Abstract
We aimed to examine whether the efficacy of the risk of poor prognosis in patients with coronary artery disease is jointly affected by total cholesterol and baseline serum albumin in a secondary analysis of previous study. We analyzed the data of 204 patients from October 2014 to October 2017 for newly diagnosed stable CAD. The outcome was major adverse cardiac events (MACE; defined as all cause mortality, non fatal myocardial infarction, and non fatal stroke). The median duration of follow-up was 783 days. Multivariable COX model was performed to revalidate the relationship between the sALB and MACE and interaction tests were conducted to find the effects of total cholesterol on their association. A total of 28 MACE occurred among the 204 participants. The risk of MACE varied by baseline serum albumin and total cholesterol. Specifically, lower serum albumin indicated higher risk of MACE (HR 3.52, 95% CI 1.30–9.54), and a test for interaction between baseline serum albumin and total cholesterol on MACE was significant (P = 0.0005). We suggested that baseline serum albumin and total cholesterol could interactively affect the risk of poor prognosis of patients with coronary artery diseases. Our findings need to be confirmed by further randomized trials.
Similar content being viewed by others
Introduction
Percutaneous coronary intervention (PCI), as an important strategy for cardiac revascularization in acute/chronic coronary artery disease (CAD), has been widely used in clinical practice. Compared with people without CAD, the CAD patients usually show higher long-term cardiovascular events, long-term revascularization events, and rehospitalization rates1,2. Some biomarkers such as high sensitivity cardiac troponin (hs-cTn), C-reactive protein (CRP), etc. are considered to be related to the long-term poor prognosis of CAD3,4. For patients with acute coronary syndrome (ACS) undergoing PCI, history of diabetes mellitus, uric acid level, blood urea nitrogen level, and activated partial thromboplastic time (APTT) were all considered risk factors of poor prognosis5. Compared with these biomarkers, serum albumin (sALB) has more and more been focused in recent years. In 2020, a study based on the CGPS (Copenhagen General Population Study) noted that lower sALB levels may be associated with an increased risk of long-term cardiovascular disease in healthy individuals6. In addition, some studies have suggested that low sALB levels may be associated with poor cardiovascular outcomes in patients with CAD7,8,9,10. Some previous studies have suggested that total cholesterol levels (TC) and low-density lipoprotein cholesterol levels (LDL-C), are risk factors for poor prognosis in CAD12,13,14. Moreover, some studies have shown that albumin is closely related to the metabolism and transport of cholesterol [23288948, 29685194, 35737216]. Therefore, we speculated that TC and serum albumin levels may jointly affect poor prognostic risk in patients with CAD. However, to date, this hypothesis has not been fully evaluated. To address this gap in knowledge, our study aimed to examine whether poor prognostic risk in patients with CAD is jointly affected by TC and serum albumin levels in baseline.
Results
Baseline characteristics of participants
The characteristics of the participants are shown in Table 1. Compared with the high sALB group (≥ 4 g/dL), the low sALB group (< 4 g/dL) had lower levels of BMI, Hb, T-chol, TG, LDL, eGFR, ALT, LVEF, and higher CRP level. Given that the low sALB group were older, the lower levels of BMI, sALB, lipoprotein, eGFR, LVEF and liver enzyme might be associated with malnutrition because of aging.
Relationship between the sALB and MACE
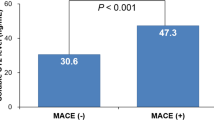
Among the 203 participants included in the analyses, a total of 28 MACE occurred, including 22 in the low sALB group and 6 in the high sALB group. Cox model was used to evaluate the association between sALB and the risk of MACE. After fully adjusting for covariates, the HR of MACE was 0.22 (95% CI 0.11–0.44, P < 0.0001) (Table 2). Cox models were established by dividing the sALB of participants into the low sALB group (< 4 g/dL) and the high sALB group (≥ 4 g/dL) according to the median level. The results showed that, compared with the high sALB group, the HR of MACE in the low sALB group was 3.52 (95% CI 1.30–9.54, P = 0.0133) in the adjusted model. which did not change markedly comparing with the previous results15. Figure 1 showed a trend in the association between sALB and the risk of MACE (logHR), indicating that the risk of MACE decreased as the baseline sALB increased. When sALB level was more than 4.0 g/dL, it remained a protective factor and the risk of MACE remained unchanged.
