Abstract
Muscle atrophy greatly affects the prognosis of patients in the intensive care unit, but the rate of change remains unclear. In this prospective observational study, we used ultrasound to measure the change in muscle thickness of the rectus femoris (RF) and vastus intermedius (VI) in 284 patients who were admitted to the SICU of Taoyuan General Hospital between January 1 and June 30, 2020. Patients were excluded if there is a wound at the right thigh which hinders the ultrasonography probe from placing. Daily rates of muscle atrophy were calculated using linear analysis and the ratios of change were plotted against the period of hospitalization. Patient characteristics were adjusted using propensity score matching and differences between men and women were analyzed. A linear mixed model was used to calculate the influence of other factors on muscle loss. The average daily atrophy rates of the RF and VI were 0.84% and 0.98%, respectively. The rate of atrophy was the highest in the third and fourth weeks. Daily atrophy rates of the RF and VI were approximately three times higher in women than in men. Protective factors of muscle atrophy included higher BMI and lower initial thickness of the RF and VI. Our study depicts the trend of muscle atrophy in the ICU and suggests more discussion in prevention to be conducted especially for women.
Similar content being viewed by others
Introduction
The incidence of intensive care unit-acquired weakness (ICUAW) is up to 80% in critically ill patients1,2. Due to its profound impact on the prognosis of patients, before or after hospital discharge3,4, developed countries have introduced early rehabilitation in the ICU to reduce the occurrence and severity of ICUAW5,6. At present, even with popularization of early rehabilitation, the rate of change in muscle mass with the number of days of hospitalization is not well-understood due to a lack of observational research in the field. Whether the rate of muscle atrophy trend is initially rapid and then gradual (i.e.: whether the slope is changed) due to improved postoperative inflammation or consistently rapid due to prolonged immobilization is unknown. This information can be acquired by observing daily muscle changes of patients under regular rehabilitation.
Physiological differences in the muscle cells of men and women affect the speed of recovery in both sexes. For example, women are more susceptible to disuse atrophy, but are better protected from inflammation-induced muscle atrophy (such as cancer cachexia)7. These differences may be due to the following reasons: (1) The proportion of type 1 muscle is higher in women8. (2) Women’s myofibrillar synthesis is faster than men’s when supplemented with nutrients9, although the muscle protein synthesis caused by intermittent exercise is worse than that of men10. (3) Men have more satellite cells and greater hypertrophy and proliferative capacity11. Studies that discuss sex differences in ICUAW are few12,13. Therefore, further research is required to determine the differences in the speed of muscle atrophy in men and women admitted in ICU.
Decrease in muscle strength is caused by polyneuropathy, myopathy, and/or muscle atrophy14. Moreover, several factors may have different effects on the speeds of atrophy of different muscle type. For example, muscle disuse can cause atrophy of type 1 muscle as they are sensitive to inactivity, decreased gravity, and denervation and tend to change from slow-twitch to fast-twitch muscles. In contrast, cachexia leads to preferential atrophy of type 2 muscle. Understanding these different rates of muscle atrophy of different types can help us better understand the pathogenesis of ICUAW15,16.
Considering the study requires multiple measurements and the ICU patients are mostly unstable, risks increase when there are more transferring to advanced neuroimaging, such as CT scan or MRI. Furthermore, acquiring samples of muscle by biopsy is invasive and causes discomfort, which may lead to a limited sample size. In comparison, ultrasonography is a convenient, non-invasive, valid measurement without radiation. We have chosen to investigate the RF and VI, which are mainly made of Type 2 and Type 1 muscle fibers17, respectively using ultrasonography. We designed a prospective observational study to determine the muscle atrophy curves of different muscle types in men and women who were admitted in the SICU.
Methods
Ethical considerations
This study was approved by the Taoyuan General Hospital Institutional Review Board (IRB number: TYGH108031) and all methods were carried out in accordance with relevant guidelines and regulations. The participants or their legal representatives provided written informed consent prior to the study. The consent of a legal representative was obtained only when the patient was unconscious.
