Abstract
Thymoma is the most common malignant tumor in thymic epithelial tumors (TETS). This study aimed to identify the changes in serum proteomics in patients with thymoma. Proteins were extracted from twenty patients with thymoma serum and nine healthy controls and prepared for mass spectrometry (MS) analysis. Data independent acquisition (DIA) quantitative proteomics technique was used to examine the serum proteome. Differential proteins of abundance changes in the serum were identified. Bioinformatics was used to examine the differential proteins. Functional tagging and enrichment analysis were conducted using the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases. The string database was used to assess the interaction of different proteins. In all, 486 proteins were found in all samples. There were differences in 58 serum proteins between patients and healthy blood donors, 35 up-regulated and 23 down-regulated. These proteins are primarily exocrine and serum membrane proteins involved in controlling immunological responses and antigen binding, according to GO functional annotation. KEGG functional annotation showed that these proteins play a significant role in the complement and coagulation cascade and the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signal pathway. Notably, the KEGG pathway (complement and coagulation cascade) is enriched, and three key activators were up-regulated: von willebrand factor (VWF), coagulation factor v (F5) and vitamin k-dependent protein c (PC). Protein–protein interaction (PPI) analysis showed that six proteins ((VWF, F5, thrombin reactive protein 1 (THBS1), mannose-binding lectin-associated serine protease 2 (MASP2), apolipoprotein B (APOB), and apolipoprotein (a) (LPA)) were up-regulated and two proteins (Metalloproteinase inhibitor 1(TIMP1), ferritin light chain (FTL)) were down-regulated. The results of this study showed that several proteins involved in complement and coagulation cascades were up-regulated in the serum of patients.
Similar content being viewed by others
Introduction
Thymoma is one of the most common tumors in the anterior mediastinum, arising from thymic epithelial cells. The incidence of thymoma is about 2.5 per million people per year1. Thymoma is closely related to the neuromuscular disease myasthenia gravis2. At present, the pathogenesis of thymoma is not clear3. Thymoma detection in the early stages of the disease is essential to improving the prognosis and survival probability. In particular, serum biomarkers are most attractive because simple blood tests are minimally invasive for patients and can provide valuable information about thymoma status. Thymoma-related biomarkers have not yet been identified and used clinically.
The molecular characteristics of different histological and clinical types of thymoma are often used to improve the diagnosis and treatment of thymoma and determine patients' prognoses4. Most of the previous studies have performed biomarker searches and tumor analysis for thymoma by comparing differential genes. These studies focused on differences in gene expression between thymoma subtypes and thymic carcinomas5,6,7,8,9. Although these studies have identified biomarkers of diagnostic or prognostic significance for thymic epithelial tumors, further validation is needed in clinical practice. The proteomic analysis provides global comparisons of proteins in almost all biological samples, enabling the identification of multiple proteins of interest in a single experiment10. Proteins are actual mediators of intracellular processes, and changes in the proteome may more accurately reflect cellular changes. Some studies have classified thymomas by comparing the proteomic characteristics of thymus and thymoma or different types of thymoma11,12,13. However, there is still a lack of research on the serum proteomic characteristics of thymoma, which hinders our understanding and treatment of the tumor.
The analysis of serum protein components in patients with thymoma may provide a basis for an in-depth understanding of the pathogenesis of thymoma and will have far-reaching significance for the research and development of the treatment of thymoma. In this study, the serum protein profiles of people with thymomas and healthy people were quantitatively examined to identify the protein components, revealing varying abundances between the two groups.
Methods
Patients
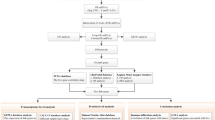
A total of twenty patients with thymoma diagnosed in Shijiazhuang people’s hospital affiliated with Hebei Medical University from February 2018 to May 2019 were chosen as the case group, and nine healthy people served as the control group. Inclusion criteria: (I) over the age of eighteen; (II) thymoma was diagnosed by postoperative pathology; (III) thymoma was classified according to the World Health Organization (WHO) histological classification. Exclusion criteria: (I) under 18 years old; (II) people were suffering from severe cardiac disease, other malignant malignancies, or unable to cooperate. All individuals, including patients and healthy controls, were of Han ethnicity. The aim of the study and the precautions that should be followed was explained to all investigators. Each participant in the study signed a documented informed consent form. The hospital's ethics committee approved the study.
