Abstract
The gut is of importance in the pathology of COVID-19 both as a route of infection, and gut dysfunction influencing the severity of disease. Systemic changes caused by SARS-CoV-2 gut infection include alterations in circulating levels of metabolites, nutrients and microbial products which alter immune and inflammatory responses. Circulating plasma markers for gut inflammation and damage such as zonulin, lipopolysaccharide and β-glycan increase in plasma along with severity of disease. However, Intestinal Fatty Acid Binding Protein / Fatty Acid Binding Protein 2 (I-FABP/FABP2), a widely used biomarker for gut cell death, has paradoxically been shown to be reduced in moderate to severe COVID-19. We also found this pattern in a pilot cohort of mild (n = 18) and moderately severe (n = 19) COVID-19 patients in Milan from March to June 2020. These patients were part of the first phase of COVID-19 in Europe and were therefore all unvaccinated. After exclusion of outliers, patients with more severe vs milder disease showed reduced FABP2 levels (median [IQR]) (124 [368] vs. 274 [558] pg/mL, P < 0.01). A reduction in NMR measured plasma relative lipid-CH3 levels approached significance (median [IQR]) (0.081 [0.011] vs. 0.073 [0.024], P = 0.06). Changes in circulating lipid levels are another feature commonly observed in severe COVID-19 and a weak positive correlation was observed in the more severe group between reduced FABP2 and reduced relative lipid-CH3 and lipid-CH2 levels. FABP2 is a key regulator of enterocyte lipid import, a process which is inhibited by gut SARS-CoV-2 infection. We propose that the reduced circulating FABP2 in moderate to severe COVID-19 is a marker of infected enterocyte functional change rather than gut damage, which could also contribute to the development of hypolipidemia in patients with more severe disease.
Similar content being viewed by others
Introduction
Very soon after the onset of the COVID-19 pandemic, the gastrointestinal (GI) tract became a research focus, despite the evident respiratory nature of the disease’s pathogenesis and lethality1. The evidence for GI involvement initiated with frequent observation of symptoms such as nausea and vomiting, diarrhoea, abdominal pain, and anorexia. A meta-analysis of 38 studies which included more than 8000 COVID-19 patients showed that 15.5% patients had at least one GI symptom, with 7.5% experiencing nausea/vomiting and 11.5% diarrhoea2. GI symptoms are even more common in critically ill patients, with a study reporting the rates of feeding intolerance, abdominal distension, vomiting, constipation and diarrhoea being 56%, 67%, 64%, 37% and 28% respectively3.
The discovery that the SARS-CoV-2 virus infects the lungs through binding the receptor angiotensin-converting enzyme 2 (ACE2) increased interest in the GI tract as it is known to be present on enterocytes in the gut epithelium4. Subsequent studies confirmed the gut infection route in COVID-195,6 and that the gut is a reservoir for viral particles1 which can persist even after clearance from the upper respiratory tract7.
Direct invasion of enterocytes leading to cellular changes and cell death is one way in which SARS-CoV-2 can damage the GI tract8. However, the GI symptoms in COVID-19 are also likely to be generated by other pathophysiological mechanisms9. ACE2 mediates intestinal functions such as the renin–angiotensin–aldosterone system by stimulating intracellular signal pathways when bound. SARS-CoV-2 binding to ACE2 can dysregulate this function10,11. Furthermore, the gut microbiome is altered in COVID-19 patients which may lead to imbalance of gut homeostasis, inflammation and dysfunction12,13. Finally, the high levels of circulating pro-inflammatory mediators, and immune cell infiltration of the gut may perturb GI function and induce pathology6. The multi-faceted role of the gut in COVID-19 infection and severity has led to efforts in treating the disease through modulation of microbiota (probiotics14,15,16 and fecal microbial transplantation17,18), diet (e.g. fibre content1) and gut function itself (e.g. through regulating plasma serotonin which influences colonic peristaltic reflexes and GI transit19).
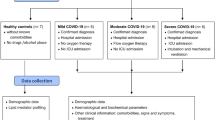
We investigated the role of the gut in affected COVID-19 patients, grouped according to mild and moderately severe disease, who were admitted to Fondazione Ca’ Grande Ospedale Maggiore Policlinico (Lombardy, Italy)20. For this pilot study we assayed commonly used plasma biomarkers of gut permeability (zonulin) and enterocyte cell death (Intestinal Fatty Acid Binding Protein / Fatty Acid Binding Protein 2, I-FABP/FABP2) as well as a range of plasma metabolites with nuclear magnetic resonance (NMR) spectroscopy21. The earliest available timepoint plasma sample (closest to date of admission) was analysed and compared to patient clinical data recorded within 3 days of the experimental sample collection. We hypothesised that circulating plasma markers of gut inflammation and damage would differ according to severity of disease and could correlate to circulating levels of metabolites as measured by NMR spectroscopy.
Results
General characteristics of the pilot study cohort
Patients were grouped based on the ultimate level of breathing support that they received. Patients receiving nasal cannula (n = 17) or VentMask (n = 1) were placed in the mild group, while those who received CPAP were grouped as moderately severe (n = 19) (Table 1). Importantly, we use the classification ‘moderately severe’ as no patients were sedated and on parenteral nutrition receiving mechanical ventilation or extracorporeal membrane oxygenation (ECMO).
Within the moderately severe group, five patients (26.3%) succumbed to death. The gender and age distributions of the two groups were similar, and the only significantly different comorbidity was obesity, which was more common in the moderately severe group, though it was not possible to ascertain in all participants (Supplementary File 1).
