Abstract
Records on the distribution of Rickettsia spp. in their natural hosts in Central Asia are incomplete. Rodents and small mammals are potential natural reservoirs for Rickettsiae in their natural lifecycle. Studies about the maintenance of Rickettsia in wild animals are available for Western nations, but—to our knowledge—no studies and data are available in the Republic of Kazakhstan so far. The first case description of Rickettsioses in Kazakhstan was made in the 1950ies in the Almaty region and now Kyzylorda, East Kazakhstan, Pavlodar and North Kazakhstan are endemic areas. The existence of murine and endemic typhus was proven in arthropod vectors in the regions Kyzylorda and Almaty. Here we show for the first time investigations on tick-borne Rickettsia species detected by a pan-rickettsial citrate synthase gene (gltA) real-time PCR in ear lobes of small mammals (n = 624) in Kazakhstan. From all analysed small mammals 2.72% were positive for Rickettsia raoultii, R. slovaca or R. conorii. Sequencing of the rickettsial gene OmpAIV and the 23S–5S interspacer region revealed a similar heritage of identified Rickettsia species that was observed in ticks in previous studies from the region. In summary, this study proves that rodents in Kazakhstan serve as a natural reservoir of Rickettsia spp.
Similar content being viewed by others
Introduction
Rickettsiae are small (0.3–0.5 by 0.8–2.0 µm) gram-negative intracellular bacteria, living in the cytosol of their host cells1. The genus Rickettsia is divided into four groups. (i) The spotted fever group (SFG) is linked predominantly with ticks and less often with fleas and mites, including Mediterranean-, Rocky Mountain- and Helvetica spotted fever. (ii) The typhus group (TG), which includes agents of epidemic typhus and murine typhus associated with lice and fleas. (iii) An ancestral group with R. bellii and R. canadensis and (iv) a transitional group with members of R. akari and R. felis.
The SFG, TG, and transitional groups include agents qualified to cause disease in human1,2. SFG is distributed worldwide and includes more than 30 species. At least 15 species cause disease, such as R. rickettsii in North America, which leads to Rocky Mountain spotted fever (RMSF) or R. conorii, causing Mediterranean spotted fever (MSF) in parts of Europe, Africa, and Asia3,4,5,6,7,8. From the TG R. typhi is causing murine/endemic typhus and more seldom R. prowazekii is inducing louse-borne or epidemic typhus in humans3,9,10. The transitional group comprises of three species transmitted by different vectors, among which R. akari is transmitted by mites (rickettsialpox), R. australis is transmitted by ticks (Queensland tick typhus), and R. felis is transmitted by fleas (flea-borne spotted fever)8,11,12,13,14,15,16.
Characteristic clinical manifestations caused by members of the SFG group include symptoms like fever, skin rash and in some cases also inoculation eschars. Moreover, other non-specific flu-like symptoms as febrile temperatures, cough, widespread lymphadenopathy, myalgia, abdominal ache and infections of the central nervous system are possible. Members of the TG group are causing epidemic typhus or murine typhus and come with symptoms such as high fever, headache and rashes on chest and extremities combined with nonspecific symptoms like cough, myalgia and malaise. In addition, neurological manifestations, like headache, meningitis and encephalitis, are also reported2,17,18.
Rickettsioses are generally distributed worldwide4. Sparse information is available on the disease and the distribution of tick-transmitted infections like Rickettsioses in Asian countries, but it is known that SFG and TG Rickettsia are present in Southeast Asia2,3,18,19,20. However, there are only incomplete records on the distribution of Rickettsia in Central Asia. In a representative country for the region, the Republic of Kazakhstan, most of the available information is based on anecdotal reports as described during an expedition by Bartoshevic to the region of Almaty in 1949–195121. In 1961 clinical symptoms of tick-borne rickettsioses were observed in South Kazakhstan, West Kazakhstan, Pavlodar, North Kazakhstan and Akmola region22. In 1961, R. sibirica was detected in Dermacentor marginatus and Haemaphysalis punctata ticks collected from the Yenbekhikazakh district in Almaty region23. Other studies have confirmed, that R. conorii ssp. caspia, R. slovaca, R. raoultii, R. aeschlimannii, R. asembonensis and R. felis are circulating throughout Kazakhstan17,24,25,26,27,28,29,30.
Official endemic regions for Rickettsioses in Kazakhstan are currently North Kazakhstan, Pavlodar, East Kazakhstan and Kyzylorda. From 1995 to 2021 a total of 4627 human cases of tick-borne rickettsioses were reported in Kazakhstan. In recent years the incident rates in Kazakhstan increased from 0.41 (per 100,000 inhabitants per year) in 1995 to the highest rates of 1.19 in 2018 and 1.12 in 2019. The highest incidence seen from 1995 to 2021 was observed in 2019 in Kyzylorda region (incidence values of 1.64–12.68) and in Pavlodar region (incidence of 1.07–9.15)31. In comparison, in the USA 5000–6000 SFG cases were recorded during 2017, 2018 and 2019 with an incidence ranging between 1.5 and 1.832.
