Abstract
We evaluated the 3-year clinical outcomes following early invasive (EI) and delayed invasive (DI) strategies in older adults with non-ST-segment elevation myocardial infarction (NSTEMI) undergoing successful new-generation drug-eluting stents (DESs) implantation to reflect current real-world practice. Overall, 2437 older adults (age, ≥ 65 years) with NSTEMI were recruited from the Korea Acute Myocardial Infarction Registry-National Institute of Health. They were divided into two groups: EI (n = 1750) and DI (n = 687). The primary clinical outcome was the occurrence of major adverse cardiac and cerebrovascular events (MACCEs), defined by all-cause death, recurrent MI, any repeat coronary revascularization, and stroke. The secondary clinical outcome was stent thrombosis (ST). After multivariable-adjusted and propensity score-matched analyses, the primary and secondary clinical outcomes were not significantly different between the EI and DI groups. Even after the analysis was confined to those having complex lesions, these major clinical outcomes were similar between these two groups. The EI and DI strategies in older adults with NSTEMI receiving new-generation DES showed comparable results.
Clinical Trial Registration: URL: http://cris.nih.go.kr/cris/en/; Unique identifier: KCT0000863.
Similar content being viewed by others
In patients with non-ST-segment elevation (STE) acute coronary syndrome (NSTE-ACS), an early invasive (EI) strategy is defined as coronary angiography (CAG) and percutaneous coronary intervention (PCI) performed within 24 h of hospital admission1,2. The European guideline recommends an EI strategy in patients with a high-risk (≥ 1) criterion1. The American College of Cardiology/American Heart Association guideline recommends an EI strategy for initially stabilized high-risk patients with NSTE-ACS and a delayed invasive (DI) strategy defined as a reasonable strategy for high/intermediate risk patients (class IIa and level of evidence B)1,2. The preference for EI strategy in patients with NSTE-myocardial infarction (NSTEMI) in the European and American guidelines are based on the result of the Timing of Intervention in Acute Coronary Syndrome (TIMACS) trial3. The data from a recent registry4 showed that in high-risk (Global Registry of Acute Coronary Events [GRACE] score ≥ 140) NSTE-ACS patients, early CAG was associated with significantly reduced mortality rate (HR 0.79; 95% CI 0.62–0.98). In another study, the EI strategy did not significantly reduce the risk of death or MI except for recurrent ischemia and the duration of in-hospital stay5. Hence, the optimal timing of PCI in NSTEMI has not been conclusively defined. For NSTE-ACS, age was an important determinant of outcomes in those patients6,7. However, the published data concerning the results of an EI strategy in the context of the older patients with NSTEMI are limited and are the subject of this study1. Tegn et al. reported that invasive strategy was superior to a conservative strategy for the reduction of MI, urgent revascularization, stroke, and death in patients aged ≥ 80 years with NSTE-ACS8. Unfortunately, the majority of the previous studies did not confine the study population to patients who received successful PCI or those who received new-generation drug-eluting stents (DESs)3,6,7. Currently, the new-generation DESs have nearly replaced bare-metal stents and first-generation DES for routine PCI; the new-generation DES is more effective than first-generation DES in reducing major clinical outcomes in patients with acute MI (AMI)9. Although we believe that these previous studies3,6,7 are valuable for estimating comparative clinical outcomes among different treatment strategies (EI, DI, or conservative treatment) in patients with NSTE-ACS, their findings have some limitations with respect to the current real-world practices. Hence, in this study, we evaluated the 3-year major clinical outcomes between the EI and DI strategies in older adults with NSTEMI undergoing successful new-generation DES implantation.
Results
Baseline characteristics
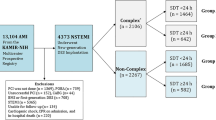
Figure 1 shows the flow chart of this study. Table 1 shows the baseline, laboratory, angiographic, and procedural characteristics of the study population. The mean values of left ventricular ejection fraction (LVEF), peak creatine kinase myocardial band (CK-MB), and peak troponin-I, and the number of current smokers, and the prescription rates of ticagrelor, angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) as discharge medications, multivessel disease and patients with pre-PCI thrombolysis in myocardial infarction (TIMI) flow grade 0/1 were higher in the EI group than in DI. In contrast, the patients who had Killip class ≥ 3, had reduced renal function (estimated glomerular filtration rate [eGFR], < 60 mL/min/1.73 m2), and received clopidogrel as discharge medication; mean value of serum creatinine and mean number of deployed stents; the use of intravascular ultrasound/optical coherent tomography/fractional flow rate were higher in the DI group than in EI (Table 1).