Multiple measurements of blood cells and molecules were at significantly different levels between the two groups in line with several studies which have reported COVID-19 associated changes (Table 1 and Supplementary File 1). For example, patients with more severe COVID-19 had elevated neutrophils, C-reactive protein (CRP), procalcitonin (Pct), ferritin and fibrinogen but reduced lymphocytes compared to milder COVID-19 patients.
Gut damage marker ELISAs
Plasma zonulin levels and FABP2 levels were measured in 17 milder COVID-19 patients and 16 more severe COVID-19 patients.
For the zonulin ELISA measurements, one and two samples were respectively excluded from the mild [ID 503, zonulin 76.3 ng/ml] and moderately severe groups [ID 506, 562, zonulin 48.4 ng/mL, 47.5 ng/mL respectively] for being more than two standard deviations from the mild and moderately severe group means (9.7 and 13.4 ng/mL respectively). Zonulin was slightly higher (not significantly, P = 0.15) in the more severe group (N = 14) than the milder group (N = 16) (Fig. 1). When the outliers were not excluded the P value was 0.46.
Plasma FABP2 but not zonulin is decreased in patients with more severe COVID-19 compared to patients with milder COVID-19. (A) Plasma FABP2 levels in patients with milder (n = 16) or more severe COVID-19 (n = 15), respectively. (B) Plasma zonulin levels in patients with milder (n = 16) or more severe (n = 14) COVID-19, respectively. Student’s t-test used for group comparisons, mean ± SEM indicated with red lines.
For the FABP2 ELISA, one sample from the milder group [ID 291, FABP2 1415.9 pg/mL] and one from the more severe group [ID 290, FABP2 406.2 pg/mL] were excluded for being more than two standard deviations from the group means (333.7 and 164.5 pg/mL respectively. FABP2 levels were significantly reduced (P = 0.002) in the more severe group (N = 15) compared to the mild group (N = 16) (Fig. 1). When the outliers were not excluded the group difference was still statistically significant P = 0.01.
Plasma NMR
Thirty-two EDTA plasma samples were available for NMR analysis (milder COVID-19 group N = 17, more severe COVID-19 group N = 15). Illustrative NMR spectra from the more severe COVID-19 group are shown in Fig. 2, noting that the NMR spectra are dominated by peaks from the sampling contaminants EDTA and ethanol.
(A,B) Illustrative expanded NMR spectra from patients in the moderately severe Covid group with differing FABP2 levels. (A) ID 112, FABP2 38 pg/mL, (B) ID 433, FABP2 195 pg/mL. The peaks marked with * were excluded from the analysis and include EDTA peaks (3.635–3.600 ppm; 3.235–3.220 ppm, 3.18–3.07 ppm; 2.72–2.68 ppm; 2.58–2.53 ppm) and ethanol peaks (quartet 3.685–3.635 ppm, triplet 1.20–1.06 ppm). Lac, lactate; L–CH2, lipid-CH2 peak; L–CH3, lipid-CH3 peak. (C) Principal Component Analysis showing no difference between the mild [1, pink] and moderately severe [2, green] groups. (D) Partial Least Squares Discriminant Analysis showing no difference between the mild [1, pink] and moderately severe [2, green] groups. (E) Variable in Importance Projection plot showing L–CH2 and L–CH3 to be the top discriminating metabolites between the mild [1] and moderately severe [2] groups.
There was no difference between the NMR spectra from milder and more severe COVID-19 groups using principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA), which was confirmed by no difference between groups on cross validation (Q2 < 0). The Variable in Importance Projection plots showed lipid-CH3 and lipid-CH2 to be the most discriminatory metabolite regions. The medians and IQRs for selected metabolites in the mild and moderately severe patient groups are presented in Supplementary Table 1. Only lipid CH3 approached significance (P = 0.06) (t-test), between groups, being reduced in the moderately severe group compared to the mild group.
NMR associations within Covid severity groups
Relative NMR metabolite levels from 32 patients (milder COVID-19 group N = 17, more severe COVID-19 group N = 15) were compared to FABP2 and zonulin concentrations using Pearson r correlation (GraphPad Prism 9.2.0). Four data points (n = 2 mild COVID-19, ID 503, 291; n = 2 moderately severe COVID-19 group, ID 506, 562; refer to ELISA exclusions above) were identified as outliers and so these data points were excluded from further analysis.
Correlation plots for the mild COVID-19 group (N = 15) and moderately severe COVID-19 group (N = 13) are shown in Fig. 3A,B respectively. The strongest positive associations in the more severe COVID-19 group, (i.e. low level when FABP2 is low) were in plasma lipids (both lipid-CH2 and lipid-CH3 regions) and lymphocytes.
(A) Correlation Pearson R matrix showing association between gut biomarkers and plasma NMR metabolites for the milder group (N = 15), after two outliers were removed, as determined by ROUT (ID 503, 291). (B) Associations between gut biomarkers and plasma NMR metabolites for the more severe group (N = 13), after two outliers were removed, as determined by ROUT analysis (ID 506, 562). Blue indicates a positive association, red indicates a negative association. Stronger associations indicated by deeper colour.