Preparation of serum samples
Before the operation, the patient drew blood. Patients and healthy participants' fasting venous blood was obtained in the morning. The serum was separated from the blood samples by centrifuging at 12,000g for 10 min at 20 °C. The resulting supernatant was stored at − 80 °C for mass spectrometry analysis. The ProteoExtract Albumin/immunoglobulin G (IgG) Removal kit by Merck Millipore (MA, USA) was used to remove albumin and IgG from the serum samples. The serum samples were treated with a reaction solution (1% sodium dodecyl sulfate (SDS), 4.5 mM (mM) dithiothreitol (DTT), 9 mM iodoacetamide (IAA)) and incubated at 55 °C for 30 min for protein denaturation, disulfide bond reduction, and sulfhydryl alkylation. The Bradford assay was used to quantify the protein concentration in samples. In order to digest the sample with enzyme, 1 mg/ml Trypsin N-tosyl-L-phenyl chloromethyl keton (TPCK) was added to the sample at 1:50 and digested overnight at 37 °C. The next day, phosphoric acid was added to stop the enzyme digestion, and the pH of the solution was adjusted to about 3.0. The peptides after enzymatic hydrolysis were desalted by SOLA™ SPE96 orifice plate. Before desalting, adjust the pH value of the sample solution to close to 7. The sample loaded onto the SOLA™ SPE 96 orifice plate using liquid–liquid extraction (LLE) method. The peptides were washed with 200 μL 5% methanol, then eluted with 150 μL methanol and dried in vacuum for MS analysis.
Data independent acquisition (DIA) analysis
For mass spectrometry (MS) analysis, DIA technology was used. The experiment was carried out using an Agilent 1100 HPLC System and a Q Exactive HF. The first step was to establish a protein map library by using the traditional data dependent acquisition (DDA) method. The DDA mass spectrometry conditions were a MS1 scan (scanning range, 350–1650 m/z; resolution, 120 K; automatic gain control (AGC), 3 × 106; maximum injection time, 100 ms) and MS2 scanning (scanning range, 200–2000 m/z; resolution, 30 K; AGC, 2 × 105; max injection time, 80 ms). We identified a total of 898 proteins in the protein library using DDA. The database searching was performed using Spectronaut Pulsar software, with a false discovery rate (FDR) of 1% at both the peptide and protein levels. The following parameters were used for the database search: missed cleavage, 2; fixed modification, carbamidomethyl; variable modification, oxidation. The second step was to use DIA technology to collect the mass spectrum data of each sample. In this experiment, all enzymolysis samples were separated by Agilent Zorbax Extend-C18 in a mobile phase with pH = 10. Both mobile phases were adjusted to a pH value of 10 using ammonia. The elution was performed with a gradient of solvent A (0.1% formic acid aqueous solution) and solvent B (0.1% hydroxyurea, 80% dicarboxamide, and 20% water), starting with 95% A and 5% B, and gradually changing the ratio to 5% A and 95% B over the course of 81 min. The detection wavelength was set at 210 nm, and the enzymolysis peptide of each sample was collected separately. The scanning range was set to 350–1250 m/z, with an isolation window of 26 m/z. The Spectronaut Pulsar processed secondary mass spectrometry files obtained from the DIA scan, which could be utilized for database retrieval. The results were used to create the spectrogram library. The key parameters of the DIA data analysis were as follows: precursor qvalue cutoff, 5%; protein qvalue cutoff, 5%; normalization strategy, local normalization; quantity MS-level, MS2. The quantitative data from the DIA analysis were screened with a 1% false discovery rate (FDR). The normalized data were transformed for difference comparison and t-test analysis to log2 data. The fold change ratio and P value were utilized to identify protein components with differing abundances between patients and the control group. Tests for differential protein were conducted using the ratio and P value. The protein was over-represented if the fold change was ≥ 1.2 and P ≤ 0.05; the protein was under-represented if the fold change was ≤ 0.833 and P ≤ 0.05.