While tick-associated Rickettsioses are monitored and reported in patients in Kazakhstan, relatively little is known about the spread of this zoonosis in the fauna of the country. A recent study on the prevalence of Rickettsia species in ticks in Almaty and Kyzylorda regions revealed a minimum infection rate (MIR) of 0.4–15.1% in Almaty region and 12.6–22.7% in Kyzylorda region. The detected species were R. raoultii, R. slovaca, a new Candidatus R. yenbekshikazakhensis, and the new genotype of R. talgarensis33.
Wild animals act as a natural reservoir for Rickettsia spp. and maintain the pathogens’ life cycle in nature34,35. Some data on the natural life cycle of Rickettsia are available from Europe, but no data from Central Asia are published so far. The European studies showed that screening ear pinnae of small mammals is a suitable tissue to detect Rickettsia species36.
The aim of this work was to identify Rickettsia spp. in ear pinnae of small mammals in West-Kazakhstan and Almaty region to study the distribution and the heritage of rickettsial pathogens in both regions.
Material and methods
Collection of tissues from small mammals
Small mammals trapping was conducted upon ethical approval of Kazakhstan local ethics committee at the National Scientific Center for Especially Dangerous Infectious in Almaty, Kazakhstan (protocol #4, 08.01.18) and the ethical committee of the Ludwig-Maximilians-University in Munich, Germany (opinion number 18-631) using snap traps in 2018 and 2019. Reporting of the animal experiments followed the recommendations in the ARRIVE guidelines. In West-Kazakhstan region, small mammals were trapped in 19 trapping sites of the four districts: Bayterek, Borili, Terekti, and Taskala. In Almaty region, small mammals were trapped in the three districts Tekeli, Rudnichniy, and Bakanas. In Almaty city small mammals were trapped in seven trapping sites (detailed location information see Supplementary Table 1 and Tukhanova et al.37). From the 624 trapped small mammals, ear pinnae were removed aseptically and stored in RNAlater (ThermoFisher Scientific, Waltham, United States), at − 20 °C. All methods were carried out in accordance with relevant guidelines and regulations.
DNA extraction
Ear pinnae from small mammals were homogenized with two stainless steel beads and 1 ml of cell culture medium (Gibco™ MEM, ThermoFisher Scientific, Massachusetts, United States) using the TissueLyser II (2 min at 30 Hz) (Qiagen, Hilden, Germany). The homogenized samples were centrifuged for 5 min at 20,000×g.
DNA was isolated from 350 µl of the supernatant using QiAmp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and stored in aliquots at − 20 °C.
Real-time PCR approach
A real time PCR assay to screen for rickettsial DNA in the rodent ear pinnae was performed using the LightCyclerFastStart DNA Master HybProbe System (Roche, Basel, Switzerland) and a Rotor-GeneQ (Qiagen, Hilden, Germany) targeting the pan-rickettsial citrate synthase gene (gltA). An Uracil-DNA-glycosylase (UDG) incubation step was added to get rid of any carry-over PCR products between the reactions36,38. The total volume of the assay was 20 µl, incorporating 5 µl sample (containing up to 500 ng of DNA). The assay included 0.5 µM of primers PanRick_gltA_2 forward (5′-ATA GGA CAA CCG TTT ATT T-3′) and PanRick_gltA_2 reverse (5′-CAA ACA TCA TAT GCA GAA A-3′) and 0.2 µM of the probe PanRick_gltA_2_taq (5′-6FAM-CCT GAT AAT TCG TTA GAT TTT ACC G-DB-3′)33,36,38.
Conventional PCR to generate DNA fragments for sequencing
Real-time PCR positive samples were further investigated using conventional PCR to amplify a fragment of the outer membrane protein OmpAIV (primers RR 190-5125: 5′-GCG GTT ACT TTA GCC AAA GG-3′, cRR 190-6013: 5′-TCT TCT GCG TTG CAT TAC CG-3′)36,38,39 and the 23S–5S interspacer region (23S forward: 5′-GAT AGG TCG GGT GTG GAA GCA C-3′, 23S reverse: 5′-GGG ATG GGA TCG TGT GTT TCA C-3′)40 according to published protocols33,36,38,39,40 for subsequent sequencing. The total volume of the PCR assay was 50 µl with a final primer concentration of 0.5 µM and 5 µl of DNA sample (containing up to 500 ng of DNA). PCR products were analysed on agarose gels, with an expected band between 378–532 bp for the 23S–5S interspacer region40 and 888 bp for the OmpAIV39.
Sequencing
All conventional PCR products targeting the partial OmpAIV and 23S–5S interspacer region were purified using the QIAquick PCR purification kit (Qiagen, Hilden, Germany). Sequencing was performed according to manufacturer’s instructions using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, USA) and a 3730xl DNA Analyzer (Applied Biosystems, Waltham, USA).