Clinical outcomes
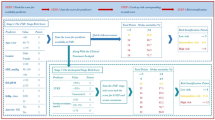
The in-hospital mortality and 3-year major clinical outcomes are summarized in Table 2 and Fig. 2. In-hospital all-cause death (hazard ratio [HR] 1.581 (95% confidence interval [CI] 0.861–2.904; p = 0.140), cardiac death (CD, HR 1.924; 95% CI 0.899–4.117; p = 0.092) and non-CD (HR 1.031; 95% CI 0.368–2.892; p = 0.954) were not significantly different between the EI and DI groups. After multivariable-adjusted analysis, the 3-year major adverse cardiac and cerebrovascular events (MACCE, adjusted HR [aHR] 1.159; 95% CI 0.960–1.398; p = 0.125), all-cause death (aHR 1.180; p = 0.192), CD (aHR 1.229; p = 0.228), non-CD (aHR 1.116; p = 0.564), recurrent MI (re-MI, aHR 1.040; p = 0.881), any repeat revascularization (aHR, 1.171; p = 0.327), stroke (aHR 1.099; p = 0.713), and stent thrombosis (ST [definite or probable], aHR 2.058; 95% CI 0.690–6.143; p = 0.196) rates were not significantly different between the EI and DI groups. (Table 2). These results were confirmed after PS-matched analysis. After PS-matched analysis, the primary and secondary clinical outcomes were not significantly different between the EI and DI groups (Table 2). For further assessment of major clinical outcomes between the EI and DI groups, we compared these major clinical outcomes by limiting the study population to patients with complex lesions (Table 3). The number of patients with complex lesions in each group was more than 50% (EI vs. DI = 51.3% vs. 56.2%, p = 0.028) (Fig. 3). The MACCE rates were similar between the EI and DI groups (aHR 1.034; 95% CI 0.810–1.320; p = 0.787) (Table 3). The ST (definite or probable) rates were also similar between the EI and DI groups (aHR 2.662; 95% CI 0.531–13.35; p = 0.234). Additionally, the all-cause death, CD, non-CD, re-MI, any repeat revascularization, and stroke rates were not significantly different between the two groups after adjustment (Table 3). Figure 4 shows the subgroup analysis for MACCE. The results of subgroup analysis using Cox logistic regression model revealed that in the all subgroups except for those showing significant p-for-interaction demonstrated comparable MACCE rates in this study. Table 4 shows predictors for all-cause mortality in the total study population, which includes reduced LVEF (< 50%, aHR 1.762; 95% CI 1.414–2.195; p < 0.001), cardiogenic shock (aHR 1.984; 95% CI 1.437–2.748; p = 0.003), intra-aortic balloon pump (IABP) or extracorporeal membrane oxygenation (ECMO, aHR 3.097; 95% CI 2.010–4.771; p < 0.001), reduced renal function (aHR 2.060; 95% CI 1.625–2.612; p < 0.001), and a high GRACE risk score (> 140, aHR 2.328; 95% CI 1.716–3.159; p < 0.001).
Kaplan–Meier curved analysis for MACCE (A), all-cause death (B), cardiac death (C), non-cardiac death (D), recurrent MI (E), any repeat revascularization (F), stroke (G), and stent thrombosis (H). MACCE major adverse cardiac and cerebrovascular events, MI myocardial infarction, PSM propensity score-matched, HR hazard ratio, aHR adjusted hazard ratio, CI confidence interval.