Design/setting/sample
This is a prospective observational study. Those were enrolled were patients admitted to the SICU of Taoyuan General Hospital between January to June 2020 and received routine and progressive rehabilitation 3–5 times per week. They had a variety of diseases affecting central nerve, respiratory and cardiovascular system. Patients who had a wound on the right thigh were excluded to avoid contact with the ultrasound probe. The observation time for each patient was from their first to the last day in the SICU or up to 28 days.
Measurements
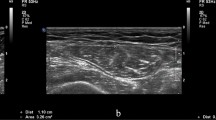
We used ultrasound1 (B-mode, linear probe, frequency of 8 MHz) to measure the thickness of the RF and VI. The patient was lying supine with their right foot extended. The ultrasound probe was placed in the middle of the anterior superior iliac spine and the upper edge of the patella on the right thigh18, perpendicular to the femoral bone. Figure 1 is an ultrasound image which shows the location of the RF and VI. To avoid changing the muscles’ diameters, additional pressure was not applied and the visible thickness of the conductive gel between the probe and the skin was maintained. One physician, who had more than 10 years of experience in skeletal muscle ultrasound, performed ultrasonography on all patients. The intra-rater reliability was 0.98, similar to that reported in other studies19.
Ultrasound measurements RF: rectus femoris muscle, VI: vastus intermedius muscle, VL: vastus lateralis muscle, VM: vastus medialis muscle, Green dotted line 1: thickness of RF, Green dotted line 2: thickness of VI, Arrow: placement of the gel to ensure that there is no pressure on the muscles to produce deformation.
Each patient underwent ultrasonography 5 days per week as each measurement was completed without data missing. The ratio of the muscle thickness measured on the day of examination to that measured on the first day was calculated for each patient. This ratio was considered a dependent variable.
Independent variables included number of days in the ICU, gender, age, height, weight, BMI, disease type, initial level of consciousness, and initial RF and VI muscle thicknesses. The disease types were categorized into central nervous system (CNS) diseases, including cerebral, subarachnoid, epidural, and subdural hemorrhage, brain tumor, and spinal cord injury; cardiovascular (CV) diseases, including rheumatic valve disease, endocarditis, coronary artery disease, and aortic dissection; thoracic diseases, including empyema, lung cancer, traumatic pneumo-hemothorax, and esophageal cancer; and infectious diseases, including empyema, limbs/intra-abdominal abscess, post-operation sepsis, and endocarditis. Disease classification was not mutually exclusive. Patients received Richmond Agitation-Sedation Scale (RASS) evaluation at hospitalization. We defined RASS of − 5 on the first day of admission as initial loss of conscious.
Analysis
Each patient’s ratio of muscle thickness on each hospitalization day was calculated and the averages of all patients’ ratios were plotted against the number of days of hospitalization days to form a trend. Linear analysis was used to express the rate of muscle atrophy and calculate the slopes for the data of 4 weeks, the first 2 weeks, and the last 2 weeks.
Past studies have shown that factors, such as age, disease, and BMI may affect the proportion of muscle loss20,21. To adjust for confounding factors between men and women, we used 1:1 propensity score (PS) matching before performing the analysis22,23. PS for each patient was calculated based on age, diagnosis, height, weight, BMI, initial level of consciousness, initial RF and VI thickness, and admission days. The proportions of categorical variables and the mean ± standard deviation of continuous variables are shown in Table 1. The differences between the men and women groups were analyzed by Chi-square analysis for categorical variables and t-test for continuous variables. Finally, we used a linear mixed model to calculate the influence of each factor on the muscle loss and the significance was defined as p < 0.05. The software used was IBM SPSS Statistics for Windows, Version 23.0.