Bioinformatics analysis
In order to perform functional annotation and enrichment analysis, the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) database was employed. The STRING database was utilized to evaluate the interaction connection of different proteins. Network analysis was carried out on the gathered data using the Python tool "networkx".
Statistical analyses
The mean ± standard deviation was used to represent continuous variables. The variations between the case and control groups and the proportion of categorical variables were compared. The chi-square test or Fisher test was used to compare the two groups' categorical variables. All statistical analyses were performed using SPSS Version 24 (Chicago, IL, USA) and R 4.1.0 (https://www.r-project.org/). P ≤ 0.05 was regarded as statistically significant.
Ethical approval
The Ethics Committee of People’s Hospital of Shijiazhuang has given this study its ethical approval (Approval number 201894). All of the patients gave their informed consent and were approved by the ethics committee. All methods were performed in accordance with the Declaration of Helsinki.
Results
Patient basic information
Primary data for each patient and healthy participant in the study are shown in Table 1. The thymoma group consisted of twelve males. This group's average age was 47.38 ± 8.18. The average body mass index (BMI) of thymoma patients was 23.27 ± 4.44. The control group included four men. The control group's average age was 40.54 ± 8.98. The average BMI in the control group was 22.78 ± 3.05. There were no significant age, gender, or BMI variations between the two groups (P > 0.05).
Proteomics analysis
486 serum proteins were found in all groups, and Table 2 summarizes the quantitative data for all of these proteins. The correlation between the protein levels and the samples was strong as evidenced by the correlation coefficients, which were all above 0.8 (Fig. 1). The ratio distribution in the case group is essentially normal (Fig. 2). Fifty-eight proteins were differentially expressed across the two groups, with 35 being up-regulated and 23 being down-regulated (Table 2, Figs. 3 and 4).
The heat map presents the results of a quantitative correlation analysis between proteins across multiple samples. The color intensity of each cell in the picture represents the intensity and direction of the correlation between protein levels in a given sample. Blue is a positive correlation and red is a negative correlation. The darker the color is, the stronger the correlation is. The software used to create the map is R 4.1.0 (https://www.r-project.org/).
Clustering heat map of all proteins. At the top is a violin diagram, a combination of a box and density diagram, indicating the probability distribution of the expression value of the sample. A flatter violin indicates a more concentrated data. Different color fillings represent different samples. The median of the data is represented by the "+" symbol in the middle, and the vertical axis shows the protein expression level. Below the violin is a column annotated heat map, where samples of the same group are represented by blocks of the same color. The clustering heat map below organizes proteins based on expression levels, with red indicating high expression and blue representing low expression, each row representing protein expression across different groups. The software used to create the map is R 4.1.0 (https://www.r-project.org/).
GO functional annotation and enrichment analysis
Three GO levels, including the biological process (BP), cellular component (CC), and molecular function (MF), were used to categorize differently regulated proteins (Fig. 5). Protein differences in the patient group for the BP category were primarily involved in receptor-mediated endocytosis (7.79%), immunological response (6.49%), and immune response control (7.79%). The extracellular exosome accounted for 20.88% of the CC, the serum membrane for 16.48%, and the extracellular space for 9.89%. In the MF category, 26.09% played a role in antigen binding, 17.39% were serine-type endopeptidase activity, and 13.04% had a zinc ion binding function.
KEEG functional annotation and enrichment analysis
We performed a KEGG pathway analysis on the identified differential proteins to better understand their involvement in healthy or pathological activities and determine the metabolic and signaling pathways involved14. According to our findings, the differential proteins primarily engage in the following biological processes: complement and coagulation cascades, phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway, and staphylococcus aureus infection. (Fig. 6). Complement and coagulation cascades have the highest KEEG enrichment15 (Fig. 7).