Discussion
The main findings of this prospective, observational study were: (1) after multivariable-adjusted and PS-matched analyses, MACCE, all-cause death, CD, non-CD, re-MI, any repeat revascularization, stroke, and ST (definite or probable) rates were similar between the EI and DI groups; (2) even after limiting the study population to patients who had complex lesions, the primary and secondary clinical outcomes were not significantly different between the EI and DI groups.
Theoretically, through the EI strategy, the operator could find significant lesions earlier in patients with NSTEMI and could have the opportunity for early revascularization, salvage of ischemic myocardium, and facilitation of earlier discharge from a facility2,10. In contrast, DI strategy may provide adequate time for optimal medical treatment in order to decrease thrombus burden and improve plaque stability10. In the recent European guideline, the recommended diagnostic and interventional strategies for older patients and younger patients are the same (class I and level of evidence B)1. However, the optimal timing of PCI in NSTEMI remains a subject of debate. The clinical presentation of NSTE-ACS in older person is atypical11,12 and the electrocardiographic changes are less frequent in older than in younger patients7,12. Despite the significant decrease in mortality and morbidities of ACS because of evidence based therapy13, these improvements in ACS treatment strategy have not equally improved outcomes for older adults2. Regarding these characteristics2,7,11,12 in older people, the information dealing with the preferred treatment option between the EI and DI strategies could be important for the interventional cardiologist. In the old report, EI strategy showed significantly improved clinical outcomes compared with conservative treatment in elderly patients with NSTE-ACS14. However, these studies were not performed in the era of new-generation DES and that did not compare clinical outcomes between the EI and DI strategies14,15. Furthermore, since the available data on this subject is limited8, the comparative results between the EI and DI strategies in older patients with NSTEMI are limited. Hence, in this study, we investigated the long-term clinical outcomes between the EI and DI strategies in older adults with NSTEMI undergoing successful new-generation DES implantation. In our study, the major clinical outcomes were not significantly different between the EI and DI groups after adjustments (multivariable or PS-matched) during a 3-year follow-up period. An EI strategy is useful but increases the risks of stroke and bleeding, which are the main complications of this strategy14,15. The key study of the current guidelines1,2 was the TIMACS trial3. Since the study was performed between April 2003 and June 2008; nearly half of the cases used bare-metal stents, and the first-generation DES might be used at that time. Moreover, less than 60% of the patients underwent PCI. At 6 months, the primary outcome (a composite of death, MI, or stroke) were similar between the EI and DI groups (HR 0.85; 95% CI 0.68–1.06; p = 0.15)3. Although this study showed valuable results for understanding the beneficial effect of EI CAG in patients with ACS3, accounting for the limitations mentioned, the results of our study could be more impactful. In the most recently published registry data, the EI strategy was associated with lower all-cause death (HR 0.61; 95% CI 0.51–0.71), CD (HR 0.52; 95% CI 0.43–0.63), and MACE (HR 0.62; 95% CI 0.54–0.71) than those in the DI strategy16. However, similarly with TIMACS trial3, this study was conducted between the years 2003 and 2017. Therefore, the type of DES did not belong to the new-generation DES.
In our study, the high number of comorbidities including hypertension, diabetes mellitus, previous MI, previous heart failure, previous stroke, reduced renal function in older adults with NSTMI (Table 1) are consistent with the previously published data8,16. This increasing prevalence of cardiovascular disease with aging has been attributed to several age-related changes including vascular wall elasticity, coagulation and hemostatic system, and endothelial dysfunction17,18,19. Therefore, age related decline in organ function increases cardiovascular diseases19.
Frailty is very common in older adults with cardiovascular diseases and frailty contributes valuable prognostic insights incremental to existing risk models and assists clinicians in defining optimal care pathways for their patients20. In elderly NSTEMI patients, frailty was independently associated with all-cause mortality at long-term follow-up of more than 6 years21. In the Australian Cooperative National Registry of Acute Coronary Care, Guideline Adherence and Clinical Events (CONCORDANCE) registry22, increased frailty was independently associated with increased post-discharge all-cause mortality. More recent study showed that an assessment of both cognitive and physical conditions should be included in the comprehensive geriatric evaluation of hospitalized older STEMI patients23. Hence, Faubert et al.24 emphasized that the management of NSTEMI in elderly patients must be individualized with regard to the patient’s goals, comorbid conditions, overall health, and cognitive status. Mone et al.25 showed the importance of thrombus aspiration in the treatment of STE-myocardial infarction (STEMI) in a group of high-risk patients such as elderly with frailty.