Results
A total of 284 patients were enrolled from January 1, 2020, to June 30, 2020 during which no patient left the study. Among them, 195 were men and 89 were women. The distribution of characteristics of the unmatched and PS-matched patients is shown in Table 1. After the 1:1 PS match, there were no significant differences in any measurable confounding factors.
We found that the proportion of the remaining RF and VI thicknesses decreased from 100% to 67.6% (SD = 21.6%) and 100% to 55.6% (SD = 23.4%), respectively within 4 weeks. The linear regression results showed that the daily atrophy rate of the RF was 0.84% and that of the VI was 0.98%. The rates of atrophy of the RF and VI in the first 2 weeks were 1.2% and 1.6%, respectively, while those in the second 2 weeks were 2.0% and 2.3%, respectively (Fig. 2). The rate of atrophy in the second 2 weeks was significantly higher than that in the first 2 weeks.
The daily muscle thickness attenuation curve for men and women after PS-match is shown in Fig. 3. We found that the daily atrophy rate of women in RF is 1.7%, which is higher than that of men’s (0.5%). The daily atrophy rate of women in VI was 2.1%, which was higher than that of men (0.8%). Women’s muscle loss rate is approximately 2.6 to 3.4 times compared with that of men. (Non-PS-match result was shown in Supplementary Fig. S1)
By using a linear mixed model, we were able to calculate percentage change of muscle thickness attributed by each factor (eg. delta thickness divided by initial thickness). In women, the RF thickness will decrease by 8% compared to men. It will decrease by 0.6% with every admission day in the ICU and 0.5% with every 1-year increase in age. Further, RF thickness will increase by 0.7% with every 1-unit increment in BMI and decrease by 4% with every 1 mm increase in the initial RF thickness. There were no significant differences in the other factors (Table 2).
Similarly, with another linear mixed model, VI thickness in women will reduce by 5.7%. The VI thickness will be lost by 0.7% with every admission day in the ICU and 0.5% with every 1-year increase in age. Further, VI thickness will increase by 0.7% with every 1-unit increase in BMI and decrease by 3.2% for every 1 mm increase in initial VI thickness. In addition, VI thickness will reduce by 14.8% in patients with infectious diseases. There were no significant differences in the other factors (Table 2). The results of non-PS match data are given in Supplementary Table S1.
Discussion
After evaluating the average daily muscle atrophy in association with the length of hospitalization, we found that the rate of muscle loss was the highest and most progressive in the 3rd and 4th weeks of the ICU stay. The VI, which is mainly constituted of slow-twitch muscles, atrophies slightly faster than the RF, which is mainly made of fast-twitch muscles. Moreover, we found that women are more susceptible to muscle loss than men, and the same results were observed for both type 1 and type 2 muscles.
Although many studies have explored the incidence and risk factors of muscle atrophy, there is a lack of discussion on the rate of muscle loss and its trend. Clair et al. and Kirby et al. described the changes in muscle loss in ICU patients over time24. However, in both studies, the sample sizes were 50 and 41, respectively, and were only observed over 7 days; hence, it was difficult to observe long-term trends in muscle loss. The sample size in the study by Puthucheary et al. was 63, but the observations were only carried out on the 1st, 3rd, 7th, and 10th day25. The study by Wolfgang et al. included 118 people and the study period was more than 28 days26. However, observations of each patient were only conducted twice, making it difficult to observe the daily changes in muscle thickness. Wolfgang et al. reported that patients' muscle loss was the most significant within 2–3 weeks of admission, after which the rate of atrophy plateaued. However, in our study, we found that the rate of muscle loss significantly increased in the 3rd and 4th weeks of admission. The results of our study suggest that more attention should be paid to preventing muscle loss for at least 1 month when managing patients in ICUs. Further studies with study periods longer than 1 month are required to explore long-term muscle thickness changes.