KEGG annotation and enrichment analysis. In the figure, the x-axis Enrichment score is the enrichment score and the y-axis is the pathway information of top20. The larger the bubble, the more the number of differential proteins, the color of the bubble changed from red–green–blue-purple, and the smaller the enrichment p value was, the greater the significance was.
Enrichment KEGG pathway diagram. In the picture, the circle represents the differential protein, the red represents the up-regulated protein, and the green represents the down-regulated protein; the size of the circle represents the degree of connectivity, and the larger the circle, the higher the connectivity.
PPI network construction
The string database was used to assess the interaction of different proteins, and the nodes with the top 25 node connectivity were visualized by the python package ‘networkx’, which was displayed by protein identification (ID) and gene name, respectively (Fig. 8). In the picture, the circle represents the differential protein/gene, the red represents the up-regulated protein/gene, and the green represents the down-regulated protein/gene; the circle's size shows the degree of connectivity; the larger the circle, the greater the connectivity.
Protein interaction network analysis. The rectangle represents the enzyme/gene, the red indicates the up-regulated protein, the green indicates the down-regulated protein, and the yellow indicates both up-regulated and down-regulated protein. The colorless rectangle represents the genes present in the map, the light green rectangle represents the species-specific genes, and the light purple rectangle represents the genes that exist in both map and ko. KEGG Pathway (KEGG, 2021).
Overexpressed proteins with significant changes are depicted in the figure, including von willebrand factor (VWF), thrombin reactive protein 1 (THBS1), coagulation factor V (F5), and mannose-binding lectin-associated serine protease 2 (MASP2), apolipoprotein B (APOB), and apolipoprotein (a) (LPA). The expression of two proteins, Metalloproteinase inhibitor 1 (TIMP1) and ferritin light chain (FTL) was significantly insufficient.
Discussion
Thymoma epithelial cells have endocrine activity16. Some reports on diagnostic or prognostic proteins in thymoma have been published in recent years. Sasaki et al. found that serum periosteal levels may indicate tumor invasion and progression of thymoma17. The results of Du et al. show that Mucin 1 (MUC1) and glucose transporter type 1 (GLUT1) staining are helpful in the differential diagnosis of thymic carcinoma and B3 thymoma with high sensitivity and specificity18. However, there are few systematic studies on the changes in serum protein components in patients with thymoma. This study is the first to conduct a global proteome map analysis using serum and liquid chromatography-mass spectrometry (LC–MS)/MS from thymoma and healthy controls. In this study, 58 distinct proteins were found in the serum samples of thymoma patients, of which 35 were overexpressed in the patient samples, and 23 were underexpressed compared with the control samples.
According to GO functional annotation, most of these proteins are factors involved in immunoreactive regulatory antigen binding in exosomes and serum membranes. These distinct proteins were predominantly connected to coagulation, complement system activation, and the PI3K-Akt signal pathway, according to KEGG functional annotation. It is worth noting that the members of the complement and coagulation cascade pathway have been enriched, which proved that VWF, F5, THBS1 and MASP-2 were overexpressed in patient samples.
The prothrombotic condition seen in cancer patients is frequently caused by an imbalance between the coagulation and fibrinolysis systems19,20,21. Many procoagulant factors, such as tissue factors and cancer procoagulants, are secreted or expressed on the cell surface of many tumors. VWF is considered to mediate tumor cell attachment to platelets and facilitate their systemic dissemination. The vascular endothelial growth factor (VEGF) increases the proliferation and differentiation of vascular endothelial cells (the principal producer of VWF) during angiogenesis, which happens during tumor growth, resulting in higher serum VWF levels in cancer patients22. This study showed that serum VWF levels in thymoma patients were higher than in healthy controls.