Even though the primary and secondary clinical outcomes were not significantly different between the EI and DI groups, after adjustment, reduced LVEF, cardiogenic shock, IABP or ECMO, reduced renal function, and a high GRACE risk score were significant predictors for all-cause mortality in this study (Table 4). Hayıroğlu et al.26 showed the mortality rate remains high despite IABP support in patients with ACS. Çinar et al.27 reported that the incidence of in-hospital mortality was significantly greater in patients with a high age, creatinine, ejection fraction score compared with the intermediate or the low score group (p < 0.005) among patients with STEMI related cardiogenic shock.
To clearly estimate the long-term clinical outcomes, we performed additional analysis as shown in Table 3. Even after considering the patients with complex lesions, the 3-year major clinical outcomes were not significantly different (Table 3). Subgroup analyses for MACCE in group A and B (Fig. 4) showed that all subgroups except for those showing significant p-for-interaction had comparable MACCE rates.
We agree with the current guideline recommendations that suggest that the management of older patients should be based on ischemic and bleeding risks, estimated life expectancy, comorbidities, the need for non-cardiac surgery, quality of life, frailty, cognitive, functional impairment, patient values and preferences, and the estimated risks and benefits of revascularization1. Our results showed that in the era of new-generation DES, the major clinical outcomes were not significantly different between the EI and DI strategies in older adults with NSTEMI after successful stent implantation during a 3-year follow-up period. Hence, we suggested that the current guideline1,2 about the management of older patients with NATE-ACS with CAG and PCI needs to be reevaluated under the era of new-generation DES. In this study, although the population may have been insufficient to provide meaningful results, 20 tertiary high-volume University hospitals participated in the registry. Therefore, we believe that our results could provide helpful information to interventional cardiologists in terms of long-term effects of EI and DI strategies in older adults with NSTEMI undergoing successful implantation of new-generation DES.
This study had other limitations. First, even though this study is a prospective, observational registry, it is not a randomized controlled study; there may have been some selection bias. Moreover, the variables that were not included in the data registry might have affected the study outcome despite the multivariable and PS-matched analyses. Second, because we set the cut-off value of older adults at age ≥ 65 years in our study, our results could change according to different cut-off ages. Third, as mentioned, although bleeding is an important complication that occurs after PCI in older adults14,15, anti-platelet therapy after 1 year index PCI was different among the physicians; we could not include bleeding complication as an outcome parameter in our study during a 3-year follow-up period. This is a major shortcoming of our study. Fourth, the 3-year follow-up duration was insufficient to evaluate long-term adverse events. Finally, contrast induced nephropathy is an important factor and acute kidney injury can effect long-term outcomes28. A recent report demonstrated that acute kidney injury was an important independent prognostic factor (HR 2.244; 95% CI 1.077–4.676; p = 0.031) for 5-year mortality among patients with STEMI complicated by cardiogenic shock and treated with primary PCI28. However, because these variables (contrast induced nephropathy and acute kidney injury) were not included in the data registry, which could have caused significant bias.
In conclusion, in the era of new-generation DES, the major clinical outcomes were not significantly different between the EI and DI strategies in older adults with NSTEMI after successful stent implantation during a 3-year follow-up period. However, further randomized, large-scale, and long-term follow-up studies are needed to clarify the differences of the clinical outcomes between these two different reperfusion strategies in those patients.