Phylogenetic analysis
Before BLAST-aided species determination and phylogenetic tree analysis the primer sequences were deleted from the sequences and then aligned in BioEdit 7.2.541. Nucleotide sequence analyses were performed with Chromas Lite, version 2.1 (Technelysium Pty Ltd, South Brisbane, Australia) and compared for similarity to sequences deposited in NCBI GenBank. Phylogenetic trees were constructed in MEGA X with the Maximum Likelihood method based on the Tamura 3-parameter model42. Obtained OmpAIV and 23S–5S interspacer nucleotide sequences were deposited in NCBI GenBank database under accession numbers ON604636–ON604650.
Results
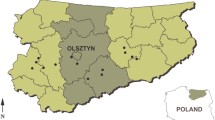
Eleven species of small mammals were collected from 29 trapping sites from West-Kazakhstan region, Almaty region and Almaty city (Fig. 1) in 2018 and 2019. The 624 small mammals were grouped into either rodents or insectivores. Members of the families Cricetidae (Microtus arvalis (n = 87), Clethrionomys glareolus (n = 13) and Microtus kirgisorum (n = 49)), of Muridae (Apodemus uralensis (n = 259), Mus musculus (n = 128), Rattus norvegicus (n = 39), Meriones meridianus (n = 2)) and of Gliridae (Dryomys nitedula (n = 15)) were examined. In addition, insectivores including Crocidura suaveolens (n = 28) and members of Sorex spp. (n = 4) (Supplementary Table 1).
Investigation of Rickettsia spp. in Kazakhstan. Rickettsioses in humans are endemic in North Kazakhstan, Pavlodar, East Kazakhstan and Kyzylorda (light grey marked areas). Small mammals and rodents were investigated in West Kazakhstan and Almaty region (dark grey marked areas) with indicated positive sampling spots (•) in 2018 and 2019. In West-Kazakhstan in the area of Bayterek and in Almaty region in Tekeli (left •) and Bakanas (right •).
From the 624 screened ear pinnae collected from small mammals, 17 (2.72%) were positive for the pan-Rickettsia citrate synthase gene gltA (Table 1). Rickettsia were detected in rodents captured in either the Bakanas district (Almaty region) from M. musculus (n = 8) and in M. meridianus (n = 1) or in the area of Tekeli from M. arvalis (n = 2) and A. uralensis (n = 1), 200 km to the east of Bakanas. In addition, in the West-Kazakhstan region five positive samples were detected from the Bayterek district, with a prevalence of 2.3% (n = 5/220) in A. uralensis (n = 4) and M. arvalis (n = 1).
The prevalence of rickettsial infection in the different species varied depending on the region. In Almaty region the prevalence is 50% for M. meridianus (n = 1/2), 12% for M. musculus (n = 8/66), 2.7% for M. arvalis (n = 2/74), and 0.76% for A. uralensis (n = 1/131), whereas in West-Kazakhstan region the prevalence of rickettsial DNA is 3.13% in A. uralensis (n = 4/128) and 7.7% in M. arvalis (n = 1/13).
Of all 17 gltA real-time PCR positive rodents, conventional PCR for detecting a part of the outer membrane protein OmpAIV and of the 23S–5S interspacer region was performed to gain more information about the exact species of Rickettsia detected. In total 18 sequences were obtained, nine partial OmpAIV-, and nine partial 23S–5S interspacer region sequences. The partial OmpAIV sequences, all obtained in 2018, are from the districts Tekeli (n = 1, AO-Tek-2018-34) and Bakanas (n = 6, AO-Bak-2018-1, -2, -3, -5, -7 and -13) in Almaty region and from the Bayterek area (WKO-Bay-2018-26 and -39) in West Kazakhstan region.
The partial 23S–5S interspacer fragments were from Bakanas- (Almaty region: AO-Bak-2018-1, -2, -3, -5, -7, -8, -13) and Bayterek districts (West Kazakhstan region: WKO-Bay-2018-20), all obtained in 2018 and one from 2019 (WKO-Bay-2019-40). Obtained sequences were compared to publicly available sequences deposited in the NCBI GenBank database using NCBI BLAST and R. raoultii, R. slovaca, or R. conorii were returned as the putative species detected in the ear lobes.
In six samples, both OmpAIV and 23S–5S interspacer sequences were obtained from the same ear lobe (AO-Bak-2018-1, -2, -3, -5, -7 and -13). However, only four of them yielded sequence reads long enough for a reliable phylogenetic analysis. Two 23S–5S interspacer sequence reads that were too short (AO-Bak-2018-5 and -7) were excluded from the phylogenetic analysis (Table 1, marked with ***).
Three of the four paired samples for both gene loci (OmpAIV and 23S–5S interspacer region) were R. raoultii (AO-Bak-2018-1, -2 and -3). However, one of the paired samples (AO-Bak-2018-13), showed the closest phylogenetic relationship to different rickettsial species for OmpAIV and 23S–5S interspacer region, respectively. The 23S–5S interspacer fragment revealed a very high sequence similarity (100% identity; 340 of 340 nt identical) to a R. raoultii isolate from Tekeli (MG974041but the partial OmpAIV sequence clustered with a very high resemblance (100% identity; 715 of 715 nt identical) to a published R. slovaca sequence from Tekeli (MG973999)33.
