Abstract
Pearlmillet-chickpea cropping system (PCCS) is emerging as an important sequence in semi-arid regions of south-Asia owing to less water-requirement. However, chickpea (dry-season crop) faces comparatively acute soil moisture-deficit over pearlmillet (wet-season crop), limiting overall sustainability of PCCS. Hence, moisture-management (specifically in chickpea) and system intensification is highly essential for sustaining the PCCS in holistic manner. Since, conservation agriculture (CA) has emerged is an important climate-smart strategy to combat moisture-stress alongwith other production-vulnerabilities. Hence, current study comprised of three tillage systems in main-plots viz., Complete-CA with residue retention (CAc), Partial-CA without residue-retention (CAp), and Conventional-tillage (ConvTill) under three cropping systems in sub-plots viz., conventionally grown pearlmillet-chickpea cropping system (PCCS) alongwith two intensified systems i.e. pearlmillet-chickpea-fodder pearlmillet cropping system (PCFCS) and pearlmillet-chickpea-mungbean cropping system (PCMCS) in split-plot design. The investigation outcomes mainly focused on chickpea (dry-season crop) revealed that, on an average, there was a significant increase in chickpea grain yield under CAc to the tune of 27, 23.5 and 28.5% under PCCS, PCFCS and PCMCS, respectively over ConvTill. NPK uptake and micronutrient (Fe and Zn) biofortification in chickpea grains were again significantly higher under triple zero-tilled CAc plots with residue-retention; which was followed by triple zero-tilled CAp plots without residue-retention and the ConvTill plots. Likewise, CAc under PCMCS led to an increase in relative leaf water (RLW) content in chickpea by ~ 20.8% over ConvTill under PCCS, hence, ameliorating the moisture-stress effects. Interestingly, CA-management and system-intensification significantly enhanced the plant biochemical properties in chickpea viz., super-oxide dismutase, ascorbate peroxidase, catalase and glutathione reductase; thus, indicating their prime role in inducing moisture-stress tolerance ability in moisture-starved chickpea. Triple zero-tilled CAc plots also reduced the N2O fluxes in chickpea but with slightly higher CO2 emissions, however, curtailed the net GHG-emissions. Triple zero-tilled cropping systems (PCFCS and PCMCS) both under CAc and Cap led to a significant improvement in soil microbial population and soil enzymes activities (alkaline phosphatase, fluorescein diacetate, dehydrogenase). Overall, the PCCS system-intensification with mungbean (PCMCS) alongwith triple zero-tillage with residue-retention (CAc) may amply enhance the productivity, micronutrient biofortification and moisture-stress tolerance ability in chickpea besides propelling the ecological benefits under semi-arid agro-ecologies. However, the farmers should preserve a balance while adopting CAc or CAp where livestock equally competes for quality fodder.
Similar content being viewed by others
Pearlmillet-chickpea cropping system (PCCS) is an important crop sequences of semi-arid regions of south-Asia owing to less water-requirement1. In arid and semi-arid regions of south-Asia, pearlmillet (Pennisetum glaucum L.) is the main food crop grown in wet-season in mono- or double-cropping systems because of its hardy nature against extreme weather conditions2. Likewise, chickpea (Cicer arietinum L.), an important dry-season (Rabi) legume crop grown on residual soil moisture, immensely contributes towards soil fertility restoration and nutritional security in this region1,3,4. However, chickpea (dry-season crop) faces comparatively acute soil moisture-deficit in dry season due to erratic precipitation distribution over pearlmillet (wet-season crop), hence, limiting chickpea productivity and quality. These production-vulnerabilities pose a great threat to the sustainability of pearlmillet-chickpea cropping system (PCCS) in the region. Therefore, appropriate soil and moisture conservation practices can mitigate the aforesaid challenges in PCCS under such vulnerable ecologies.