In this study, we used PS matching to exclude other interference factors and found that the muscle loss in women in the ICU was significantly higher than that in men, i.e., the decrease in rates of muscle loss of the RF and VI was 2.6 and 3.6 times higher, respectively. These results indicate that sex is an independent factor affecting muscle atrophy in the ICU, similar to the previous study conducted by De Jonghe B et al. in which Medical Research Council Scale was used to detect ICUAW in 200214.
Similar to previous studies16, we did not find a significant difference between type 1 and type 2 muscle atrophies. Earlier studies have reported that disuse atrophy may be more obvious in type 1 muscles15; however, faster degeneration of the VI was not observed in patients with CNS injury or initial loss of conscious. This may be because many factors affect the patient's activity status. For example, in the ICU, restricted mobility in patients may result from CNS damage, general weakness, or sedation.
Suetta et al. reported that elderly patients lose muscle strength faster but lose muscle volume slower when compared to those in the young27. However, in our study, results showed that the older the person, the faster was the loss of muscle thickness. This may be due to the differences in BMI and other characteristics between older and younger patients. In the study by Suetta et al., the BMIs of the older patients were higher and the initial quadriceps muscle thicknesses were lower than those of the younger patients. These two factors were found to be protective factors for muscle atrophy in our study. We speculate that analyzing the differences between different age groups directly without adjusting for confounding factors is likely to yield confusing results. In contrast, in the study by Urso28, there was no significant difference in the initial muscle thickness between the elderly and the younger patients. Similar to our study, the results of the study by Urso showed that the rate of muscle atrophy was higher in the elderly compared to that of the younger patients.
Patients with higher muscle content than adipose tissue may be more likely to undergo muscle breakdown to meet the energy needs29. Our study showed that an increase in BMI is a protective factor for muscle atrophy, which implies that patients with higher proportions of adipose tissue have more energy resources to meet the body’s stress and recovery needs. This also suggests that we must suspect muscle atrophy more in lower BMI patients. However, in the study by Segaran et al., no difference was found in the degree of muscle loss between patients with higher and lower BMI30. This may be due to their small sample size and short observation time. Further studies are required to investigate the effect of BMI on muscle atrophy.
The limitations of this study are as follows: (1) Although past reviews have reported that medications, such as corticosteroids and neuromuscular blockers, were risk factors of ICUAW2, we did not analyze patients’ medication history in our study. As this is a longitudinal observational study, we cannot confirm the causal relationship between non-sustained medication status and daily changes in muscle ratios. Similarly, we did not consider laboratory data that can reveal possible changes in muscle breakdown, such as inflammation or infection, as independent variables. However, the lack of the abovementioned factors in our analysis is unlikely to affect the results as the aim of this study was to describe the differences in the rates of atrophy of different muscles between men and women. (2) The ICU setting in our study may not be similar to that in other countries. The mode and intensity of rehabilitation treatment and the attributes of patients may differ across different regions and countries. Considering the great variability of ICU admission, there are some factors (ex: fluid status, etiology) we overlooked which may affect the precision of measurement. (3) As we depicted the atrophy rate for as long as 4 weeks, it is likely the decline in the 3rd and 4th week was mostly attributed by, instead of all the patients in the ICU, those with more severe illness requiring longer admission days. (4) The observation time span (i.e. 6 months) is relatively arbitrary subject to funding and research time, although which is still longer than previous studies. (5) The number of men and women is disproportionate and beyond control of current study method. The effect of sex on muscle atrophy should be further verified with studies with higher level of evidence.
Conclusions
In conclusion, the rate of atrophy of the RF in the first month in the ICU was 0.84% per day and that of VI was 0.98%, with the highest atrophy rates in the 3rd and 4th weeks compared to the first 2 weeks. The rate of muscle atrophy in women was approximately three times higher than that in men, which suggests that more attention must be paid to women in terms of prevention and treatment of muscle atrophy. Future studies should explore the factors and treatment effects of muscle atrophy in men and women separately.