This ambiguity of the species can also be observed in the phylogenetic trees in Figs. 2 and 3, where representatives of worldwide distributed Rickettsia species and also published Rickettsia sequences from Kazakhstan like R. raoultii from Tekeli (Almaty region) and Kyzylorda region as well as the recently recorded “Candidatus Rickettsia yenbekshikazakhensis” and “genotype Rickettsia talgarensis”33 are included. AO-Bak-2018-13 clusters in Fig. 2 for OmpAIV with other strains of R. slovaca (MG973999 and CP002428) and in Fig. 3 for 23S–5S interspacer region with representatives of R. raoultii from Tekeli (MG974041 and MG974047).
Maximum likelihood phylogenetic tree based on 68 partial OmpAIV DNA sequences. Nine sequences are originating from amplificates from small rodents from Kazakhstan and 59 from the GenBank database. Eight of the new generated sequences from Kazakhstan were 100% identical to R. raoulti and one were 100% identical to R. slovaca. In addition, 30 sequences form the Candidatus Rickettsia yenbekshikazakhensis and three sequences form the “genotype Rickettsia talgarensis” cluster. The tree with the highest log-likelihood (− 2445.21) is shown. There are in total 720 positions in the final dataset.
Maximum likelihood phylogenetic tree based on 46 partial 23S–25S interspacer DNA sequences. Six sequences originating from amplificates from small rodents from Kazakhstan and 40 from the GenBank database. Four of the new generated sequences from Kazakhstan were 90–100% identical to R. raoulti, one was 99% identical to R. slovaca and one was 99% identical to R. conorii. In addition, nine sequences form the Candidatus Rickettsia yenbekshikazakhensis and two sequences form the “genotype Rickettsia talgarensis” cluster. Three sequences form the cluster of Rickettsia helvetica. The tree with the highest log-likelihood (− 1639.61) is shown. There are in total 411 positions in the final dataset.
For some gltA-positive ear lobes only one sequence, either from OmpAIV or from the 23S–5S interspacer region could be generated. Three partial sequences for OmpAIV (AO-Tek-2018-34, WKO-Bay-2018-26, and WKO-Bay-2018-39) showed high similarity to R. raoultii (MG973997 and AH015610, sequence identity 100%, Table 1). In addition, three individual sequences for the 23S–5S interspacer region (AO-Bak-2018-8, WKO-Bay-2018-20, WKO-Bay-2019-40) clustered with R. raoultii (CP010969, sequence identity 99.7–100%), R. slovaca (MG450332, sequence identity 99.44%) or R. conorii (AY125012, sequence identity 99.73%), respectively (Fig. 2 and Table 1).
Discussion
To our knowledge, this study shows the first large-scale investigation of the prevalence of tick-transmitted Rickettsia in rodents in Kazakhstan. It is a follow-up study to recently published investigations on Rickettsia in ticks33 as well as agents for fever of unknown origin in patients43. Hence, it closes the gap between missing vector information and disease data in humans since it investigates the prevalence in natural hosts. Two regions of Kazakhstan were part of the study, the West-Kazakhstan region and the Almaty region in the south-east of the country including Almaty city. Both regions are not yet listed as endemic areas of rickettsiosis in Kazakhstan. Currently officially endemic areas for SFG rickettsioses in Kazakhstan are North-Kazakhstan, Pavlodar, East-Kazakhstan and Kyzylorda regions (Fig. 1). Only in these endemic areas the numbers of infections and incidences are recorded and listed in annual reports on case numbers43,44.
Here we show that Rickettsia species can be detected in the ear pinnae of several families of small mammals such as Cricetidae (M. arvalis) and Muridae (M. musculus and A. uralensis). From 624 screened small mammals, 17 were positive in a gltA screening PCR. This is a surprisingly high number given the fact that a screening based on PCR identifies only animals that have an acute infection with rickettsia. The rickettsial bacteraemia in rodents is rather short45, however, this is the critical phase for transmission to other ticks that might become infected while feeding. The rodents that yielded a positive gltA PCR such as M. musculus or M. arvalis are typical hosts of Dermacentor marginatus, a tick reported to carry R. raoultii and R. slovaca in previous studies in the investigated areas46. In comparison in Europe and Africa small mammals have a prevalence of Rickettsia spp. ranging from 5.2 to 17.6%, however those screenings were from areas that were suspected as rickettsia hotspots36,47,48. In our study randomly selected sampling spots were also included that had no previous history of rickettsioses.
To gain an idea on the genotypes circulating in small mammals in Kazakhstan we further amplified and sequenced two partial gene loci, OmpAIV and the 23S–5S interspacer region, from the gltA positive samples. Of 17 positive ear pinnae we could retrieve six partial fragments of 23S–5S interspacer regions and nine partial OmpAIV fragments. Using the NCBI BLAST nucleotide algorithm it was possible to gain more information on the genotype of the Rickettsia infecting the rodents. All identified sequences had high similarities to either R. raoultii, R. slovaca, or R. conorii. All three have been reported previously to reside in ticks in Kazakhstan46 and are of the SFG group. R. slovaca and R. raoultii are human pathogens that may cause scalp eschar and neck lymph adenopathy after a tick bite (SENLAT) syndrome that was also reported in Kazakhstan44.