Discussion
From onset of the COVID-19 pandemic the gut has repeatedly been implicated in infection, progression and severity of the disease. We and others considered the role of microbial translocation in exacerbating the hyperinflammation in moderate to severe COVID-1922,23. There is now strong evidence for increasing microbial translocation in COVID-19 which correlates with inflammation24,25,26 and may even influence mortality27. Mechanistically, it is unclear whether the increased translocation is due to gut damage caused by enterocyte infection5,6,28, or whether SARS-CoV-2 infected lung induces systemic hyperinflammation, microbial dysbiosis, and/or coagulopathy which in turn causes the gut damage13,29,30. One way to answer this question is to compare the levels of plasma biomarkers of gut permeability and damage with markers of inflammatory responses across the different disease stages. Gut permeability markers such as LPS, LBP, zonulin and cCD14 have been shown to be higher in COVID-19 patients than healthy controls24,25,31,32. Giron et al., (2021) examined gut-associated biomarkers and metabolites in a cohort of patients with mild (outpatients), moderate (inpatients hospitalised in regular wards) and severe (inpatients hospitalised in ICU) COVID-19 (18–20 in each group)24. They observed large and significant increases in the tight junction permeability marker zonulin between the mild and moderate/severe groups, but no difference between the latter two. Therefore, in this cohort at least the biggest increases in gut permeability occur between the timepoints of infection and hospitalisation, with little further increase in the more severe stages which are associated with hyperinflammation. Our study of relatively mild and more severe COVID-19 patients (as determined by the level of respiratory support) admitted in medicine units, also showed little increase in zonulin between these stages.
As gut permeability and enterocyte cell death go hand in hand in conditions such as sepsis and intestinal ischaemia/reperfusion33, and as SARS-CoV-2 infection has been observed to kill gut epithelial cells8, we expected that plasma FABP2 would also increase in COVID-19 patients. However, we saw the opposite, with a significant reduction in more severe compared to milder COVID-19 patients. The literature on plasma FABP2 in COVID-19 is conflicted, with some studies reporting increased FABP2 in COVID-19 patients compared to healthy controls25,32, some no difference24,31,34 and some a reduction35,36. Studies which classified disease condition as mild (outpatients), moderate (inpatients hospitalised in regular wards) and severe (hospitalisation in ICU) have either shown no increases34,35 or a similar decrease in severe compared to moderate as we saw25,36.
Further studies are therefore needed to confirm the relationship between plasma FABP2 levels and COVID-19 severity. However, the surprising decrease in the more severe patients deserves consideration as it gives insight into the state of the gut at the most critical timepoint for patient survival. A straightforward explanation could be that the expression of FABP2 is reduced in these patients due to reduced food intake or malabsorption3,37,38. However, since in the present study we did not examine the most severe patients who were on mechanical ventilation, and therefore sedated and on parenteral nutrition, we ruled out a possible source of major bias in previous reports. Alternatively, or additionally, plasma FABP2 levels may be reduced as the gene’s expression is downregulated in enterocytes due to infection or an aspect of hyperinflammation itself. In vitro experiments with human small intestinal organoids found that fat metabolism and biosynthesis were some of the most significantly downregulated processes in response to SARS-CoV-2 infection5 (FABP2 itself was reduced but non-significantly). This metabolic change has been proposed to be an anti-viral measure, as SARS-CoV-2 infection and replication makes extensive use of cellular lipids39,40. This alteration of gut lipid metabolism may even have importance for disease survival as the drug Ezetimibe which reduces fat uptake in the gut41 has been reported to possibly reduce COVID-19 severity42.
Comparison of plasma FABP2 levels with other circulating metabolites and patient clinical data in the more severe COVID-19 group showed the strongest negative associations (i.e. high level when FABP2 is low) with CRP, Il–6 and zonulin. These data suggest that plasma FABP2 is especially low in patients with the highest levels of inflammation and gut permeability. The anti-correlation between plasma FABP2 and systemic inflammation fits with the observation that the 3 lowest FABP2 levels in the study were recorded in patients who died (out of a total of 5 who died). However, the significant difference in FABP2 levels between the 2 study groups was still seen if these patients were excluded. The strongest positive associations in the more severe COVID-19 group, (i.e. low level when FABP2 is low) were in plasma lipids (both lipid intramolecular CH2 and CH3 groups), and lymphocytes, both of which have been frequently observed in severe COVID-1940,43,44. However, considering the low sample numbers of these comparisons, care must be taken in their interpretation.
Several studies have focussed on the plasma lipid changes in COVID-19 patients39,40,45,46,47,48,49,50. It is important to note that patients with type 2 diabetes, obesity, or users of lipid lowering drugs are more likely to progress to severe COVID-19 due to their comorbidities, so this needs to be taken into account when stratifying patients into severity groups in order to investigate lipids in COVID-1951. Accordingly, low HDL cholesterol and high triglycerides prior to infection are risk factors for progression to severe COVID-1946. Nonetheless, the most commonly reported lipid changes in COVID-19 patients at all stages of disease are reduced circulating cholesterol (LDL and HDL) and elevated triglycerides21,47,48,49,50,52. Reported metabolite changes includes increased levels of acetoacetic acid, 3-hyroxybutyric acid, acetone and 2-hdroxybutyic acid; changes have also been reported in porphyrin levels, branched chain amino acids and tryptophan pathways21,45,49,53. The most severely affected patients have a further reduction of HDL compared to patients with moderate disease46,47, with increases of cholesterol and triglycerides upon recovery in survivors52. As FABP2 expression level is an important determinant of lipoprotein production in the gut it is possible that it may contribute to the reduction in HDL in the more severely affected patients54,55,56,57,58,59.
The extreme and progressive immunological response induced by SARS-CoV-2 infection explains many of the differences in systemic inflammatory markers between the milder and more severe groups43. Higher neutrophil to lymphocyte ratio, CRP and procalcitonin have all been used to stratify severity of COVID-1944. Elevated ferritin has also been reported in many COVID-19 studies60. Ferritin correlates with pulmonary involvement in SARS-CoV-2 infection61, which fits with our use of pulmonary support to separate the two patient groups. Higher levels of the clotting factor fibrinogen are also associated with severe COVID-1962. Overall, these data indicate that there is a higher systemic inflammation response, particularly of the acute phase reaction in the more severe patient group than the mild group.