Methods
Study population
A total of 13,104 patients with AMI between November 2011 and December 2015 were recruited from Korea AMI Registry-National Institute of Health (KAMIR-NIH)29. KAMIR-NIH is a nation-wide prospective multicenter registry integrated from 20 high-volume centers in the Republic of Korea. Detailed information on this registry can be found on the website (http://www.kamir.or.kr). All patients aged ≥ 18 years at the time of hospital admission were included. Patients who did not receive PCI (n = 1369, 10.4%) or who received unsuccessful PCI (failed PCI [n = 61, 0.5%] and suboptimal PCI [n = 94, 0.7%]), received plain old balloon angioplasty (n = 739, 5.6%), were treated with bare-metal stent or first-generation DES (n = 563, 4.3%), underwent coronary artery bypass graft (n = 38, 0.3%), had STE MI (STEMI) (n = 5342, 40.8%), and were unavailable for follow-up (n = 157, 1.2%) were excluded. Moreover, the patients aged less than 65 years (n = 2310, 48.7%) were excluded. Overall, 2437 patients with NSTEMI who underwent successful new-generation DES implantation were included (Fig. 1). The types of new-generation DES used are listed in Table 1. The definition of older adults is controversial. In general, a person is considered old if their civil age is ≥ 60 or 65 years30. The average age at which individuals experience a first heart attack is 65.8 years for men and 70.4 years for women12. Additionally, based on the Consensus Development Conference on Diabetes and Older Adults (age ≥ 65 years) convened by the American Diabetes Association in Feb 201231 and other report32 showed that multimorbidity and polypharmacy are highly prevalent among adults aged ≥ 65 years, we set the cut-off value at ≥ 65 years for older adults in our study. These patients were divided into two groups: EI (n = 1750, 71.8%) and DI (n = 687, 28.2%) (Fig. 1). Trained research coordinators at each center collected patient data using a web-based report form on the Internet-based Clinical Research and Trial management system, supported by a grant from the Korean Centers for Disease Control and Prevention since November 2011 (URL: http://cris.nih.go.kr/cris/en/; Unique identifier: KCT0000863; First registration: 01/11/2011). The study was conducted in accordance with the ethical guidelines of the 2004 Declaration of Helsinki. The study was approved by the ethics committee of each participating center and the Chonnam National University Hospital Institutional Review Board ethics committee (CNUH-2011-172). All patients included in the study provided written informed consent prior to enrollment. They were followed-up via face-to-face interviews, phone calls, or chart reviews and they completed a 3-year follow-up schedule. All clinical events were evaluated by an independent event adjudication committee. The event adjudication process has previously been described by the KAMIR investigators29.
PCI procedure and medical treatment
CAG and PCI were performed via a transfemoral or transradial approach in accordance with the general guidelines33. Aspirin (200–300 mg) and clopidogrel (300–600 mg), ticagrelor (180 mg), or prasugrel (60 mg) were prescribed to the patients as loading doses before PCI. After PCI, all patients were recommended to take aspirin (100 mg/day) along with clopidogrel (75 mg/day), ticagrelor (90 mg twice a day), or prasugrel (5–10 mg/day) for at least 1 year. The access site, revascularization strategy, and selection of DES were left to the discretion of the individual operators.
Study definitions and clinical outcomes
NSTEMI was defined as the absence of persistent STE with increased levels of cardiac biomarkers and appropriate clinical context1,2. A successful PCI was defined as residual stenosis of < 30% and thrombolysis in MI (TIMI) flow grade 3 in the infarct-related artery. Glomerular function for estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation34. The GRACE risk score35 was calculated for all the patients. Complex lesions were defined as PCI for unprotected left main coronary disease, multivessel PCI, multiple stents implantation (≥ 3 stents per patient), and those with the total length of deployed stent being over 38 mm36,37. The primary clinical outcome was the occurrence of major adverse cardiac and cerebrovascular events (MACCE), which was defined by all-cause death, recurrent MI (re-MI), any repeat coronary revascularization, including target lesion revascularization, target vessel revascularization (TVR), non-TVR, and stroke. According the American Heart Association/American Stroke Association guideline38, an acute cerebrovascular event resulting in death or neurological deficit for > 24 h or the presence of acute infarction demonstrated by imaging studies was defined as a stroke. An all-cause death was considered a cardiac death (CD) unless an undisputed non-cardiac cause was present39. The secondary clinical outcome was definite or probable stent thrombosis (ST) during a 3-year follow-up period. Stent thrombosis was defined according to the definition provided by the Academic Research Consortium40. The definitions of re-MI, TLR, TVR, and non-TVR have been published previously41.