A phylogenetic analysis of the obtained sequences with other sequences deposited in NCBI GenBank shows that the amplified fragments cluster closely with other rickettsia sequences that were obtained from ticks or small mammals in the region. Sequences from rodents in the Bakanas district had a close phylogenetic relationship to sequences obtained from ticks isolated in Tekeli, a city from the same region in Kazakhstan. In the Almaty oblast area R. raoultii is dominant and was mostly isolated from Mus musculus. Previous studies found R. raoultii in this region in Dermacentor ticks33, a tick that is known to feed on M. musculus. We are the first to sequence partial rickettsial genomes in the West Kazakhstan region, more than 2000 km to the west from the sampling sites in Almaty region. Still the phylogenetic distance is very short. This either proves that the genome of Rickettsia is highly evolutionarily conserved49,50, or allows the alternative explanation that the respective Rickettsia strains only recently moved to West Kazakhstan by migratory small mammals, birds or ticks they carry. This assumption may be supported by the fact that in Almaty region, the rate animals infected with rickettsia was about 3%, while in West Kazakhstan Oblast it was slightly lower at 2.2%.
In one ear lobe isolate (AO-Bak-2018-13) conflicting results were obtained from the OmpAIV and 23S–5S interspacer sequencing. The OmpAIV returned as a R. slovaca and the 23S–5S interspacer sequence grouped with R. raoultii. Theoretically it is possible, that this rodent was infected with two Rickettsia species at the same time. To explain this, it would be necessary to perform multi locus sequence typing (MLST) on seven or more loci of the rickettsia genome, howsoever this was not practical in the scope of this research project.
Unfortunately, not all positive gltA samples yielded amplicons for the OmpAIV or 23S–5S interspacer region to obtain sequences for phylogeny. Other studies already showed that conventional PCR assays are less sensitive than real-time PCR assays51. This explains, why some lysates yielded positive results in the real time PCR but failed to produce an amplicon product in the conventional PCR.
The role of rodents and small mammals in the life-cycle of Rickettsia is far from being fully understood8,36,52,53. Ticks may transmit Rickettsia transovarially and also transstadially, which empowers the spread of the bacteria within the tick population without any additional vertebrate reservoir54. Co-feeding might serve also as a transmission route for Rickettsia spp.55. However, infection of vertebrates during tick feeding probably still plays a significant role. Indeed studies highlight that small animals—living in the wild or in laboratories—act as potential reservoir hosts for Rickettsia species51,56,57. Other studies, however, claim that rodents and small mammals do not carry any rickettsial DNA suggesting they do not play a role53,58,59,60,61. However, these findings should be taken with caution, as the selection of the organs examined and the capture sites may not have been optimal.
The ear lobes are a favourable region for ectoparasites like ticks and fleas that are feeding on rodents and other small mammals36. However, here we could not investigate whether rodents with Rickettsia-infected ears would also yield a positive PCR result when screening alternative organ tissues from the same animals. Other studies showed that rickettsial DNA can be detected in blood and skin biopsies (like ear pinnae), however with stark differences47,51. It is reported that the amount of rickettsial DNA in skin biopsies is threefold higher compared to the rickettsial DNA content of blood. Spleen samples have even lower DNA contents in infected animals47.
This screen for rickettsial DNA in small mammals and rodents completes other investigations on Rickettsia in Central Asia. A previous study in Kazakhstan on fever patients enrolled in Kyzylorda, an endemic region, and Almaty region, a non-endemic region, showed that in both regions 1.4% of 802 patients had acute SFG rickettsioses and 2.7% acute TG rickettsioses. A previous infection with SFG or TG rickettsia was detected in approximately 30% of the participating patients43. This study on patients was backed-up by a further investigation of ticks collected in the same regions (Almaty and Kyzylorda). Here, several Rickettsia species were identified in the arthropod vectors33. The MIR for rickettsia in the investigated ticks (Dermacentor marginatus, D. reticulatus, Haemaphysalis punctata, Hyalomma asiaticum, and Rhipicephalus turanicus) in Kyzylorda region was 12.6–22.7%, and in the non-endemic Almaty region 0.4–15.1%. In those ticks R. raoultii and R. slovaca, the new “Candidatus R. yenbekshikazakhensis” and a new genotype “genotype R. talgarensis” were detected46. The role of other vectors was assessed in additional studies. For instance, several Rickettsia species were detected in ticks and fleas collected all over Kazakhstan including Kyzylorda, East Kazakhstan, West-Kazakhstan and Almaty region17,24,25,26,28,29. At the Kazakhstan-China border in the Chinese province of Xinjiang several Rickettsia species (R. raoultii, Candidatus R. barbariae and genotype Babesia) were detected in Haemaphysalis ticks that were collected from Vormela peregusna (marbled polecats)62. These publications showed, that Rickettsiae are more widely distributed in Kazakhstan than officially reported and also reside in non-endemic areas such as the Almaty region. Moreover, microorganisms reside in dynamic borders and their prevalence in certain regions is heavily influenced by many factors such as climatic conditions, environmental changes, differences in urbanisation or land-use and other factors affecting both the bacteria themselves and their hosts. It is therefore essential to close the gaps in prevalence and vector data and keep a vigilant eye on changes. Continuous monitoring and surveillance are needed to keep track of any variations in these multi-faceted rickettsial ecosystems.