Limitations to our study were linked to the low number of patients examined and the low volume of plasma available. The period of sample collection was during a particularly challenging time in Milan, since it was the first major European city to experience the COVID-19 pandemic. Consequently, patient samples for highly selected research purposes were scarce 63,64. The low sample volume available meant we had to adjust the routine replicate experimental practices into singlicate analysis and use a minimal volume of 70 μl of plasma for NMR. A more extensive study of circulating markers of gut function, such as citrulline and gut cell death, is needed to confirm that the FABP2 levels in severe COVID-19 are indicative of altered lipid import and metabolism rather than apoptosis. Additionally, sample collection used EDTA as an anticoagulant, so a number of NMR peaks were obscured by the EDTA resonances, including those that would have given information on choline-containing compounds.
Another consequence of low sample numbers is that, unlike some published studies with larger patient groups, the NMR metabolites were not significantly different between the mild and moderately severe groups, with only lipid CH3 approaching significance with a reduction in the moderately severe group (P = 0.06) compared to the mild group. Plasma NMR spectroscopy is high in information content65. Therefore, detailed interpretation of plasma NMR findings may require information from a range of clinical and lifestyle factors, to allow for the impact of confounding factors on metabolite levels. It is therefore possible that increased sample numbers would have enabled subgroups to be analysed to fully account for confounding variables, such as gender, co-morbidities, medication and nutritional status. The presence of correlations between the ELISA measured parameters and NMR datasets seems to confirm that possibility. As previously discussed, there is an abundance of literature on reductions in plasma lipid species in COVID-19 patients21, and our observed trends of formate53,66, phenylalanine21,66,67,68,69 and 3-hydroxybutyrate21,49,66,67,68,69 increasing with COVID-19 severity is in agreement with previous plasma NMR studies21.
In conclusion our data strengthen the possibility that downregulation of enterocyte fat metabolism in patients with moderate to severe COVID-19 is contributing to the reduced circulating cholesterol, potentially as an anti-infection response. As has been proposed by others, our data therefore support the idea that manipulation of gut lipid absorption is a useful therapeutic approach to manage COVID-1942,70.
Methods
Clinical parameters
All experiments were performed in accordance with the ethics committee guidelines and regulations (COVID-19 and non-COVID patients). Informed consent was obtained from all participants and/or their legal guardians (COVID-19 and non-COVID patients).
Ethics was obtained for the “Fondazione Genomic SARS-CoV-2 study” (ethics number 109365) to Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico Milano. Blood samples were obtained with informed consent and plasma samples were collected into EDTA tubes. All studies were performed in accordance with guidelines and regulations. The clinical metadata was compiled by the Biological Resource Center and Translational Medicine Unit, Dipartimento di Medicina Trasfusionale e di Ematologia, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Università degli Studi di Milano.
Non-COVID patient samples for assay optimisation were recruited at King’s College Hospital after admission to the ward or from the hepatology out-patient clinic. The study was granted ethics approval by the UK NHS national research ethics committee (Apprival number 12/LO/1417) and local research and development department at King’s College Hospital (Approval number KCH12-126).
Laboratory measurements
Laboratory measurements were made in 33 plasma samples from COVID-19 patients in a CL2 laboratory housed at the Institute of Liver Studies, Kings College Hospital. All assays were performed using plasma which was not treated to inactivate the virus (e.g. heat-inactivation) so as to avoid denaturation of proteins which can impair antibody detection.
To ensure safety while handling samples which potentially contained live SARS-CoV-2 several steps and procedures were undertaken. Tubes containing patient plasma were aliquoted within a biosafety hood in the CL3 laboratory (at the Roger Williams Institute of Hepatology). The initial steps of ELISAs were performed in the Mowat Laboratories biosafety hoods for blood handling with 96-well plates only removed from the hoods after the post-primary antibody incubation wash stage.
Active (uncleaved) zonulin was measured using a sandwich enzyme immunoassay (Human Zonulin ELISA Kit MyBioSource MBS706368) with manufacturer’s instructions.
Plasma Intestinal fatty acid-binding protein (I-FABP/FABP2) were measured using a sandwich enzyme immunoassay (Human FABP2/I-FABP Quantikine ELISA Kit, R&D systems DFBP20). The manufacturer’s instructions were followed with plasma diluted fivefold in sample diluent (22 μl plasma added to 88 μl diluent). Prior to analysis with COVID-19 samples we investigated whether heat-inactivation of the plasma, a commonly used method to destroy SARS-CoV-2 virus, could influence FABP2 assay fidelity. We performed the ELISA using plasma samples from four patients with decompensated liver disease and one healthy control. An aliquot of each sample underwent heat inactivation of the SARS-CoV-2 virus with incubation at 56 °C for 15 min. As expected without heat inactivation the healthy control sample (298.1 pg/mL) was lower than the samples from patients with decompensated liver cirrhosis (mean 789.3 ± 182.1 pg/mL SEM)71. However, heat inactivation drastically reduced the levels detected in all samples, even to below the level of detection in two samples, presumably by denaturing FABP2 protein and destroying the immunological detection site. We therefore examined FABP2 and zonulin in the COVID-19 samples without heat inactivation.
In the analysis of COVID-19 patient samples both zonulin and FABP2 were measured only once from the samples due to limitations of available sample volume. End-point analysis for both ELISAs was performed using a FLUOstar Omega microplate reader.
Plasma NMR studies
EDTA plasma samples were collected in sufficient volume for NMR study from 32 patients. Samples were stored frozen at − 80 °C until NMR analysis at the Centre for Biomolecular Spectroscopy, King’s College London. NMR results from the earliest time point (day 1 for all N = 32 subjects) have been included.