In addition, F5 also increased in the same pathway. F5 is a circulating high molecular weight (330 KDa) factor, which plays an essential role in the coagulation cascade reaction. When F5 is activated, it acts as a cofactor to activate factor X, which converts prothrombin into thrombin23. F5 gene polymorphism is associated with the risk of breast cancer, colorectal cancer, gastric cancer and other cancers24,25. THBS is a multifunctional extracellular matrix protein family involved in tissue remodelling and has been linked to cancer, wound healing, and embryonic development26. The first member to be identified is THBS1, which inhibits endogenous angiogenesis. THBS1 binds to several cellular receptors to fulfil various tasks27. According to earlier research, THBS1 is abundantly expressed in a variety of tumor types and encourages tumor cell adhesion, proliferation, apoptosis, invasion, and metastasis28,29,30,31.
The complement-activated mannan-binding lectin (MBL) pathway is an inherent immune protection mechanism that reacts to infections directly. MBL and MBL-related serine proteases circulate in the blood. MASP-2 is a protease that activates complement cascades. The binding of MBL initiates the complement-activated MBL pathway to carbohydrates present on the surface of microorganisms. This leads to the activation of C4 and C2 mediated by MASP-2 to produce C3 invertase (C4bC2b), which leads to the neutralization of pathogens32,33,34,35. High MASP-2 concentration is associated with poor prognosis in patients with colorectal cancer36. In addition, the MASP-2 gene is also highly associated with lung and endometrial cancer37,38. MASP-2 expression was higher in patients with lung adenocarcinoma than in people with lung squamous cell carcinoma39.
In this study, the serum TIMP-1 of patients with thymoma was lower than that of normal controls. TIMPs were first discovered as a serum protein linked to the formation of erythroid progenitor cells and the inhibition of collagenase40. TIMPs are tissue-specific proteins that block matrix metalloproteinases (MMPs). MMPs are proteolytic enzymes that break down basement membrane and extracellular matrix41. The increased expression of MMPs in various cancers is associated with tumor aggressiveness. Metallopeptidase inhibitors (TIMPs) are negative regulators of tumor invasion and metastasis. However, the role of TIMP-1 in cancer is controversial because it can have both pro- and antitumoral effects. In addition to inhibiting matrix metalloproteinases, TIMP-1 can also promote cell growth and proliferation and inhibit cell apoptosis, which may be involved in the regulation of angiogenesis. Clinical investigations have demonstrated a strong correlation between high levels of TIMP-1 expression and poor prognosis or tumor growth in various malignancies, including lung cancer, brain prostate cancer, breast cancer, colon cancer, and others42,43,44,45,46,47,48. Yukiue et al. found that the expression level of TIMP-1 was increased in invasive thymoma tissues and speculated that TIMP-1 and MMP-1 played an essential role in the invasion process of thymoma49. In studies of cellular events associated with TIMP-induced cell growth, TIMP-1 was found to stimulate the Rat sarcoma viral oncogene homolog (RAS) protein activation50. RAS protein is the upstream regulator of the Tyrosine Kinase/Mitogen-Activated Protein Kinase (TyK/MAPK) cascade. In other words, TIMP-1 can activate the tyrosine kinase/mitogen-activated protein kinase signal pathway. The location of TIMP-1 may hold the key to understanding its dual function: intracellular TIMP-1 boosts cancer cell proliferation and survival by interfering with various signal pathways, whereas extracellular TIMP-1 is restricted to the extracellular matrix to prevent metastasis by inhibiting nearby MMPs. It can be speculated that TIMP-1 inhibits invasion and metastasis mainly by inhibiting the remodelling of the extracellular matrix in thymoma. Further research is needed to confirm this.
This research has certain limitations. First, the study's sample size was limited, which may have resulted in selection bias. Secondly, the differential proteins identified were not confirmed by other detection methods. As a result, if conditions permit, subsequent investigations with bigger sample sizes may be done, and other analytical techniques will be required to confirm the different proteins found.
Conclusion
In the patient's serum, several complement and coagulation-related proteins were up-regulated. These serum proteins may become serological biomarkers of thymoma in the future. Extracellular TIMP-1 of thymoma may prevent invasion and metastasis mainly by inhibiting the remodelling of the extracellular matrix.
Data availability
The data will be accessible by contacting the corresponding author of this study.