In summary this study highlights that rickettsial bacteria can be detected in small animals in non-endemic areas like Almaty region and West-Kazakhstan region. In areas where rickettsial infections are not monitored, the number of patients with rickettsiosis will be underestimated, as already postulated in a previous patient study in Almaty region43. Hence, physicians and policy makers in the Republic of Kazakhstan should be aware that rickettsioses are more widespread than previously thought.
Data availability
The data used and/or analysed during the current study are available from the corresponding author on reasonable request. All generated sequences were uploaded to NCBI Genbank and are accessible as ON604636, ON604637, ON604638, ON604639, ON604640, ON604641, ON604642, ON604643, ON604644, ON604645, ON604646, ON604647, ON604648, ON604649 and ON604650.
References
Blanton, L. S. The rickettsioses: A practical update. Infect. Dis. Clin. North Am. 33, 213–229 (2019).
Parola, P. et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 26, 657–702 (2013).
Robinson, M. T., Satjanadumrong, J., Hughes, T., Stenos, J. & Blacksell, S. D. Diagnosis of spotted fever group Rickettsia infections: The Asian perspective. Epidemiol. Infect. 147, e286 (2019).
Graves, S. & Stenos, J. Rickettsioses in Australia. Ann. N. Y. Acad. Sci. 1166, 151–155 (2009).
Niang, M. et al. Prevalence of antibodies to Rickettsia conorii, Ricketsia africae, Rickettsia typhi and Coxiella burnetii in Mauritania. Eur. J. Epidemiol. 14, 817–818 (1998).
Parola, P. Tick-borne rickettsial diseases: Emerging risks in Europe. Comp. Immunol. Microbiol. Infect. Dis. 27, 297–304 (2004).
Nanayakkara, D. M., Rajapakse, R. P. V. J., Wickramasinghe, S. & Kularatne, S. A. M. Serological evidence for exposure of dogs to Rickettsia conorii, Rickettsia typhi, and Orientia tsutsugamushi in Sri Lanka. Vector Borne Zoon. Dis. Larchmt. N 13, 545–549 (2013).
Brown, L. D. & Macaluso, K. R. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr. Trop. Med. Rep. 3, 27–39 (2016).
Newton, P. N. et al. A prospective, open-label, randomized trial of doxycycline versus azithromycin for the treatment of uncomplicated murine typhus. Clin. Infect. Dis. 68, 738–747 (2019).
Vallee, J. et al. Contrasting spatial distribution and risk factors for past infection with scrub typhus and murine typhus in Vientiane City, Lao PDR. 4 (2010).
Akram, S. M., Jamil, R. T. & Gossman, W. G. Rickettsia Akari (2021).
Dong, X., El Karkouri, K., Robert, C., Raoult, D. & Fournier, P.-E. Genome sequence of Rickettsia australis, the agent of Queensland tick typhus. J. Bacteriol. 194, 5129 (2012).
Fournier, P.-E. & Raoult, D. Current knowledge on phylogeny and taxonomy of Rickettsia spp. Ann. N. Y. Acad. Sci. 1166, 1–11 (2009).
Legendre, K. P. & Macaluso, K. R. Rickettsia felis: A review of transmission mechanisms of an emerging pathogen. Trop. Med. Infect. Dis. 2, E64 (2017).
Murray, G. G. R., Weinert, L. A., Rhule, E. L. & Welch, J. J. The phylogeny of rickettsia using different evolutionary signatures: How tree-like is bacterial evolution?. Syst. Biol. 65, 265–279 (2016).
Shpynov, S. N., Fournier, P., Pozdnichenko, N. N., Gumenuk, A. S. & Skiba, A. A. New approaches in the systematics of rickettsiae. New Microbes New Infect. 23, 93–102 (2018).
Shpynov, S. et al. Detection of a rickettsia closely related to Rickettsia aeschlimannii, ‘Rickettsia heilongjiangensis’, Rickettsia sp. strain RpA4, and Ehrlichia muris in ticks collected in Russia and Kazakhstan. J. Clin. Microbiol. 42, 2221–2223 (2004).
Aung, A. K., Spelman, D. W., Murray, R. J. & Graves, S. Review article: Rickettsial infections in Southeast Asia: Implications for local populace and febrile returned travelers. Am. J. Trop. Med. Hyg. 91, 451–460 (2014).
Rodkvamtook, W. et al. Scrub typhus outbreak in Chonburi Province, Central Thailand, 2013. Emerg. Infect. Dis. 24, 361–365 (2018).