On the day of NMR analysis, the EDTA plasma samples were gently thawed. 70 µl of plasma and 110 µl of 75 mM sodium phosphate buffer solution (with 6.2 mM sodium azide and adjusted to pH 7.4) were aliquoted into 3 mm SampleJet NMR tubes (Bruker BioSpin, Germany). NMR data were also acquired from three samples of fetal calf serum to illustrate and confirm stability and reproducibility of the NMR spectrometer. The samples were transported at 4 °C locally to the Centre for Biomolecular Spectroscopy at Guy’s Campus, King’s College London.
On delivery to the NMR facility, the SampleJet racks were immediately placed on the SampleJet holder for sample storage at 4 °C prior to data collection. Proton (1H) NMR spectra were acquired at 37 °C (310 K) using a Bruker 600 MHz (AVANCE NEO) NMR spectrometer and a 1H/13C/15 N TCI Prodigy probe (nitrogen-cooled). Proton shimming was done under automation such that a 1.0–1.5 Hz linewidth for one of the alanine doublet peaks was routinely achieved. Pulse-collect and spin-echo 1D NMR data sets were acquired using PURGE water suppression and the PROJECT spin-echo sequences, as previously described72. Both data sets were acquired with 4 dummy scans, 64 data collects, constant receiver gain, 64 K points, acquisition time 2.62 s and recycle delay of 4 s. The spin-echo time for the PROJECT sequence is 78 ms (64 loops). For confirmation of peak assignment, a TOCSY spectrum and an HSQC spectrum was acquired on a representative plasma sample.
The 1D NMR data sets were processed using 0.3 Hz exponential line broadening filter. Metabolite assignments were made based on chemical shift and coupling patterns with reference to published databases and confirmed by the TOCSY and HSQC NMR studies73.
Mulitvariate analyses of the NMR spectral region 10.00–0.50 ppm were undertaken using spectral binning methodologies as previously published74,75. Specifically, KnowItAll software (Wiley Science Solutions KnowItAll Spectroscopy Edition software version 17.0.117.0 (https://sciencesolutions.wiley.com/knowitall-spectroscopy-software/) and MetaboAnalyst v5.0 (https://www.metaboanalyst.ca) were used. Certain regions of all NMR data sets were excluded from multivariate analyses because of confounding effects, which included the residual water region (5.00–4.50 ppm), EDTA peaks (3.635–3.600 ppm; 3.235–3.220 ppm, 3.18–3.07 ppm; 2.72–2.68 ppm; 2.58–2.53 ppm) and ethanol contamination (quartet 3.685–3.635 ppm, triplet 1.20–1.06 ppm). Both fixed width spectral bins (0.02 ppm bucket widths) and variable width spectral bins (defined using the proprietary InetillibucketTM software of Wiley Sciences Solutions KnowItAll, which is based on defining local minima) were used to define metabolite regions. Following on from variable width spectral bucketing, specific regions were targeted to minimise any peak overlap, including formate, 8.468–8.455 ppm; phenylalanine, 7.452–7.414 ppm; N-acetyl glycoproteins, 2.05–2.03 ppm; alanine, 1.495–1.470 ppm; lactate, 1.34–1.32 ppm; lipid CH2, 1.32–1.24 ppm; lipid CH3, 0.90–0.82 ppm; valine, 1.057–1.023 ppm. Peaks were also quantified for acetone (2.06–2.03 ppm) and 3-hydroxybutyrate (region not overlapping with ethanol triplet, 1.21–1.20 ppm) and assigned to leucine and isoleucine.
Statistics
Differences for specific parameters between clinical groupings were assessed using Student’s t-tests (IBM SPSS Statistics v28.0.1.1).
MetaboAnalyst v15 (https://www.metaboanalyst.ca) was used to compare NMR changes between clinical groupings using principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA).
Alterations in relative levels of circulating metabolites were correlated with measures of gut permeability (FABP2 and zonulin) using Pearson r Correlation (GraphPad Prism 9.2.0, Graphstats Technologies Private Limited, Karnataka, India). Outliers were determined by the ROUT algorithm in GraphPad Prism.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alberca, G. G. F., Solis-Castro, R. L., Solis-Castro, M. E. & Alberca, R. W. Coronavirus disease-2019 and the intestinal tract: An overview. World J. Gastroenterol. 27, 1255–1266. https://doi.org/10.3748/wjg.v27.i13.1255 (2021).
Ghimire, S. et al. Diarrhea is associated with increased severity of disease in COVID-19: Systemic review and meta-analysis. SN Compr. Clin. Med. 3, 28–35. https://doi.org/10.1007/s42399-020-00662-w (2021).
Liu, R. et al. Feeding intolerance in critically ill patients with COVID-19. Clin. Nutr. https://doi.org/10.1016/j.clnu.2021.03.033 (2021).
Hamming, I. et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 203, 631–637. https://doi.org/10.1002/path.1570 (2004).
Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54. https://doi.org/10.1126/science.abc1669 (2020).
Xiao, F. et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158, e1833. https://doi.org/10.1053/j.gastro.2020.02.055 (2020).
Zhang, T. et al. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J. Med. Virol. 92, 909–914. https://doi.org/10.1002/jmv.25795 (2020).
Lehmann, M. et al. Human small intestinal infection by SARS-CoV-2 is characterized by a mucosal infiltration with activated CD8(+) T cells. Mucosal. Immunol. 14, 1381–1392. https://doi.org/10.1038/s41385-021-00437-z (2021).
Wang, M. K. et al. COVID-19 and the digestive system: A comprehensive review. World J. Clin. Cases 9, 3796–3813. https://doi.org/10.12998/wjcc.v9.i16.3796 (2021).