Rainfed agriculture already contributes ~ 82% of global arable area (1.223 billion ha) while hosting ~ 40% global population5. Increasing population in south-Asia further exerts an exorbitant pressure to enhance the food production in arid and semi-arid regions. With the advent of short-duration pearlmillet varieties, it has though become possible to go in for triple cropping systems with appropriate soil and moisture management practices. Here, mungbean and fodder pearlmillet can be the appropriate short-duration summer season crops for the intensification of PCCS in semi-arid regions to meet both pulse and fodder requirement, respectively6. Hence, appropriate moisture-management (specifically in chickpea) coupled with system intensification may play an important role in sustaining the PCCS in holistic manner. Conservation agriculture (CA) has emerged as an important climate-smart strategy to mitigate the moisture stress in vulnerable agro-ecologies besides its positive impact on crop productivity, quality, moisture-stress tolerance and soil health7,8,9.
Residue retention on the soil surface under CA-management prevents the soil erosion and evaporation losses, and regulates the soil thermal dynamics which maintains soil moisture, and consequently improves water- and nutrient-use efficiency and crop productivity10. CA systems also curtail the nutrient (~ 30%), labour (~ 50%), and fuel (~ 65%) requirement in various crops11,12,13,14. CA management also promotes the soil organic carbon (SOC) storage, aggregate stability, and soil biological activity over intensive tillage1,15,16,17. In addition, improved soil physico-chemical and biological properties and micro-climate modulation under CA-management9,10,18,19, may also prove helpful in inducing the moisture-stress tolerance ability in rainfed crops20. Hence, it is obligatory to generate robust scientific evidences for CA based agronomic interventions for the water-scarce semi-arid regions that too under triple-zero tilled cropping systems, as most of the research till date is largely confined to irrigation-based rice–wheat cropping system (RWCS) in south-Asia21. As, Rabi season chickpea is more vulnerable to moisture-stress due to meager and erratic rainfall distribution5. Hence, we studied the impact of CA practices and the system intensification on the productivity, quality and moisture-stress tolerance ability of moisture-starved chickpea as an indicator crop under intensified PCCS. Current experimentation was therefore based on the hypothesis that the triple zero-till based system intensification of PCCS would lead to enhanced productivity, micronutrient biofortification and plant biochemical properties with reduced green-house gas (GHG) emissions under chickpea besides improved soil microbial population and soil enzymatic activities in semi-arid agro-ecologies of south-Asia.
Results and discussion
Soil microbial population
In current study, soil microbial population was significantly (p < 0.05) affected by various tillage practices and the intensified cropping systems. The colony forming unit (CFU) counts were significantly higher under complete conservation agriculture (CA) with residue retention (CAc) followed by partial CA without residue retention (CAp) treatments and conventional tillage (ConvTill), respectively (Fig. 1). Highest number of bacteria, fungi and actinomycete were seen under CAc followed by CAp. Across the tillage practices, pearlmillet-chickpea–mungbean cropping system (PCMCS) had significantly higher microbial CFU count followed by pearlmillet-chickpea cropping system (PCCS) while pearlmillet-chickpea–fodder pearlmillet cropping system (PCFCS) reported least microbial population. Among various treatment combinations, highest microbial counts of bacteria (82.2 × 104 CFU g−1 soil), fungi (63.2 × 102 CFU g−1 soil) and actinomycetes (49.5 × 104 CFU g−1 soil) were observed in CAc plots with PCMCS system (CAc_PCMCS) followed by CAc with PCCS system (CAc_PCCS) and CAc with PCFCS system (CAc_PCFCS), respectively. Lowest population of bacteria (53.3 × 104 CFU g−1 soil), fungi (41.5 × 102 CFU g−1 soil) and actinomycetes (29.3 × 104 CFU g−1 soil) were perceived under ConvTill_PCFCS which were 35.2, 52.2 and 16.2% lower than the best treatment combination CAc_PCMCS. This may be accrued to the reason the high organic biomass addition under CAc plots improved the soil structure, aggregate stability and uniform soil moisture availability, which in turn might have allowed microbial populations to grow and sustain in the rhizosphere15,16,17,18,22. Combining the CAc practice with legume-intensification also enhanced the SOC input owing to adequate leaf litterfall and root biomass additions from legumes with narrow C:N ratio18,19; which in turn, enhanced the soil microbial diversity17,23,24,25. Legume roots also release the root exudates which harbor the microbial diversity in the rhizosphere4,20,26, that’s why the double-legumes system i.e. PCMCS had highest microbial counts of bacteria, fungi and actenomycetes in current study.