Data availability
The data that support the findings of this study are available on request from the corresponding author, HJY. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Abbreviations
- BMI:
-
Body mass index
- CNS:
-
Central nervous system
- CV:
-
Cardiovascular
- ICUAW:
-
Intensive care unit-acquired weakness
- RASS:
-
Richmond agitation-sedation scale
- RF:
-
Rectus femoris
- PS:
-
Propensity score
- SICU:
-
Surgical intensive care unit
- VI:
-
Vastus intermedius
References
Kress, J. P. & Hall, J. B. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 371, 287–288. https://doi.org/10.1056/NEJMc1406274 (2014).
Jolley, S. E., Bunnell, A. E. & Hough, C. L. ICU-acquired weakness. Chest 150, 1129–1140. https://doi.org/10.1016/j.chest.2016.03.045 (2016).
Sidiras, G. et al. Long term follow-up of quality of life and functional ability in patients with ICU acquired weakness - A post hoc analysis. J. Crit. Care 53, 223–230. https://doi.org/10.1016/j.jcrc.2019.06.022 (2019).
Hermans, G. et al. Predictive value for weakness and 1-year mortality of screening electrophysiology tests in the ICU. Intensive Care Med. 41, 2138–2148. https://doi.org/10.1007/s00134-015-3979-7 (2015).
Balas, M. C., Devlin, J. W., Verceles, A. C., Morris, P. & Ely, E. W. Adapting the ABCDEF bundle to meet the needs of patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting: Historical perspectives and practical implications. Semin. Respir. Crit. Care Med. 37, 119–135. https://doi.org/10.1055/s-0035-1570361 (2016).
Devlin, J. W. et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 46, e825–e873. https://doi.org/10.1097/CCM.0000000000003299 (2018).
Rosa-Caldwell, M. E. & Greene, N. P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex Differ. 10, 43. https://doi.org/10.1186/s13293-019-0257-3 (2019).
Haizlip, K. M., Harrison, B. C. & Leinwand, L. A. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda) 30, 30–39. https://doi.org/10.1152/physiol.00024.2014 (2015).
Horstman, A. M. H. et al. The muscle protein synthetic response to whey protein ingestion is greater in middle-aged women compared with men. J. Clin. Endocrinol. Metab. 104, 994–1004. https://doi.org/10.1210/jc.2018-01734 (2019).
Scalzo, R. L. et al. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J. 28, 2705–2714. https://doi.org/10.1096/fj.13-246595 (2014).
Murach, K. A. et al. Starring or supporting role? satellite cells and skeletal muscle fiber size regulation. Physiology (Bethesda) 33, 26–38. https://doi.org/10.1152/physiol.00019.2017 (2018).
Yang, T., Li, Z., Jiang, L., Wang, Y. & Xi, X. Risk factors for intensive care unit-acquired weakness: A systematic review and meta-analysis. Acta Neurol. Scand. 138, 104–114. https://doi.org/10.1111/ane.12964 (2018).
Vanhorebeek, I., Latronico, N. & Van den Berghe, G. ICU-acquired weakness. Intensive Care Med. 46, 637–653. https://doi.org/10.1007/s00134-020-05944-4 (2020).
De Jonghe, B. et al. Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA 288, 2859–2867. https://doi.org/10.1001/jama.288.22.2859 (2002).
Wang, Y. & Pessin, J. E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 16, 243–250. https://doi.org/10.1097/MCO.0b013e328360272d (2013).
Puthucheary, Z. A. et al. Qualitative ultrasound in acute critical illness muscle wasting. Crit. Care Med. 43, 1603–1611. https://doi.org/10.1097/CCM.0000000000001016 (2015).
Suzuki, A. & Tamate, H. Distribution of myofiber types in the hip and thigh musculature of sheep. Anat. Rec. 221, 494–502. https://doi.org/10.1002/ar.1092210106 (1988).
Tillquist, M. et al. Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. JPEN J. Parenter. Enteral Nutr. 38, 886–890. https://doi.org/10.1177/0148607113501327 (2014).