Vaduganathan, M. et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 382, 1653–1659. https://doi.org/10.1056/NEJMsr2005760 (2020).
Rysz, S. et al. COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system. Nat. Commun. 12, 2417. https://doi.org/10.1038/s41467-021-22713-z (2021).
Zuo, T. et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159, e948. https://doi.org/10.1053/j.gastro.2020.05.048 (2020).
Segal, J. P. et al. The gut microbiome: An under-recognised contributor to the COVID-19 pandemic?. Therap. Adv. Gastroenterol. 13, 1756284820974914. https://doi.org/10.1177/1756284820974914 (2020).
Mullish, B. H. et al. Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: Should we be considering probiotics during viral pandemics?. Gut Microbes 13, 1–9. https://doi.org/10.1080/19490976.2021.1900997 (2021).
Gutierrez-Castrellon, P. et al. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 14, 2018899. https://doi.org/10.1080/19490976.2021.2018899 (2022).
d’Ettorre, G. et al. Challenges in the management of SARS-CoV2 infection: The role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front. Med. Lausanne 7, 389. https://doi.org/10.3389/fmed.2020.00389 (2020).
Liu, F. et al. Gastrointestinal disturbance and effect of fecal microbiota transplantation in discharged COVID-19 patients. J. Med. Case Rep. 15, 60. https://doi.org/10.1186/s13256-020-02583-7 (2021).
Wu, L. H. et al. Efficacy and safety of washed microbiota transplantation to treat patients with mild-to-severe COVID-19 and suspected of having gut microbiota dysbiosis: Study protocol for a randomized controlled trial. Curr. Med. Sci. 41, 1087–1095. https://doi.org/10.1007/s11596-021-2475-2 (2021).
Jin, B. et al. Pathophysiological mechanisms underlying gastrointestinal symptoms in patients with COVID-19. World J. Gastroenterol. 27, 2341–2352. https://doi.org/10.3748/wjg.v27.i19.2341 (2021).
Bandera, A. et al. COVID-19 Network: the response of an Italian reference institute to research challenges about a new pandemia. Clin. Microbiol. Infect. 26, 1576–1578. https://doi.org/10.1016/j.cmi.2020.06.028 (2020).
Ghini, V. et al. Profiling metabolites and lipoproteins in COMETA, an Italian cohort of COVID-19 patients. PLoS Pathog. 18, e1010443. https://doi.org/10.1371/journal.ppat.1010443 (2022).
Assante, G., Williams, R. & Youngson, N. A. Is the increased risk for MAFLD patients to develop severe COVID-19 linked to perturbation of the gut-liver axis?. J. Hepatol. 74, 487–488. https://doi.org/10.1016/j.jhep.2020.05.051 (2021).
Vignesh, R. et al. Could perturbation of gut microbiota possibly exacerbate the severity of COVID-19 via cytokine storm?. Front. Immunol. 11, 607734. https://doi.org/10.3389/fimmu.2020.607734 (2020).
Giron, L. B. et al. Plasma markers of disrupted gut permeability in severe COVID-19 patients. Front. Immunol. 12, 686240. https://doi.org/10.3389/fimmu.2021.686240 (2021).
Oliva, A. et al. Persistent systemic microbial translocation and intestinal damage during Coronavirus Disease-19. Front. Immunol. 12, 708149. https://doi.org/10.3389/fimmu.2021.708149 (2021).
Venzon, M. et al. Gut microbiome dysbiosis during COVID-19 is associated with increased risk for bacteremia and microbial translocation. Res. Sq. https://doi.org/10.21203/rs.3.rs-726620/v1 (2021).
Teixeira, P. C. et al. Increased LPS levels coexist with systemic inflammation and result in monocyte activation in severe COVID-19 patients. Int. Immunopharmacol. 100, 108125. https://doi.org/10.1016/j.intimp.2021.108125 (2021).
Nayak, T., De, D. & Dhal, P. K. The differences in SARS-CoV and SARS-CoV-2 specific co-expression network mediated biological process in human gut enterocytes. Infect. Genet. Evol. 92, 104892. https://doi.org/10.1016/j.meegid.2021.104892 (2021).
Polidoro, R. B., Hagan, R. S., de Santis Santiago, R. & Schmidt, N. W. Overview: Systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front. Immunol. 11, 1626. https://doi.org/10.3389/fimmu.2020.01626 (2020).
Troisi, J. et al. COVID-19 and the gastrointestinal tract: Source of infection or merely a target of the inflammatory process following SARS-CoV-2 infection?. World J. Gastroenterol. 27, 1406–1418. https://doi.org/10.3748/wjg.v27.i14.1406 (2021).
Hoel, H. et al. Elevated markers of gut leakage and inflammasome activation in COVID-19 patients with cardiac involvement. J. Intern. Med. 289, 523–531. https://doi.org/10.1111/joim.13178 (2021).
Prasad, R. et al. Plasma microbiome in COVID-19 subjects: An indicator of gut barrier defects and dysbiosis. Int. J. Mol. Sci. 23, 9141. https://doi.org/10.3390/ijms23169141 (2022).
Subramanian, S., Geng, H. & Tan, X. D. Cell death of intestinal epithelial cells in intestinal diseases. Sheng. Li. Xue. Bao. 72, 308–324 (2020).
Syed, F. et al. Excessive matrix metalloproteinase-1 and hyperactivation of endothelial cells occurred in COVID-19 patients and were associated with the severity of COVID-19. J. Infect. Dis. 224, 60–69. https://doi.org/10.1093/infdis/jiab167 (2021).
Guedj, K. et al. I-FABP is decreased in COVID-19 patients, independently of the prognosis. PLoS ONE 16, e0249799. https://doi.org/10.1371/journal.pone.0249799 (2021).