Robinson, M. T., Vongphayloth, K., Hertz, J. C., Brey, P. & Newton, P. N. Tick-transmitted human infections in Asia. Microbiol. Aust. 39, 203–206 (2018).
Bartoshevic, E. To the issue of rickettsioses. Health Care Kazakhstan 3, 20–24 (1952) (in Russian).
Kereyev, N. Human natural focal diseases in Kazakhstan. Alma-ata (1961) (in Russian).
Arkhangelskiy, D. Experimental study of tick-borne rickettsial pathogen in Almaty region. In Collection of Scientific Papers of the Institute of Microbiology and Virologoy Vol 4. Physiology and ecology of micro-organisms. Almta-ata 176–85 (1961) (in Russian).
Kyraubayev, K. et al. Study of Dermacentor marginatus ticks for Rickettsiae in Central Kazakhstan. Proc. ASM (2014).
Shpynov, S. et al. Detection and identification of spotted fever group Rickettsiae in dermacentor ticks from Russia and Central Kazakhstan. Eur. J. Clin. Microbiol. Infect. Dis. 20, 903–905 (2001).
Shpynov, S., Rudakov, N. & Yastrebov, V. Identification of new genotypes of rickettsia tick-borne spotted fever group in the south of the Ural, Siberia, Far East and Kazakhstan. Epidemiol. Infect. Dis. 1, 23–27 (2005).
Hay, J. et al. Biosurveillance in Central Asia: Successes and challenges of tick-borne disease research in Kazakhstan and Kyrgyzstan. Front. Public Health 4, 4 (2016).
Yegemberdiyeva, R. & Shapieva, Z. Clinical and epidemiological characteristic of tick-borne rickettsiosis in Kazakhstan. Abstract Book of the International Conference on Zoonoses. Ulaanbaatar 48–51 (2008).
Rudakov, N. V., Shpynov, S. N., Samoilenko, I. E. & Tankibaev, M. A. Ecology and epidemiology of spotted fever group Rickettsiae and new data from their study in Russia and Kazakhstan. Ann. N. Y. Acad. Sci. 990, 12–24 (2003).
Sansyzbayev, Y. et al. Survey for Rickettsiae within fleas of Great Gerbils, Almaty Oblast, Kazakhstan. Vector Borne Zoon. Dis. Larchmt. N 17, 172–178 (2017).
Kazakhstan Scientific Practical Center of Sanitary Epidemiological Expertise and Monitoring. Almaty. Epidemiological situation of infectious diseases in the Republic of Kazakhstan from 2016. Annual Report (2016) (in Russian).
CDC. https://www.cdc.gov/vhf/omsk/index.html (2022).
Turebekov, N. et al. Prevalence of Rickettsia species in ticks including identification of unknown species in two regions in Kazakhstan. Parasit. Vectors 12, 1–16 (2019).
Jones, K. E. et al. Global trends in emerging infectious diseases. Nature 451, 990–993 (2008).
Tomassone, L. et al. Neglected vector-borne zoonoses in Europe: Into the wild. Vet. Parasitol. 251, 17–26 (2018).
Schex, S., Dobler, G. & Riehm, J. Rickettsia spp. in wild small mammals in Lower Bavaria, South-Eastern Germany. Vector Borne Zoon. Dis. 11, 493–502 (2011).
Tukhanova, N. et al. Molecular characterisation and phylogeny of Tula virus in Kazakhstan. Viruses 14, 1258 (2022).
Wölfel, R., Essbauer, S. & Dobler, G. Diagnostics of tick-borne rickettsioses in Germany: A modern concept for a neglected disease. Int. J. Med. Microbiol. 298, 368–374 (2008).
Fournier, P. E., Roux, V. & Raoult, D. Phylogenetic analysis of spotted fever group Rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol. 48(Pt 3), 839–849 (1998).
Jado, I. et al. Molecular method for identification of Rickettsia species in clinical and environmental samples. J. Clin. Microbiol. 44, 4572–4576 (2006).
Hall, T. A. BioEdit a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549 (2018).
Turebekov, N. et al. Occurrence of anti-Rickettsia spp. antibodies in hospitalized patients with undifferentiated febrile illness in the southern region of Kazakhstan. Am. J. Trop. Med. Hyg. 104, 2000–2008 (2021).
SPC SEEM. Kazakhstan Scientific Practical Center of Sanitary Epidemiological Expertise and Monitoring, Almaty, Kazakhstan (2021).
Yamamoto, Y. PCR in diagnosis of infection: detection of bacteria in cerebrospinal fluids. Clin. Vaccine Immunol. 9, 508–514 (2002).
Turebekov, N. et al. Prevalence of Rickettsia species in ticks including identification of unknown species in two regions in Kazakhstan. Parasit. Vectors 12, 197 (2019).
Gajda, E. et al. Spotted fever Rickettsiae in wild-living rodents from south-western Poland. Parasit. Vectors 10, 413 (2017).
Essbauer, S., Hofmann, M., Kleinemeier, C., Wölfel, S. & Matthee, S. Rickettsia diversity in southern Africa: A small mammal perspective. Ticks Tick-Borne Dis. 9, 288–301 (2018).