Statistical analysis
For continuous variables, the differences between the groups were evaluated using unpaired t-tests. Data are expressed as the mean ± standard deviation, or median (interquartile range). For discrete variables, the differences between the groups were expressed as counts and percentages and were analyzed using the chi-squared or Fisher’s exact test. Univariate analysis was performed for all variables of EI and DI groups with the p-value set at < 0.05. Subsequently, we performed a multicollinearity test42 between the included variables to confirm non-collinearity between them (Supplementary Table S1). Variance inflation factor (VIF) values were calculated to measure the degree of multicollinearity among the variables. A VIF of > 5 indicated a high correlation43. When the tolerance value was < 0.144 or the condition index was > 1043, the presence of multicollinearity was considered. The variables included in the multivariable Cox regression analysis were: male sex, age, LVEF, body mass index, systolic blood pressure, diastolic blood pressure, cardiogenic shock, symptom-to-door time, hypertension, diabetes mellitus, dyslipidemia, previous MI, previous PCI, current smoker, CK-MB, peak troponin-I, serum creatinine, eGFR < 60 mL/min/1.73 m2, high-density lipoprotein cholesterol, and GRACE risk score > 140. Moreover, to adjust for potential confounders, propensity score (PS)-matched analysis was performed using a logistic regression model. We tested all potentially relevant variables such as baseline clinical, angiographic, and procedural factors (Table 1). The c-statistic for the PS-matched (PSM) analysis in this study was 0.724. Patients in the EI group were matched to those in the DI group (1:1) using the nearest available pair-matching method according to PSs. The subjects were matched with a caliper width of 0.01. This procedure yielded 1314 well-matched pairs (Table 1). Various clinical outcomes were estimated using a Kaplan–Meier curve analysis, and group differences were compared using the log-rank test. Statistical significance was defined as a 2-tailed p-value of < 0.05. All statistical analyses were performed using SPSS software v. 20 (IBM; Armonk, NY, USA).
Data availability
Data is contained with the article or supplementary material.
References
Collet, J. P. et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 42, 1289–1367 (2021).
Amsterdam, E. A. et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 64, e139–e228 (2014).
Mehta, S. R. et al. Early versus delayed invasive intervention in acute coronary syndromes. N. Engl. J. Med. 360, 2165–2175 (2009).
Álvarez Álvarez, B. et al. Early revascularization and long-term mortality in high-risk patients with non-ST-elevation myocardial infarction. The CARDIOCHUS-HUSJ registry. Rev. Esp. Cardiol. Engl. Ed. 73, 35–42 (2020).
Bonello, L. et al. Timing of coronary invasive strategy in non-ST-segment elevation acute coronary syndromes and clinical outcomes: An updated meta-analysis. JACC Cardiovasc. Interv. 9, 2267–2276 (2016).
Fox, K. A., Eagle, K. A., Gore, J. M., Steg, P. G. & Anderson, F. A. The global registry of acute coronary events, 1999 to 2009–GRACE. Heart 96, 1095–1101 (2010).
Rosengren, A. et al. Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur. Hear. J. 27, 789–795 (2006).
Tegn, N. et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (After Eighty study): An open-label randomised controlled trial. Lancet 387, 1057–1065 (2016).
Kim, Y. H. et al. Impact of stent generation on 2-year clinical outcomes in ST-segment elevation myocardial infarction patients with multivessel disease who underwent culprit-only or multivessel percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 95, E40–E55 (2020).
Mahendiran, T. et al. Optimal timing of invasive coronary angiography following NSTEMI. J. Interv. Cardiol. 2020, 8513257 (2020).
Alexander, K. P. et al. Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: A scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: In collaboration with the Society of Geriatric Cardiology. Circulation 115, 2549–2569 (2007).
Brieger, D. et al. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: insights from the Global Registry of Acute Coronary Events. Chest 126, 461–469 (2004).
Fox, K. A. et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA 297, 1892–1900 (2007).
Bauer, T. et al. Effect of an invasive strategy on in-hospital outcome in elderly patients with non-ST-elevation myocardial infarction. Eur. Heart J. 28, 2873–2878 (2007).