References
den Bakker, M. A., Roden, A. C., Marx, A. & Marino, M. Histologic classification of thymoma: A practical guide for routine cases. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 9, S125-130. https://doi.org/10.1097/JTO.0000000000000297 (2014).
Gilhus, N. E. Myasthenia gravis. N. Engl. J. Med. 375, 2570–2581. https://doi.org/10.1056/NEJMra1602678 (2016).
Travis, W. D., Brambilla, E., Burke, A. P., Marx, A. & Nicholson, A. G. Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 10, 1240–1242. https://doi.org/10.1097/JTO.0000000000000663 (2015).
Marx, A., & Ströbel, P. Update on thymoma pathology. Lessons from molecular and translational studies. Ann. Pathol. 29(1), S22–S24 (2009). https://doi.org/10.1016/j.annpat.2009.07.016.
Girard, N. et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 15, 6790–6799. https://doi.org/10.1158/1078-0432.CCR-09-0644 (2009).
Kojika, M. et al. Immunohistochemical differential diagnosis between thymic carcinoma and type B3 thymoma: diagnostic utility of hypoxic marker, GLUT-1, in thymic epithelial neoplasms. Mod. Pathol. Off. J. U S Can. Acad. Pathol. 22, 1341–1350. https://doi.org/10.1038/modpathol.2009.105 (2009).
Petrini, I. et al. Copy number aberrations of genes regulating normal thymus development in thymic epithelial tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 19, 1960–1971. https://doi.org/10.1158/1078-0432.CCR-12-3260 (2013).
Radovich, M. et al. A large microRNA cluster on chromosome 19 is a transcriptional hallmark of WHO type A and AB thymomas. Br. J. Cancer 114, 477–484 (2016).
Pfister, F. et al. Vascular architecture as a diagnostic marker for differentiation of World Health Organization thymoma subtypes and thymic carcinoma. Histopathology 70, 693–703. https://doi.org/10.1111/his.13114 (2017).
Veenstra, T. D. Global and targeted quantitative proteomics for biomarker discovery. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 847, 3–11. https://doi.org/10.1016/j.jchromb.2006.09.004 (2007).
Sun, Q.-L. et al. Proteome analysis and tissue array for profiling protein markers associated with type B thymoma subclassification. Chin. Med. J. (Engl) 125, 2811–2818 (2012).
Wang, L., Branson, O. E., Shilo, K., Hitchcock, C. L. & Freitas, M. A. Proteomic signatures of thymomas. PLoS ONE 11, e0166494 (2016).
Zhao, T. et al. Diagnosis of thymic epithelial tumor subtypes by a quantitative proteomic approach. Analyst 143, 2491–2500 (2018).
Kanehisa, M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457–D462. https://doi.org/10.1093/nar/gkv1070 (2016).
Savino, W. et al. Thymoma epithelial cells secrete thymic hormone but do not express class II antigens of the major histocompatibility complex. J. Clin. Invest. 76, 1140–1146. https://doi.org/10.1172/JCI112069 (1985).
Sasaki, H. et al. Serum level of the periostin, a homologue of an insect cell adhesion molecule, in thymoma patients. Cancer Lett. 172, 37–42. https://doi.org/10.1016/s0304-3835(01)00633-4 (2001).
Du, M. J., Shen, Q., Yin, H., Rao, Q. & Zhou, M. X. Diagnostic roles of MUC1 and GLUT1 in differentiating thymic carcinoma from type B3 thymoma. Pathol. Res. Pract. 212, 1048–1051. https://doi.org/10.1016/j.prp.2016.09.005 (2016).
Bick, R. L. Alterations of hemostasis associated with malignancy: Etiology, pathophysiology, diagnosis and management. Semin. Thromb. Hemost. 5, 1–26. https://doi.org/10.1055/s-0028-1087142 (1978).
Lip, G. Y. H., Chin, B. S. P. & Blann, A. D. Cancer and the prothrombotic state. Lancet Oncol. 3, 27–34. https://doi.org/10.1016/s1470-2045(01)00619-2 (2002).