Statistical interaction between sALB and TC on MACE
Figure 2 presents the association between sALB level and the risk of MACE (logHR), stratified by TC. The solid line, which represents those whose TC less than 200 mg/dL, shows a decline in MACE risk with increasing sALB level after adjusting for covariates. Contrarily, there is no such association in those whose TC more than 200 mg/dL. Because of sample size limitation, our primary outcomes were the tests for interaction between sALB and TC on MACE. The result was statistically significant in crude model (P = 0.0005), and compared with crude model, the effect of TC on the relationship between sALB and MACE did not change markedly after adjusting for age, sex, BMI, ALT, eGFR in the multivariable analysis (P = 0.0005) (Table 3).
Discussion
As some previous studies reported, baseline TC and sALB seem to be risk factors of poor prognosis in CAD patients. We conducted this study mainly to explore whether there is an interaction between them. We analyzed the interaction between TC and sALB and adjusted for covariates that may have influenced the results based on literature reports and clinical practice. Our analysis showed an interaction between TC and sALB levels after adjusting for covariates, which may suggested that baseline TC may influence the effect of sALB on the risk of poor prognosis in patients with coronary artery disease undergoing percutaneous coronary intervention. The relationship between sALB and cardiovascular risk has ever been reported by some previous studies. The study by Suzuki et al. suggested that the baseline sALB was a risk factor of long-term MACE in patients undergoing PCI15. Shih-Chieh Chien et al. pointed out that sALB is one of the reliable risk factors of CVD prognosis16. Oduncu et al. retrospectively analyzed 1706 patients with ST-segment elevation myocardial infarction (STEMI) who underwent PCI and suggested that lower sALB was independent risk factor of long-term mortality17. The potential mechanisms linking TC with sALB in cardiovascular prognostic risk have not been clearly elucidated. The molecular mechanism of albumin's effect on the prognosis of CVD may be multifactorial, which may include maintaining vascular endothelial integrity, regulating vasodilation, binding toxins, anticoagulation, and antioxidation, etc.7. Burl R Don et al. pointed out that the poor nutritional status reflected by low sALB may also be one of the risk factors of poor prognosis18. Studies have pointed out that albumin plays an important role in the transport of serum and cellular cholesterol and the maintenance of cholesterol homeostasis11,16,19,20. Albumin can shuttle free cholesterol among various receptors, which will increase the movement of free cholesterol under concentration gradients between the numerous cholesterol pools present in plasma and tissues, thereby promoting and maintaining cholesterol metabolism to restore steady-state levels20. The improving of cholesterol efflux may improve the prognosis of CAD21. What’s more, Deepak Kumar et al. found that the pseudoesterase activity of albumin might be an important determinant of cholesterol biosynthesis22. These study results may suggest that the influence of albumin level on the risk of poor prognosis may be closely related to cholesterol level. In conclusion, based on our analysis, we believe that TC level may influence the effect of sALB on the risk of poor prognosis in CAD patients treated by percutaneous coronary intervention. The potential limitations of the current analysis should also be considered in interpreting the study results. First, the data of our study came from a single-center sample, mainly elderly patients with CAD who underwent PCI. Therefore, for the extension of the research conclusions, further research is still needed. Second, the patients with chronic liver diseases, end stage renal diseases, malabsorption disease can effect serum albumin and cholesterol levels, but our data did not include these situation of the participants. Thus, we do not know whether TC affects the association of sALB with MACE in these patients, so our conclusions should be applied with caution in this group of patients. Finally, we have to emphasize that our results are merely hypothesis-generating; confirmation of our findings in an independent randomized trial is essential.
Method
Research design and population
This was a secondary scientific analysis of a retrospective, single-center cohort study where the dataset was collected by Sho et al. and is now available on Dryad (via:https://doi.org/10.5061/dryad.fn6730j)15. As previously described, the cohort included hospitalized patients newly diagnosed with stable coronary artery disease and treated with PCI at Shinonoi general hospital from October 2014 to October 2017 in Shinonoi General Hospital. Patients with old myocardial infarction or malignancy were excluded. All participants were enrolled after the approval of Shinonoi General Hospital Ethics Committee and written informed consent. The research was consistent with the principles outlined in Helsinki Declaration. The outcome was major adverse cardiac events (MACE; defined as all cause mortality, non fatal myocardial infarction, and non fatal stroke). Events were validated by chart review and the median duration of follow-up was 783 days15. In our analysis, we included all the cases to revalidate the relationship between the sALB and MACE by adjusting more covariates than the previous research15, and more importantly, we tried to find the effects of TC on their association by interaction tests.