Bach, R. G. et al. The effect of routine, early invasive management on outcome for elderly patients with non-ST-segment elevation acute coronary syndromes. Ann. Intern. Med. 141, 186–195 (2004).
González Ferrero, T. et al. Early angiography in elderly patients with non-ST-segment elevation acute coronary syndrome: The cardio CHUS-HUSJ registry. Int. J. Cardiol. 351, 8–14 (2022).
Abbate, R., Prisco, D., Rostagno, C., Boddi, M. & Gensini, G. F. Age-related changes in the hemostatic system. Int. J. Clin. Lab. Res. 23, 1–3 (1993).
Brandes, R. P., Fleming, I. & Busse, R. Endothelial aging. Cardiovasc. Res. 66, 286–294 (2005).
Usta, C. & Bedel, A. Update on pharmacological treatment of acute coronary syndrome without persistent ST segment elevation myocardial infarction in the elderly. J. Geriatr. Cardiol. 14, 457–464 (2017).
Afilalo, J. et al. Frailty assessment in the cardiovascular care of older adults. J. Am. Coll. Cardiol. 63, 747–762 (2014).
Ekerstad, N. et al. Frailty as an instrument for evaluation of elderly patients with non-ST-segment elevation myocardial infarction: A follow-up after more than 5 years. Eur. J. Prev. Cardiol. 25, 1813–1821 (2018).
Patel, A. et al. Frailty and outcomes after myocardial infarction: Insights from the CONCORDANCE registry. J. Am. Heart Assoc. 7, e009859 (2018).
Mone, P. et al. Cognitive dysfunction correlates with physical impairment in frail patients with acute myocardial infarction. Aging Clin. Exp. Res. 34, 49–53 (2022).
Faubert, C., Heckman, G. & McKelvie, R. Management of non-ST-elevation myocardial infarction in elderly patients: Time to consider frailty and quality of life. Can. J. Cardiol. 34, 241–243 (2018).
Mone, P. et al. Impact of thrombus aspiration in frail STEMI patients. Aging Clin. Exp. Res. 33, 3081–3089 (2021).
Hayıroğlu, M. İ et al. Clinical characteristics and outcomes of acute coronary syndrome patients with intra-aortic balloon pump inserted in intensive cardiac care unit of a tertiary clinic. Turk. Kardiyol. Dern. Ars. 46, 10–17 (2018).
Çinar, T. et al. The predictive value of age, creatinine, ejection fraction score for in-hospital mortality in patients with cardiogenic shock. Coron. Artery Dis. 30, 569–574 (2019).
Hayıroğlu, M. İ, Bozbeyoglu, E., Yıldırımtürk, Ö., Tekkeşin, A. İ & Pehlivanoğlu, S. Effect of acute kidney injury on long-term mortality in patients with ST-segment elevation myocardial infarction complicated by cardiogenic shock who underwent primary percutaneous coronary intervention in a high-volume tertiary center. Turk. Kardiyol. Dern. Ars. 48, 1–9 (2020).
Kim, J. H. et al. Multicenter cohort study of acute myocardial infarction in Korea—Interim analysis of the korea acute myocardial infarction registry-national institutes of health registry. Circ. J. 80, 1427–1436 (2016).
Kim, Y. H. et al. Outcomes between prediabetes and type 2 diabetes mellitus in older adults with acute myocardial infarction in the era of newer-generation drug-eluting stents: A retrospective observational study. BMC Geriatr. 21, 653 (2021).
Kirkman, M. S. et al. Diabetes in older adults. Diabetes Care 35, 2650–2664 (2012).
Barnett, K. et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380, 37–43 (2012).
Grech, E. D. ABC of interventional cardiology: Percutaneous coronary intervention. II: The procedure. BMJ 326, 1137–1140 (2003).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Pieper, K. S. et al. Validity of a risk-prediction tool for hospital mortality: The Global Registry of Acute Coronary Events. Am. Heart J. 157, 1097–1105 (2009).
Choi, K. H. et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc. Interv. 12, 607–620 (2019).
Valgimigli, M. et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 39, 213–260 (2018).