Kwaan, H. C. & Lindholm, P. F. Fibrin and fibrinolysis in cancer. Semin. Thromb. Hemost. 45, 413–422. https://doi.org/10.1055/s-0039-1688495 (2019).
Senger, D. R. Molecular framework for angiogenesis: a complex web of interactions between extravasated plasma proteins and endothelial cell proteins induced by angiogenic cytokines. Am. J. Pathol. 149, 1–7 (1996).
Cramer, T. J. & Gale, A. J. The anticoagulant function of coagulation factor V. Thromb. Haemost. 107, 15–21. https://doi.org/10.1160/th11-06-0431 (2012).
Tinholt, M., Sandset, P. M. & Iversen, N. Polymorphisms of the coagulation system and risk of cancer. Thromb. Res. 140(Suppl 1), S49-54. https://doi.org/10.1016/s0049-3848(16)30098-6 (2016).
Tinholt, M. et al. Coagulation factor V is a marker of tumor-infiltrating immune cells in breast cancer. Oncoimmunology 9, 1824644 (2020).
Huang, T., Sun, L., Yuan, X. & Qiu, H. Thrombospondin-1 is a multifaceted player in tumor progression. Oncotarget 8, 84546–84558. https://doi.org/10.18632/oncotarget.19165 (2017).
Jeanne, A., Schneider, C., Martiny, L. & Dedieu, S. Original insights on thrombospondin-1-related antireceptor strategies in cancer. Front. Pharmacol. 6, 252. https://doi.org/10.3389/fphar.2015.00252 (2015).
Kamijo, H. et al. Thrombospondin-1 promotes tumor progression in cutaneous T-cell lymphoma via CD47. Leukemia 34, 845–856. https://doi.org/10.1038/s41375-019-0622-6 (2020).
Horiguchi, H., Yamagata, S., Rong Qian, Z., Kagawa, S. & Sakashita, N. Thrombospondin-1 is highly expressed in desmoplastic components of invasive ductal carcinoma of the breast and associated with lymph node metastasis. J. Med. Investig. JMI 60, 91–96. https://doi.org/10.2152/jmi.60.91 (2013).
Jayachandran, A. et al. Thrombospondin 1 promotes an aggressive phenotype through epithelial-to-mesenchymal transition in human melanoma. Oncotarget 5, 5782–5797. https://doi.org/10.18632/oncotarget.2164 (2014).
Pal, S. K. et al. THBS1 is induced by TGFB1 in the cancer stroma and promotes invasion of oral squamous cell carcinoma. J. Oral. Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral. Pathol. 45, 730–739. https://doi.org/10.1111/jop.12430 (2016).
Weis, W. I., Taylor, M. E. & Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev. 163, 19–34. https://doi.org/10.1111/j.1600-065x.1998.tb01185.x (1998).
Thiel, S. et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature 386, 506–510. https://doi.org/10.1038/386506a0 (1997).
Chen, C.-B. & Wallis, R. Two mechanisms for mannose-binding protein modulation of the activity of its associated serine proteases. J. Biol. Chem. 279, 26058–26065. https://doi.org/10.1074/jbc.M401318200 (2004).
Turner, M. W. & Hamvas, R. M. Mannose-binding lectin: structure, function, genetics and disease associations. Rev. Immunogenet. 2, 305–322 (2000).
Ytting, H., Christensen, I. J., Thiel, S., Jensenius, J. C. & Nielsen, H. J. Serum mannan-binding lectin-associated serine protease 2 levels in colorectal cancer: Relation to recurrence and mortality. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 11, 1441–1446. https://doi.org/10.1158/1078-0432.ccr-04-1272 (2005).
Zengin, T. & Önal-Süzek, T. Analysis of genomic and transcriptomic variations as prognostic signature for lung adenocarcinoma. BMC Bioinformatics 21, 368. https://doi.org/10.1186/s12859-020-03691-3 (2020).
Sun, P. et al. Distinct clinical and genetic mutation characteristics in sporadic and Lynch syndrome-associated endometrial cancer in a Chinese population. Cancer Epidemiol. 73, 101934. https://doi.org/10.1016/j.canep.2021.101934 (2021).