Statistical analysis
The baseline characteristics of the participants were analyzed and divided into two groups according to the median baseline sALB level. The continuous variables with normal distribution were presented as Mean ± SD (comparison between groups by t test), the continuous variables with abnormal distribution were presented as Median (IQR) (comparison between groups by Kruskal Wallis rank sum test) and the categorical variables were presented as N (%) (comparison between groups by χ2 test).
The primary outcome was to determine the effect of TC on the association between MACE and baseline sALB level. Therefore, the results of unadjusted and adjusted covariates model analysis were presented based on STROBE recommendations. When the covariate was included or excluded from the model, the hazard ratio of the match was changed by at least 10%, and this covariate needed to be adjusted23. Besides, if it was related to serum albumin and MACE in clinical practice, covariate wound be included with other congener covariates excluded. Moreover, covariates adjusted in previous congener studies were also included24. And finally we adjusted, if not stratified, for age, sex, BMI, TC, eGFR, ALT in the multivariable model.
In the multivariable regression analysis, baseline sALB level was first analyzed as a continuous variable and then divided into two groups according to the median level. Interaction tests were conducted according to TC (< 200 mg/dL and ≥ 200 mg/dL), and the models were divided into crude model and adjusted model according to the adjusted covariates.
P values less than 0.05 (two-tailed) were considered statistically significant. EmpowerStats ver.3.0 (http://www.empowerstats.net/analysis/, X&Y Solutions,Inc., Boston, MA) and the R software ver.3.3.1 (http://www.R-project.org/, The R Foundation), were used for all statistical analyses.
Data availability
The dataset was collected by Sho et al. and is now available on Dryad (via: https://doi.org/10.5061/dryad.fn6730j). The datasets generated or analysed during the current study are available from the corresponding author on reasonable request.
References
Eisen, A. et al. Angina and future cardiovascular events in stable patients with coronary artery disease: Insights from the reduction of atherothrombosis for continued health (REACH) registry. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.116.004080 (2016).
Fox, K. A. A., Metra, M., Morais, J. & Atar, D. The myth of “stable” coronary artery disease. Nat. Rev. Cardiol. 17, 9–21. https://doi.org/10.1038/s41569-019-0233-y (2020).
Yayan, J. Emerging families of biomarkers for coronary artery disease: Inflammatory mediators. Vasc. Health Risk Manag. 9, 435–456. https://doi.org/10.2147/VHRM.S45704 (2013).
Rusnak, J. et al. Biomarkers in stable coronary artery disease. Curr. Pharm. Biotechnol. 18, 456–471. https://doi.org/10.2174/1389201018666170630120805 (2017).
Wang, R. et al. Determination of risk factors affecting the in-hospital prognosis of patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention. BMC Cardiovasc. Disord. 17, 243. https://doi.org/10.1186/s12872-017-0660-9 (2017).
Ronit, A. et al. Plasma albumin and incident cardiovascular disease: Results from the CGPS and an updated meta-analysis. Arterioscler. Thromb. Vasc. Biol. 40, 473–482. https://doi.org/10.1161/ATVBAHA.119.313681 (2020).
Chien, S. C. et al. Association of low serum albumin concentration and adverse cardiovascular events in stable coronary heart disease. Int. J. Cardiol. 241, 1–5. https://doi.org/10.1016/j.ijcard.2017.04.003 (2017).
Huang, H. et al. Independent and joint effects of high-sensitivity c-reactive protein and hypoalbuminemia on long-term all-cause mortality among coronary artery disease: A prospective and multicenter cohort study. BMC Cardiovasc. Disord. 21, 613. https://doi.org/10.1186/s12872-021-02431-6 (2021).
Polat, N. et al. Prognostic significance of serum albumin in patients with acute coronary syndrome. Angiology 71, 903–908. https://doi.org/10.1177/0003319720941747 (2020).
Demir, M., Özbek, M., Aktan, A. & Ertaş, F. J. D. T. D. Fibrinogen to albumin ratio predicts burden of coronary artery disease in patients with NSTEMI. BMC Cardiovasc. Disord. 48, 688–695 (2021).