Bunnell, A., Ney, J., Gellhorn, A. & Hough, C. L. Quantitative neuromuscular ultrasound in intensive care unit-acquired weakness: A systematic review. Muscle Nerve 52, 701–708. https://doi.org/10.1002/mus.24728 (2015).
English, C., McLennan, H., Thoirs, K., Coates, A. & Bernhardt, J. Loss of skeletal muscle mass after stroke: A systematic review. Int. J. Stroke 5, 395–402. https://doi.org/10.1111/j.1747-4949.2010.00467.x (2010).
Goossens, C. et al. Premorbid obesity, but not nutrition, prevents critical illness-induced muscle wasting and weakness. J. Cachexia Sarcopenia Muscle 8, 89–101. https://doi.org/10.1002/jcsm.12131 (2017).
Yeh, H. J., Chou, Y. J., Yang, N. P., Cheng, C. C. & Huang, N. Association between physical therapy and risk of coronary artery disease and Dyslipidemia among Osteoarthritis patients: A nationwide database study. Arch. Phys. Med. Rehabil. 97, 8–16. https://doi.org/10.1016/j.apmr.2015.08.410 (2016).
Amoah, J. et al. Comparing propensity score methods versus traditional regression analysis for the evaluation of observational data: A case study evaluating the treatment of gram-negative bloodstream infections. Clin. Infect. Dis. 71, e497–e505. https://doi.org/10.1093/cid/ciaa169 (2020).
Mayer, K. P. et al. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit. Care 24, 637. https://doi.org/10.1186/s13054-020-03355-x (2020).
Puthucheary, Z. A. et al. Acute skeletal muscle wasting in critical illness. JAMA 310, 1591–1600. https://doi.org/10.1001/jama.2013.278481 (2013).
Gruther, W. et al. Muscle wasting in intensive care patients: Ultrasound observation of the M. quadriceps femoris muscle layer. J. Rehabil. Med. 40, 185–189. https://doi.org/10.2340/16501977-0139 (2008).
Suetta, C. et al. Effects of aging on human skeletal muscle after immobilization and retraining. J. Appl. Physiol. 1985(107), 1172–1180. https://doi.org/10.1152/japplphysiol.00290.2009 (2009).
Urso, M. L., Clarkson, P. M. & Price, T. B. Immobilization effects in young and older adults. Eur. J. Appl. Physiol. 96, 564–571. https://doi.org/10.1007/s00421-005-0109-1 (2006).
Dickerson, R. N., Boschert, K. J., Kudsk, K. A. & Brown, R. O. Hypocaloric enteral tube feeding in critically ill obese patients. Nutrition 18, 241–246. https://doi.org/10.1016/s0899-9007(01)00793-6 (2002).
Segaran, E., Wandrag, L., Stotz, M., Terblanche, M. & Hickson, M. Does body mass index impact on muscle wasting and recovery following critical illness? a pilot feasibility observational study. J. Hum. Nutr. Diet. 30, 227–235. https://doi.org/10.1111/jhn.12401 (2017).
Funding
This project was sponsored by the Ministry of Science and Technology (Project Number: MOST 108 2314 B 087 001 MY2).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by R.-Y. W., and H.-J. Y. The first draft of the manuscript was written by H.-J. Y. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, RY., Sung, WH., Cheng, HC. et al. Investigating the rate of skeletal muscle atrophy in men and women in the intensive care unit: a prospective observational study. Sci Rep 12, 16629 (2022). https://doi.org/10.1038/s41598-022-21052-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-21052-3
This article is cited by
-
Muscle mass in critically ill patients: analysis using ultrasound, traditional bioelectrical impedance analysis, and wearable devices: a prospective observational study
European Journal of Medical Research (2026)
-
Methodologies and clinical applications of lower limb muscle ultrasound in critically ill patients: a systematic review and meta-analysis
Annals of Intensive Care (2024)