Bao, M. et al. Proteomic analysis of plasma exosomes in patients with non-small cell lung cancer. Transl. Lung Cancer Res. 11, 1434–1452. https://doi.org/10.21037/tlcr-22-467 (2022).
Cabral-Pacheco, G. A. et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 21, E9739. https://doi.org/10.3390/ijms21249739 (2020).
Stetler-Stevenson, W. G., Aznavoorian, S. & Liotta, L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell Biol. 9, 541–573. https://doi.org/10.1146/annurev.cb.09.110193.002545 (1993).
Inoue, H. et al. Expression of tissue inhibitor of matrix metalloproteinase-1 in human breast carcinoma. Oncol. Rep. 7, 871–874. https://doi.org/10.3892/or.7.4.871 (2000).
Groft, L. L. et al. Differential expression and localization of TIMP-1 and TIMP-4 in human gliomas. Br. J. Cancer 85, 55–63. https://doi.org/10.1054/bjoc.2001.1854 (2001).
Schrohl, A.-S., Look, M. P., Meijer-van Gelder, M. E., Foekens, J. A. & Brünner, N. Tumor tissue levels of Tissue Inhibitor of Metalloproteinases-1 (TIMP-1) and outcome following adjuvant chemotherapy in premenopausal lymph node-positive breast cancer patients: A retrospective study. BMC Cancer 9, 322. https://doi.org/10.1186/1471-2407-9-322 (2009).
Roy, R. et al. Urinary TIMP-1 and MMP-2 levels detect the presence of pancreatic malignancies. Br. J. Cancer 111, 1772–1779. https://doi.org/10.1038/bjc.2014.462 (2014).
Bodnar, M., Szylberg, Ł, Kazmierczak, W. & Marszalek, A. Tumor progression driven by pathways activating matrix metalloproteinases and their inhibitors. J. Oral. Pathol. Med. Off. Publ. Int. Assoc. Oral. Pathol. Am. Acad. Oral. Pathol. 44, 437–443. https://doi.org/10.1111/jop.12270 (2015).
Lugowska, I. et al. Serum markers in early-stage and locally advanced melanoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 36, 8277–8285. https://doi.org/10.1007/s13277-015-3564-2 (2015).
Yi, Y.-C. et al. Genetic polymorphism of the tissue inhibitor of metalloproteinase-1 is associated with an increased risk of endometrial cancer. Clin. Chim. Acta Int. J. Clin. Chem. 409, 127–131. https://doi.org/10.1016/j.cca.2009.09.015 (2009).
Yukiue, H. et al. Clinical significance of tissue inhibitor of metalloproteinase and matrix metalloproteinase mRNA expression in thymoma. J. Surg. Res. 109, 86–91. https://doi.org/10.1016/S0022-4804(02)00040-9 (2003).
Wang, T., Yamashita, K., Iwata, K. & Hayakawa, T. Both tissue inhibitors of metalloproteinases-1 (TIMP-1) and TIMP-2 activate Ras but through different pathways. Biochem. Biophys. Res. Commun. 296, 201–205. https://doi.org/10.1016/s0006-291x(02)00741-6 (2002).
Funding
Shijiazhuang Science and Technology Bureau funded this research (Grant No. 201460603). This work was also supported by financial grants from the National Natural Science Foundation of China (82274582), Hebei Provincial Natural Science Foundation (H2019106063), Hebei Province Innovation capability Improvement plan project (20577715D) and the S&T Program of Hebei (19277701D).
Author information
Authors and Affiliations
Contributions
J.D., Y.L., and G.Y.: conceptualization, methodology, draft preparation, and supervision. J.D. and Y.L.: writing-reviewing and editing, data collection, and draft preparation. J.D. and G.Y.: visualization, investigation, software, data collection, and draft preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Qi, G. & Liu, Y. Proteomics analysis of serum from thymoma patients. Sci Rep 13, 5117 (2023). https://doi.org/10.1038/s41598-023-32339-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32339-4