Arques, S. Human serum albumin in cardiovascular diseases. Eur. J. Intern. Med. 52, 8–12. https://doi.org/10.1016/j.ejim.2018.04.014 (2018).
Navarese, E. P. et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: A systematic review and meta-analysis. JAMA 319, 1566–1579. https://doi.org/10.1001/jama.2018.2525 (2018).
Packard, C., Chapman, M. J., Sibartie, M., Laufs, U. & Masana, L. Intensive low-density lipoprotein cholesterol lowering in cardiovascular disease prevention: Opportunities and challenges. Heart 107, 1369–1375. https://doi.org/10.1136/heartjnl-2020-318760 (2021).
Sabatine, M. S. PCSK9 inhibitors: Clinical evidence and implementation. Nat. Rev. Cardiol. 16, 155–165. https://doi.org/10.1038/s41569-018-0107-8 (2019).
Suzuki, S. et al. Prognostic significance of serum albumin in patients with stable coronary artery disease treated by percutaneous coronary intervention. PLoS ONE 14, e0219044. https://doi.org/10.1371/journal.pone.0219044 (2019).
Chien, S. C., Chen, C. Y., Lin, C. F. & Yeh, H. I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark. Res. 5, 31. https://doi.org/10.1186/s40364-017-0111-x (2017).
Oduncu, V. et al. The prognostic value of serum albumin levels on admission in patients with acute ST-segment elevation myocardial infarction undergoing a primary percutaneous coronary intervention. Coron. Artery Dis. 24, 88–94. https://doi.org/10.1097/MCA.0b013e32835c46fd (2013).
Don, B. R. & Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 17, 432–437. https://doi.org/10.1111/j.0894-0959.2004.17603.x (2004).
Meierhofer, T., van den Elsen, J. M., Cameron, P. J., Munoz-Berbel, X. & Jenkins, A. T. The interaction of serum albumin with cholesterol containing lipid vesicles. J. Fluoresc. 20, 371–376. https://doi.org/10.1007/s10895-009-0522-7 (2010).
Sankaranarayanan, S. et al. Serum albumin acts as a shuttle to enhance cholesterol efflux from cells. J. Lipid Res. 54, 671–676. https://doi.org/10.1194/jlr.M031336 (2013).
Brownell, N. & Rohatgi, A. Modulating cholesterol efflux capacity to improve cardiovascular disease. Curr. Opin. Lipidol. 27, 398–407. https://doi.org/10.1097/MOL.0000000000000317 (2016).
Kumar, D., Behal, S., Bhattacharyya, R. & Banerjee, D. Pseudoesterase activity of albumin: A probable determinant of cholesterol biosynthesis. Med. Hypotheses 115, 42–45. https://doi.org/10.1016/j.mehy.2018.03.018 (2018).
Vandenbroucke, J. P. et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Ann. Intern. Med. 147, W163-194. https://doi.org/10.7326/0003-4819-147-8-200710160-00010-w1 (2007).
Seidu, S., Kunutsor, S. K. & Khunti, K. Serum albumin, cardiometabolic and other adverse outcomes: Systematic review and meta-analyses of 48 published observational cohort studies involving 1,492,237 participants. Scand. Cardiovasc. J. 54, 280–293. https://doi.org/10.1080/14017431.2020.1762918 (2020).
Acknowledgements
Thanks for the kind work and sincere suggestions of editor and reviewers.
Funding
Shenzhen Science and Technology R&D Fund Project (JCYJ20150330102720161); Shenzhen Second People’s Hospital Clinical Research Fund of Guangdong Province High-level Hospital Construction Project (No. 20193357009); Shenzhen Second People’s Hospital Clinical Research Fund of Guangdong Province High-level Hospital Construction Project (No. 20213357016).
Author information
Authors and Affiliations
Contributions
Y.Y. contributed to the conception, data analyses and manuscript preparation of the study; Z.C. contributed to the conception, data analyses and wrote the manuscript; H.C. and T.L. helped perform the analysis with constructive discussions. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yao, YF., Chen, ZY., Luo, TY. et al. Cholesterol affects the relationship between albumin and major adverse cardiac events in patients with coronary artery disease: a secondary analysis. Sci Rep 12, 12634 (2022). https://doi.org/10.1038/s41598-022-16963-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16963-0