Effect of tillage practices and cropping systems on soil microbial populations (Soil samples taken from 0 to 15 cm soil layer after 2nd years of cropping cycle). Vertical bars indicate the LSD at p = 0.05. [Note: PCCS = Pearlmillet-chickpea cropping system; PCFCS = Pearl millet–chickpea–fodder pearlmillet cropping system; PCMCS = Pearlmillet-chickpea–mungbean cropping system; CAc = Complete conservation agriculture with residue retention; CAp = Partial conservation agriculture without residues; ConvTill = Conventional tillage].
Soil microbial enzymatic activities
Different tillage practices had significant (p < 0.05) effect on acid and alkaline phosphatase, glucosidase, dehydrogenase and fluorescein diacetate (FDA) activities (Fig. 2). These enzymatic activities were significantly (p < 0.05) higher under CAc followed by CAp, and ConvTill. Compared to ConvTill, the acid phosphatase, alkaline phosphatase, glucosidase, dehydrogenase and FDA activities were higher by 55.6, 64.3, 16.7, 105.3 and 83.8%, respectively under CAc. The system-intensification had significant effect on alkaline phosphatase, dehydrogenase and FDA activities. Highest activities of alkaline phosphatase (152 μmol p-nitrophenol g−1 h−1), dehydrogenase (454 μg TPF g−1 24 h−1) and FDA (24.2 μg fluorescein g−1 h−1) were observed under PCMCS, whereas PCFCS had least activities of these enzymes. As, legume-inclusion enhances the SOM4,20,26, thus, resulting in higher soil enzyme activities under double-legume system PCMCS21,27. Likewise, higher SOC enrichment both under CA-based tillage systems and the legume-intensification might have enhanced the FDA activity in our study16,21. The CA practices and legume-intervention also enhanced the dehydrogenase activity due to higher microbial nutrient bioavailability in the rhizosphere16,28,29.
Effect of tillage practices and cropping systems on soil microbial enzyme activities (Soil samples taken from 0 to 15 cm soil layer after 2nd years of cropping cycle). Vertical bars indicate the LSD at p = 0.05. [Note: PCCS = Pearlmillet-chickpea cropping system; PCFCS = Pearl millet–chickpea–fodder pearlmillet cropping system; PCMCS = Pearlmillet-chickpea–mungbean cropping system; CAc = Complete conservation agriculture with residue retention; CAp = Partial conservation agriculture without residues; ConvTill = Conventional tillage].