Weinert, L. A., Werren, J. H., Aebi, A., Stone, G. N. & Jiggins, F. M. Evolution and diversity of Rickettsia bacteria. BMC Biol. 7, 6 (2009).
El Karkouri, K., Ghigo, E., Raoult, D. & Fournier, P.-E. Genomic evolution and adaptation of arthropod-associated Rickettsia. Sci. Rep. 12, 3807 (2022).
Zemtsova, G. E., Montgomery, M. & Levin, M. L. Relative sensitivity of conventional and real-time PCR assays for detection of SFG Rickettsia in blood and tissue samples from laboratory animals. PLoS One 10, e0116658 (2015).
Burri, C., Schumann, O., Schumann, C. & Gern, L. Are Apodemus spp. mice and Myodes glareolus reservoirs for Borrelia miyamotoi, Candidatus Neoehrlichia mikurensis, Rickettsia helvetica, R. monacensis and Anaplasma phagocytophilum?. Ticks Tick-Borne Dis. 5, 245–251 (2014).
Tadin, A. et al. Molecular survey of zoonotic agents in rodents and other small mammals in Croatia. Am. J. Trop. Med. Hyg. 94, 466–473 (2015).
Karbowiak, G., Biernat, B., Stańczak, J., Szewczyk, T. & Werszko, J. The role of particular tick developmental stages in the circulation of tick-borne pathogens affecting humans in Central Europe. 3. Rickettsiae. Ann. Parasitol. 62, 89–100 (2016).
Zemtsova, G., Killmaster, L. F., Mumcuoglu, K. Y. & Levin, M. L. Co-feeding as a route for transmission of Rickettsia conorii israelensis between Rhipicephalus sanguineus ticks. Exp. Appl. Acarol. 52, 383–392 (2010).
Rehácek, J., Urvölgyi, J., Kocianová, E. & Jedlicka, L. Susceptibility of some species of rodents to Rickettsiae. Folia Parasitol. (Praha) 39, 265–284 (1992).
Rehácek, J., Zupancicová, M., Kovácová, E., Urvölgyi, J. & Brezina, R. Study of rickettsioses in Slovakia. III. Experimental infection of Apodemus flavicollis Melch. by Rickettsiae of the spotted fever (SF) group isolated in Slovakia. J. Hyg. Epidemiol. Microbiol. Immunol. 21, 306–313 (1976).
Biernat, B., Stańczak, J., Michalik, J., Sikora, B. & Wierzbicka, A. Prevalence of infection with Rickettsia helvetica in Ixodes ricinus ticks feeding on non-rickettsiemic rodent hosts in sylvatic habitats of west-central Poland. Ticks Tick-Borne Dis. 7, 135–141 (2016).
Stańczak, J. et al. Prevalence of infection with Rickettsia helvetica in feeding ticks and their hosts in western Poland. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 15(Suppl 2), 328–329 (2009).
Barandika, J. F. et al. Tick-borne zoonotic bacteria in wild and domestic small mammals in northern Spain. Appl. Environ. Microbiol. 73, 6166–6171 (2007).
Spitalská, E., Boldis, V., Kostanová, Z., Kocianová, E. & Stefanidesová, K. Incidence of various tick-borne microorganisms in rodents and ticks of central Slovakia. Acta Virol. 52, 175–179 (2008).
Guo, L.-P. et al. Rickettsia raoultii in Haemaphysalis erinacei from marbled polecats, China-Kazakhstan border. Parasit. Vectors 8, 461 (2015).
Acknowledgements
We thank the staff members of the Taldykorgan, Bakans and Oral Antiplague Stations for excellent assistance in gathering samples.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study is supported by the German Biosecurity Programme of the German Federal Foreign Office. The authors also express gratitude to the German Federal Ministry for Economic Cooperation and Development (BMZ) and the German Academic Exchange Services (DAAD) through the CIH LMU—Center for International Health, Ludwig-Maximilian University, Munich, Germany.
Author information
Authors and Affiliations
Contributions
E.W., L.P. and S.E. conceived the layout of the project. N.Tk., A.Si., N.Tu. performed homogenization and DNA-extraction. A.Si., N.Tk., E.W. performed experimental work, A.Se. was in charge of the sequencing, EW performed analysis of data, created figures and tables and wrote the manuscript. A.Si., N.Tk., N.Tu., Z.S., A.Sh., T.N., V.S., A.B., N.M., I.L., K.F., R.E. and C.E. contributed additional information and reviewed the manuscript. S.E. and L.P. supervised the project. L.P. was in charge of the revision process.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wagner, E., Tukhanova, N., Shin, A. et al. Incidence of tick-borne spotted fever group Rickettsia species in rodents in two regions in Kazakhstan. Sci Rep 12, 14872 (2022). https://doi.org/10.1038/s41598-022-19145-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-19145-0
This article is cited by
-
Genetic diversity and prevalence of emerging Rickettsiales in Yunnan Province: a large-scale study
Infectious Diseases of Poverty (2024)