Teng, X. et al. comprehensive profiling of inflammatory factors revealed that growth differentiation factor-15 is an indicator of disease severity in COVID-19 patients. Front Immunol 12, 662465. https://doi.org/10.3389/fimmu.2021.662465 (2021).
Keur, M. B., Beishuizen, A. & van Bodegraven, A. A. Diagnosing malabsorption in the intensive care unit. F1000 Med. Rep. 2, 1–7. https://doi.org/10.3410/M2-7 (2010).
Auinger, A. et al. Human intestinal fatty acid binding protein 2 expression is associated with fat intake and polymorphisms. J. Nutr. 140, 1411–1417. https://doi.org/10.3945/jn.109.118034 (2010).
Abu-Farha, M. et al. The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int. J. Mol. Sci. 21, 3544. https://doi.org/10.3390/ijms21103544 (2020).
Rezaei, A., Neshat, S. & Heshmat-Ghahdarijani, K. Alterations of lipid profile in COVID-19: A narrative review. Curr. Probl. Cardiol. 47, 100907. https://doi.org/10.1016/j.cpcardiol.2021.100907 (2021).
Nakano, T. et al. Ezetimibe impairs transcellular lipid trafficking and induces large lipid droplet formation in intestinal absorptive epithelial cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1865, 158808. https://doi.org/10.1016/j.bbalip.2020.158808 (2020).
Israel, A. et al. Identification of drugs associated with reduced severity of COVID-19—A case-control study in a large population. Elife 10, e68165. https://doi.org/10.7554/eLife.68165 (2021).
Karimi, A., Shobeiri, P., Kulasinghe, A. & Rezaei, N. Novel Systemic Inflammation Markers to Predict COVID-19 Prognosis. Front Immunol 12, 741061. https://doi.org/10.3389/fimmu.2021.741061 (2021).
Ghahramani, S. et al. Laboratory features of severe vs non-severe COVID-19 patients in Asian populations: A systematic review and meta-analysis. Eur. J. Med. Res. 25, 30. https://doi.org/10.1186/s40001-020-00432-3 (2020).
Loo, R. L. et al. Quantitative in vitro diagnostic NMR Spectroscopy for lipoprotein and metabolite measurements in plasma and serum: Recommendations for analytical artifact minimization with special reference to COVID-19/SARS-CoV-2 Samples. J. Proteome Res. 19, 4428–4441. https://doi.org/10.1021/acs.jproteome.0c00537 (2020).
Masana, L. et al. Low HDL and high triglycerides predict COVID-19 severity. Sci. Rep. 11, 7217. https://doi.org/10.1038/s41598-021-86747-5 (2021).
Wei, X. et al. Hypolipidemia is associated with the severity of COVID-19. J. Clin. Lipidol. 14, 297–304. https://doi.org/10.1016/j.jacl.2020.04.008 (2020).
Jin, H. et al. Altered lipid profile is a risk factor for the poor progression of COVID-19: From two retrospective cohorts. Front Cell Infect. Microbiol. 11, 712530. https://doi.org/10.3389/fcimb.2021.712530 (2021).
Ballout, R. A. et al. The NIH Lipo-COVID study: A pilot NMR investigation of lipoprotein subfractions and other metabolites in patients with severe COVID-19. Biomedicines 9, 1090. https://doi.org/10.3390/biomedicines9091090 (2021).
Mahat, R. K. et al. Lipid profile as an indicator of COVID-19 severity: A systematic review and meta-analysis. Clin Nutr. ESPEN 45, 91–101. https://doi.org/10.1016/j.clnesp.2021.07.023 (2021).
Fogacci, F., Borghi, C. & Cicero, A. F. G. Misinterpreting data in lipidology in the era of COVID-19. J. Clin. Lipidol. 14, 543–544. https://doi.org/10.1016/j.jacl.2020.07.004 (2020).
Roccaforte, V. et al. Altered lipid profile in patients with COVID-19 infection. J. Lab. Precis. Med. 6, 2–2 (2020).
Kimhofer, T. et al. Integrative modeling of quantitative plasma lipoprotein, metabolic, and amino acid data reveals a multiorgan pathological signature of SARS-CoV-2 infection. J. Proteome Res. 19, 4442–4454. https://doi.org/10.1021/acs.jproteome.0c00519 (2020).
Lagakos, W. S. et al. Different functions of intestinal and liver-type fatty acid-binding proteins in intestine and in whole body energy homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G803-814. https://doi.org/10.1152/ajpgi.00229.2010 (2011).
Lackey, A. I. et al. Mechanisms underlying reduced weight gain in intestinal fatty acid-binding protein (IFABP) null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 318, G518–G530. https://doi.org/10.1152/ajpgi.00120.2019 (2020).
Levy, E. et al. Localization, function and regulation of the two intestinal fatty acid-binding protein types. Histochem. Cell Biol. 132, 351–367. https://doi.org/10.1007/s00418-009-0608-y (2009).
Soayfane, Z. et al. Exposure to dietary lipid leads to rapid production of cytosolic lipid droplets near the brush border membrane. Nutr. Metab. Lond. 13, 48. https://doi.org/10.1186/s12986-016-0107-9 (2016).
Gajda, A. M. et al. Direct comparison of mice null for liver or intestinal fatty acid-binding proteins reveals highly divergent phenotypic responses to high fat feeding. J. Biol. Chem. 288, 30330–30344. https://doi.org/10.1074/jbc.M113.501676 (2013).
Gajda, A. M. & Storch, J. Enterocyte fatty acid-binding proteins (FABPs): Different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot. Essent. Fat Acids 93, 9–16. https://doi.org/10.1016/j.plefa.2014.10.001 (2015).