Crop productivity
Different Tillage practices and cropping systems had significant (p < 0.05) influence on the number of pods plant−1 during both years (Table 1). Pods plant−1 during 2020–2021 were 19% lesser than the year 2019–2020. Highest pods plant−1 (40.5 and 32.8) were obtained under CAc_PCMCS compared to rest of the treatments combinations during both years where ConvTill_PCFCS had least pod count plant−1 (31.4 and 26.6). Crop residue-retention improves the soil fertility and moisture holding capacity owing to SOM enrichment and nutrient bioavailability after biomass decomposition13,21,30, which accelerate the plant growth and dry matter accumulation and finally economic yield31. The CA practices are intended to increase carbon inputs, nutrient bioavailability with better physical rhizo-ecology (aggregate formation, moisture permeability and conservation) which directly proliferate the soil microbial diversity with higher crop yields13,16. Similarly, the interaction tillage and legume-inclusion (mungbean) showed significant (p < 0.05) grain and straw yield enhancement in chickpea during both years (Table 1). In general, chickpea grain and straw yield was comparatively higher during 2019–2020 than 2020–2021 owing to uniform rainfall distribution during 2019–2020 compared to 2020–2021 (Fig. 6). Significantly highest grain (1.23; 0.74 t ha−1) and straw yield (3.6; 2.06 t ha−1) of chickpea were recorded from the combination of CAc with PCMCS system over other combinations during 2019–2020 and 2020–2021, respectively. The CAc practice compared to ConvTill had a respective average grain yield increase by ~ 27, 23.5 and 28.5% and average straw yield increase by ~ 48.5, 47.5 and 56% under PCCS, PCFCS and PCMCS in our study. Again, the conventionally tilled PCFCS system had least grain and stover yield over other cropping systems. As, residue retention under CA plots was highly effective in reducing the evaporation losses and conserving more soil moisture, thus, resulting in better crop growth and yield over ConvTill plots13,14. Moreover, chickpea is a deep-rooted crop, therefore, which efficiently utilized the conserved soil moisture under CA plots for realizing higher yields4,32. There existed a significant positive and strong correlation between chickpea productivity and pods plant-1 during 2019–2020 (R2 = 0.96) and 2020–2021 (R2 = 0.77) (Fig. 3). The overall improvement in chickpea yield under CA plots (CAc and CAp) could be ascribed to pivotal role of crop residues in several physiological, biochemical, chemical and physical processes15,16,17,23,24,33.
Nutrient uptake
The experimental results revealed that both CA practices (CAc and CAp) improved the total (grain + stover) NPK uptake in chickpea over conventional tillage (Table 2). Significantly (p < 0.05) higher total N (73.3 l; 43 kg ha−1), P (7.5; 4.3 kg ha−1) and K uptake (53.3; 30.2 kg ha−1) were obtained under CAc_PCMCS during 2019–2020 and 2020–2021. Greater nutrient bioavailability as a result of optimal moisture conditions under CA plots could be the major factor for such observations34. Higher NPK uptake may also be accrued to higher yield under CAc owing to improved soil physico-chemical and biological properties9,10. Lowest NPK uptake was recorded from ConvTill_PCFCS owing to poor crop growth and biomass production in ConvTill plots compared to CAc18,19,35. Higher NPK uptake under PCMCS may also be accrued to inclusion of two legumes (chickpea and mungbean) in the system which greatly improved the soil biofertility over the PCCS and PCFCS systems4.
Micronutrient biofortification
Tillage practices and system-intensification had significant (p < 0.05) effect on micronutrient (Zn, Fe) biofortification in chickpea grains and straw (Table 3). Among tillage treatments, significantly (p < 0.05) greatest micronutrient content in chickpea grains as well as straw were obtained under CAc and CAp followed by ConvTill. The Fe and Zn content increased by ~ 2.5 and 1.56; and 8.3 and 10.1% in chickpea grains; and 3.4 and 3.8; and 3.7 and 6.2% in straw during 2019–2020 and 2020–2021, respectively over ConvTill. The improvement in micronutrient content under CAc may be attributed to enhanced microbial activity and synchronous nutrient release during SOM decomposition process of the crop residues16,24,36,37. Likewise, the highest micronutrient content (Zn, Fe) in chickpea grains and straw were observed under PCMCS owing to higher nutrient acquisition and biomass productivity under the influence of two legumes i.e. chickpea and mungbean4. Significant enhancement in micronutrients (2-years’ av.) under different cropping systems was found to be 1.60 and 1.80 (Fe); 3.9 and 3.5 (Zn) mg kg−1 in grain and stover in PCCS and PCMCS, respectively over PCFCS. As, legume-imbedded systems fixed more N with sufficient biomass additions having narrow C: N ratio18,19; thus, speeding-up the biomass decomposition with more C-sequestration vis-à-vis more micronutrient acquisition4,27. The resultant SOM might have also helped in synthesis of organic acids in rhizosphere27, which in turn, acted as micronutrient chelates, influencing translocation and remobilization of micronutrients37,38.
