅SO42− at intertube groove (Fig. 7c)23; the (i) supports Figs. 4 and 6.
Conclusion
Diode structure is successfully created in composite films made of PVA, Fe2(SO4)3(aq) and CNTs. DL and electrical rectification are verified by elemental analyses and I–V measurements. Calculations confirm E induced film polarization and charge separation through hydration with H2O acting as carriers. Diode character is preserved in strained composite films.
Data availability
The raw data of Figs. 4 and 5 and simulation videos in this study are available from zenodo.org (https://doi.org/10.5281/zenodo.6570681). Other data and material used and analyzed in this study are available from the corresponding author on reasonable request.
References
Wilson, P. R. Measurements on the depletion layer properties of planar diodes. Solid·State Electron, 12, 539–547. https://doi.org/10.1016/0038-1101(69)90109-9(1969).
Jung, Y. H., Zhang, H. L., Gong, S. Q. & Ma, Z. Q. High-performance green semiconductor devices: Materials, designs, and fabrication. Semicond. Sci. Technol. https://doi.org/10.1088/1361-6641/aa6f88 (2017).
Roth, S. & Filzmoser, M. Conducting polymers—Thirteen years of polyacetylene doping. Adv. Mater. 2, 356–360. https://doi.org/10.1002/adma.19900020804 (1990).
Andrews, R. & Weisenberger, M. C. Carbon nanotube polymer composites. Curr. Opin. Solid State Mater. Sci. 8, 31–37. https://doi.org/10.1016/j.cossms.2003.10.006 (2004).
Lee, J. S., Oh, J., Kim, S. G. & Jang, J. Highly sensitive and selective field-effect-transistor NonEnzyme dopamine sensors based on Pt/conducting polymer hybrid nanoparticles. Small 11, 2399–2406. https://doi.org/10.1002/smll.201403263 (2015).
Lucas, A. A., Lambin, P. H. & Smalley, R. E. On the energetics of tubular fullerenes. J. Phys. Chem. Solids 54, 587–593. https://doi.org/10.1016/0022-3697(93)90237-L (1993).
Schadler, L. S., Giannaris, S. C. & Ajayan, P. M. Load transfer in carbon nanotube epoxy composites. Appl. Phys. Lett. 73, 3842–3844. https://doi.org/10.1063/1.122911 (1998).
Long, R. Q. & Yang, R. T. Carbon nanotubes as superior sorbent for dioxin removal. JACS 123, 2058–2059. https://doi.org/10.1021/ja003830l (2001).
Krause, B. et al. Influence of dry grinding in a ball mill on the length of multiwalled carbon nanotubes and their dispersion and percolation behaviour in melt mixed polycarbonate composites. Compos. Sci. Technol. 71, 1145–1153. https://doi.org/10.1016/j.compscitech.2011.04.004 (2011).
Na, P. S. et al. Investigation of the humidity effect on the electrical properties of single-walled carbon nanotube transistors. Appl. Phys. Lett. 87, 093101. https://doi.org/10.1063/1.2032594 (2005).
Peter Atkins, J. d. P. Physical Chemistry. P.980 (W. H. Freeman and Company, 2002).
Fujii, R., Gotoh, Y., Liao, M. Y., Tsuji, H. & Ishikawa, J. Work function measurement of transition metal nitride and carbide thin films. Vacuum 80, 832–835. https://doi.org/10.1016/j.vacuum.2005.11.030 (2006).
Lien, D. H., Hsu, W. K., Zan, H. W., Tai, N. H. & Tsai, C. H. Photocurrent amplification at carbon nanotube-metal contacts. Adv. Mater. 18, 98–103. https://doi.org/10.1002/adma.200500912 (2006).
Baughman, R., Zakhidov, A. & Heer, W. Carbon Nanotubes-The Route Toward Applications. Science (New York, N.Y.) 297, 787–792, doi:https://doi.org/10.1126/science.1060928 (2002).
Li, S. et al. A nanoarchitectured Na6Fe5(SO4)8/CNTs cathode for building a low-cost 3.6 V sodium-ion full battery with superior sodium storage. J. Mater. Chem. A 7, 14656–14669. https://doi.org/10.1039/C9TA03089A (2019).
Watts, P. C. P. et al. Carbon nanotubes as polymer antioxidants. J. Mater. Chem. 13, 491–495. https://doi.org/10.1039/B211328G (2003).
Mamontov, E. Diffusion dynamics of water molecules in a LiCl solution: A low-temperature crossover. J. Phys. Chem. B 113, 14073–14078. https://doi.org/10.1021/jp904734y (2009).
Byrne, M. T. & Gun’ko, Y. K. Recent advances in research on carbon nanotube-polymer composites. Adv. Mater. 22, 1672–1688. https://doi.org/10.1002/adma.200901545 (2010).
Martí, A., Balenzategui, J. L. & Reyna, R. F. Photon recycling and Shockley’s diode equation. J. Appl. Phys. 82, 4067–4075. https://doi.org/10.1063/1.365717 (1997).
McKelvey, J. P. Solid State and Semiconductor Physics. (Harper & Row, 1966).
Chan, V. et al. in CICC: Proceedings of the IEEE 2005 Custom Integrated Circuits Conference 667–674 (2005).
Zhu, Y.-F. et al. Alignment of multiwalled carbon nanotubes in bulk epoxy composites via electric field. J. Appl. Phys. 105, 054319. https://doi.org/10.1063/1.3080243 (2009).
Adu, C. K. W., Sumanasekera, G. U., Pradhan, B. K., Romero, H. E. & Eklund, P. C. Carbon nanotubes: A thermoelectric nano-nose. Chem. Phys. Lett. 337, 31–35. https://doi.org/10.1016/S0009-2614(01)00159-2 (2001).
Acknowledgements
Authors thank the Ministry of Science and Technology of Taiwan for the financial support (MOST-109-2811-M-007-570). This work is also supported by "High Entropy Materials Centre" from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (108QR001J4). And thanks for the assistance from Hsuan-Hao Huang with reference collection.
Author information
Authors and Affiliations
Contributions
H.-J.T., C.-Y.K., and Y.-K.Y. handle the total experiments and collect data. W.-K.H. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Supplementary Video 2.
Supplementary Video 3.
Supplementary Video 4.
Supplementary Video 5.
Supplementary Video 6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsai, HJ., Ke, CY., Yang, YK. et al. Electric field created p–n junction in composite films made from carbon nanotubes, iron (III) sulfate and polyvinyl alcohol. Sci Rep 12, 11203 (2022). https://doi.org/10.1038/s41598-022-15294-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15294-4