Gomez-Pastora, J. et al. Hyperferritinemia in critically ill COVID-19 patients—Is ferritin the product of inflammation or a pathogenic mediator?. Clin. Chim. Acta 509, 249–251. https://doi.org/10.1016/j.cca.2020.06.033 (2020).
Carubbi, F. et al. Ferritin is associated with the severity of lung involvement but not with worse prognosis in patients with COVID-19: data from two Italian COVID-19 units. Sci. Rep. 11, 4863. https://doi.org/10.1038/s41598-021-83831-8 (2021).
Sui, J., Noubouossie, D. F., Gandotra, S. & Cao, L. elevated plasma fibrinogen is associated with excessive inflammation and disease severity in COVID-19 patients. Front. Cell Infect. Microbiol. 11, 734005. https://doi.org/10.3389/fcimb.2021.734005 (2021).
Ruscica, M. et al. Prognostic parameters of in-hospital mortality in COVID-19 patients—An Italian experience. Eur. J. Clin. Invest. 51, e13629. https://doi.org/10.1111/eci.13629 (2021).
Saccon, E. et al. Distinct metabolic profile associated with a fatal outcome in COVID-19 patients during the early epidemic in Italy. Microbiol. Spectr. 9, e0054921. https://doi.org/10.1128/Spectrum.00549-21 (2021).
Bliziotis, N. G. et al. Preanalytical pitfalls in untargeted plasma nuclear magnetic resonance metabolomics of endocrine hypertension. Metabolites 12, 679. https://doi.org/10.3390/metabo12080679 (2022).
Bruzzone, C. et al. SARS-CoV-2 infection dysregulates the metabolomic and lipidomic profiles of serum. iScience. 23, 101645. https://doi.org/10.1016/j.isci.2020.101645 (2020).
Meoni, G. et al. Metabolomic/lipidomic profiling of COVID-19 and individual response to tocilizumab. PLoS Pathog. 17, e1009243. https://doi.org/10.1371/journal.ppat.1009243 (2021).
Masuda, R. et al. Integrative modeling of plasma metabolic and lipoprotein biomarkers of SARS-CoV-2 infection in Spanish and Australian COVID-19 patient cohorts. J. Proteome Res. 20, 4139–4152. https://doi.org/10.1021/acs.jproteome.1c00458 (2021).
Bizkarguenaga, M. et al. Uneven metabolic and lipidomic profiles in recovered COVID-19 patients as investigated by plasma NMR metabolomics. NMR Biomed. 35, e4637. https://doi.org/10.1002/nbm.4637 (2022).
Balgoma, D., Gil-de-Gomez, L. & Montero, O. Lipidomics issues on human positive ssRNA virus infection: An update. Metabolites 10, 356. https://doi.org/10.3390/metabo10090356 (2020).
Riva, A. et al. Faecal cytokine profiling as a marker of intestinal inflammation in acutely decompensated cirrhosis. JHEP Rep. 2, 100151. https://doi.org/10.1016/j.jhepr.2020.100151 (2020).
Cox, I. J. et al. Metabolomics and microbial composition increase insight into the impact of dietary differences in cirrhosis. Liver Int. 40, 416–427. https://doi.org/10.1111/liv.14256 (2020).
Vignoli, A. et al. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 58, 968–994. https://doi.org/10.1002/anie.201804736 (2019).
Probert, F. et al. (1)H NMR metabolic profiling of plasma reveals additional phenotypes in knockout mouse models. J. Proteome Res. 14, 2036–2045. https://doi.org/10.1021/pr501039k (2015).
Cox, I. J. et al. Stool microbiota show greater linkages with plasma metabolites compared to salivary microbiota in a multinational cirrhosis cohort. Liver Int. https://doi.org/10.1111/liv.15329 (2022).
Acknowledgements
We thank the Foundation for Liver Research for funding the laboratory and NMR aspects of this study (N.Y., I.J.C., G.A., K.T., V.C.P., S.C.). Other funding was received from the Italian Ministry of Health (Ministero della Salute), Ricerca Finalizzata RF-2016-02364358 (“Impact of whole exome sequencing on the clinical management of patients with advanced nonalcoholic fatty liver and cryptogenic liver disease”) (L.V.); Italian Ministry of Health (Ministero della Salute), Rete Cardiologica “CV-PREVITAL” (DP, LV); Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Ricerca corrente (L.V., D.P., F.P.); Fondazione IRCCS Ca’ Granda core COVID-19 Biobank (RC100017A), “Liver BIBLE” (PR-0391) (L.V.); Innovative Medicines Initiative 2 joint undertaking of European Union’s Horizon 2020 research and innovation programme and EFPIA European Union (EU) Programme Horizon 2020 (under grant agreement No. 777377) for the project LITMUS (L.V.); The European Union, programme “Photonics” under grant agreement “101016726” (L.V.); Gilead_IN-IT-989-5790 (L.V.).
Author information
Authors and Affiliations
Contributions
N.Y., L.V. and I.J.C. planned the study. L.V., R.C., F.F., D.P., F.B., F.P., A.B., N.Y., G.A., K.T. obtained ethics, consented the patients for the study and curated all the clinical information. A.G. and I.J.C. are responsible for N.M.R. data acquisition and N.M.R. data analysis. G.A., K.T., S.C., V.C.P. and N.Y. undertook all the laboratory measurements.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assante, G., Tourna, A., Carpani, R. et al. Reduced circulating FABP2 in patients with moderate to severe COVID-19 may indicate enterocyte functional change rather than cell death. Sci Rep 12, 18792 (2022). https://doi.org/10.1038/s41598-022-23282-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23282-x