Relative water content
Various treatment combinations significantly (p < 0.05) improved the relative water content (RWC) in fully expanded chickpea leaves at flowering (Fig. 4). The highest RWC (86.3%) was achieved under CAc in PCMCS system. This treatment combination improved the RWC by ~ 20.76% over ConvTill_PCFCS system. The improved RWC under CAc was a consequence of higher moisture retention and comparatively lower moisture stress in residue-retained CAc plots9,10. As, the legume intervention in the crop sequences enhances the water holding capacity due to better physical and biological rhizospheric environment, hence, resulting in favorable plant-soil–water relations with higher RWC18,21,25.
Effect of tillage practices and cropping systems on relative water content (RWC) in chickpea leaves (Pooled mean of 2-years). Vertical bars indicate the LSD at p = 0.05. [Note: PCCS = Pearlmillet-chickpea cropping system; PCFCS = Pearl millet–chickpea–fodder pearlmillet cropping system; PCMCS = Pearlmillet-chickpea–mungbean cropping system; CAc = Complete conservation agriculture with residue retention; CAp = Partial conservation agriculture without residues; ConvTill = Conventional tillage].
Soil moisture content
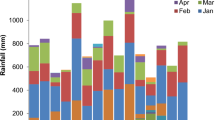
Diverse tillage and cropping system treatment combinations remarkably affected the soil moisture content (0–15 cm soil profile), at monthly intervals during both chickpea growing seasons (Fig. 5). The differences in soil moisture contents were relatively lesser during the crop period 2019–20 as the crop received 336.7 mm rainfall, in comparison to 2nd year when the winter season rainfall was merely 73.7 mm. Maximum soil moisture content were recorded under conservation agricultural systems, specifically under CAc plots. Whereas, under ConvTill treatments, the soil moisture remained lowest. The reduced moisture losses owing to lesser evaporation and greater moisture retention under crop residue retained plots might have resulted in more moisture availability under CA systems2,10. Likewise, the legume component in PCMCS under different tillage systems might have enhanced the water holding capacity due to better physical and biological rhizospheric environment, hence, resulting in greater moisture content in the soil profile18,21.
Soil moisture content at monthly interval during the chickpea crop growing period in the 0–15 cm soil profile corresponding to the year (A) 2019–20 and (B) 2020–21. [Note: PCCS = Pearlmillet-chickpea cropping system; PCFCS = Pearl millet–chickpea–fodder pearlmillet cropping system; PCMCS = Pearlmillet-chickpea–mungbean cropping system; CAc = Complete conservation agriculture with residue retention; CAp = Partial conservation agriculture without residues; ConvTill = Conventional tillage].
Biochemical properties vis-à-vis moisture-stress tolerance ability
Tillage practices and system-intensification had significant (p < 0.05) influence on biochemical properties vis-à-vis moisture-stress tolerance ability of chickpea (Fig. 6), except ascorbate peroxidase (APX) and catalase (CAT) activity. Treatments, CAc_PCMCS (22.9%), a combination of complete CA and double legume imbedded cropping system exhibited highest grain protein content (Fig. 6A), with ~ 3.6% higher protein content compared to ConvTill_PCCS. Higher N content in chickpea under CAc_PCMCS may be attributed to increased N-bioavailability in the soil due to double legume-inclusion1,4,18. Higher decomposition rate of crop residues in CAc system might have also enhanced the N-acquisition and protein content in the plants18,39. Grain protein content was least in ConvTill_PCFCS be due to extensive N removal by two cereal components in the system9,10. The proline content was found to be inversely related with RWC. The maxima of proline content (9.5 μmol g-1 FW) was obtained under ConvTill_PCFCS (Fig. 6B). This treatment combination remained at par with ConvTill_PCCS (7.98 μmol g−1 FW) and ConvTill_PCMCS (6.84 μmol g−1 FW) and CAp_PCFCS (7.15 μmol g−1 FW). The least proline content was noticed in CAc_PCMCS and CAc_PCCS. The reduced proline levels in chickpea leaves in CAc might