Sacco, R. L. et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44, 2064–2089 (2013).
Lee, J. M. et al. Multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction with cardiogenic shock. J. Am. Coll. Cardiol. 71, 844–856 (2018).
Cutlip, D. E. et al. Clinical end points in coronary stent trials: A case for standardized definitions. Circulation 115, 2344–2351 (2007).
Kim, Y. H. et al. Impact of renin–angiotensin system inhibitors on long-term clinical outcomes in patients with acute myocardial infarction treated with successful percutaneous coronary intervention with drug-eluting stents: Comparison between STEMI and NSTEMI. Atherosclerosis 280, 166–173 (2019).
Vatcheva, K. P., Lee, M., McCormick, J. B. & Rahbar, M. H. Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiology (Sunnyvale) 6, 227 (2016).
Kim, J. H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 72, 558–569 (2019).
Kalantari, S. et al. Predictors of early adulthood hypertension during adolescence: A population-based cohort study. BMC Public Health 17, 915 (2017).
Acknowledgements
Investigators of KAMIR‐NIH (Korea Acute Myocardial Infarction Registry‐National Institutes of Health). Myung Ho Jeong, Chonnam National University Hospital, Gwangju, Korea, Young Jo Kim, Yeungnam University Medical Center, Daegu, Korea, Chong Jin Kim, Kyunghee University Hospital at Gangdong, Seoul, Korea, Myeong Chan Cho, Chungbuk National University Hospital, Cheongju, Korea, Hyo‐Soo Kim, Seoul National University Hospital, Seoul, Korea, Hyeon‐Cheol Gwon, Samsung Medical Center, Seoul, Korea, Ki Bae Seung, Seoul St. Mary’s Hospital, Seoul, Korea, Dong Joo Oh, Korea University Guro Hospital, Seoul, Korea, Shung Chull Chae, Kyungpook National University Hospital, Daegu, Korea, Kwang Soo Cha, Pusan National University Hospital, Busan, Korea, Junghan Yoon, Wonju Severance Christian Hospital, Wonju, Korea, Jei‐Keon Chae, Chonbuk National University Hospital, Jeonju, Korea, Seung Jae Joo, Jeju National University Hospital, Jeju, Korea, Dong‐Ju Choi, Seoul National University Bundang Hospital, Bundang, Korea, Seung‐Ho Hur, Keimyung University Dongsan Medical Center, Daegu, Korea, In Whan Seong, Chungnam National University Hospital, Daejeon, Korea, Doo-II Kim, Inje University Haeundae Paik Hospital, Busan, Korea, Seok Kyu Oh, Wonkwang University Hospital, Iksan, Korea, Tae Hoon Ahn, Gachon University Gil Medical Center, Incheon, Korea, Jin‐Yong Hwang, Gyeongsang National University Hospital, Jinju, Korea.
Funding
This research was supported by a fund (2016-ER6304-02) by Research of Korea Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Contributions
Y.H.K. and A.-Y.H. researched data and wrote the manuscript. Y.H.K., A.-Y.H., S.-W.R., C.U.C., B.G.C., J.B.K., J.Y.P., and S.-H.P. contributed to study design. S.-W.R., C.U.C., B.G.C., J.B.K., S.P., D.O.K., and M.H.J. contributed to the collection research data. Y.H.K., A.-Y.H., S.-W.R., C.U.C., B.G.C., J.B.K., J.Y.P., S.-H.P., and M.H.J contributed to provide intellectual inputs for the discussion. Y.H.K., A.-Y.H., B.G.C., S.P., D.O.K., J.Y.P., and S.-H.P. contributed to data analysis and edited the manuscript. Y.H.K., S.-W.R., and M.H.J contributed to provide supervisor role during the full processes of manuscript submitting and editing. All authors have read and approved the manuscript, and all authors take full responsibility for this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y.H., Her, AY., Rha, SW. et al. Outcomes of early versus delayed invasive strategy in older adults with non-ST-segment elevation myocardial infarction. Sci Rep 12, 11429 (2022). https://doi.org/10.1038/s41598-022-15593-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-15593-w