be due to the increased moisture retention under crop residues, which resulted in low plant moisture-stress25,40. Similarly, chickpea plants grown with CA practices in PCMCS showed significantly higher biochemical properties like superoxide dismutase (SOD) activity (28.9 Ug−1 FW−1), and glutathione reductase (GR) activity (0.63 U mg−1 protein−1 min−1) which were ~ 11 and 30% higher over ConvTill_PCCS (Fig. 6C,D). Statistically non-significant increase was noticed in the CAT and APX activities under CAc (Fig. 6E,F). Least proline and higher values of SOD, GR, CAT and APX activity in chickpea under CAc indicate the ability of CA-management on moisture-stress tolerance in the current study2,18. It is evident from various studies that moisture or drought stress causes oxidative stress by decreasing stomatal conductivity in the plants which confines CO2 influx in to the leaves40. Hence, there is reduction in the leaf internal CO2, causing formation of reactive oxygen species (ROS) mainly in plant cell, mitochondria, chloroplasts and peroxisomes41. In our study, there was higher production of SOD, GR, CAT and APX activity in chickpea under CAc. As, higher ROS production induces deleterious impact on plant cells; the plant defense system becomes active against ROS42; and releases non-enzymatic antioxidants (proline) and antioxidant enzymes (like CAT, SOD) and ascorbate–glutathione (AsA–GSH) cycle enzymes (like GR and APX) for detoxification of ROS and plant cell protection40,41,42,43. It indicates that enhanced SOD, GR, CAT and APX activities under CAc inducts drought-stress tolerance ability in chickpea plants in semi-arid environment.
Effect of tillage practices and cropping systems on biochemical properties of chickpea (Pooled mean of 2-years), (A) Protein content; (B) Proline content; (C) Superoxide dismutase (SOD); (D) Ascorbate peroxidase (APX); (E) Catalase (CAT), and (F) Glutathione reductase (GR). Vertical bars indicate the LSD at p = 0.05. [Note: PCCS = Pearlmillet-chickpea cropping system; PCFCS = Pearl millet–chickpea–fodder pearlmillet cropping system; PCMCS = Pearlmillet-chickpea–mungbean cropping system; CAc = Complete conservation agriculture with residue retention; CAp = Partial conservation agriculture without residues; ConvTill = Conventional tillage; Vertical bars indicate the LSD at p = 0.05].
GHG-emissions
In current study, the CO2 and N2O emissions ranged between 1757 and 2246 kg ha−1 and 332–345 kg ha−1, respectively under various tillage treatments (Table 4). The CAc system emitted relatively larger amount of CO2 followed by CAp and ConvTill. The CAp and ConvTill remained statistically at par in terms of CO2 emissions. Contrary to CO2, the N2O emission was the larger under ConvTill and lowest in CAc plots; however, net GHG-emissions were least under CAc compared to ConvTill. Likewise, the system-intensification (PCMCS and PCFCS) led to slight enhancement in CO2 emissions both of which remained statistically at par. Intensive cropping (PCMCS/PCFCS) didn’t affect N2O emissions where all the cropping systems behaved statistically similar. Zero-tillage with residue retention in intensive cropping systems increased the availability of organic carbon that might have resulted in enhanced soil respiration and CO2 release44,45. Presence of residue-cover reduces the N2O emissions46, and therefore, the slightly lower N2O flux was observed in the CA-systems47.
Conclusions
In current study, the triple zero-till based system-intensification coupled with residue retention (CAc) may enhance the chickpea grain yield by ~ 25% over conventional tillage (ConvTill) systems in the moisture-starved semi-arid ecologies. Likewise, the double legume bound triple cropping system i.e. pearlmillet-chickpea–mungbean cropping system (PCMCS) under CAc significantly enhanced the relative leaf water content (~ 21%), total NPK uptake, protein content, micronutrient (Fe, Zn) biofortification, soil microbial population and soil enzyme activities compared to ConvTill. The micronutrient biofortification (Fe, Zn) in chickpea grains followed the trend of CAc > Cap > ConvTill. The N2O emissions remained unaffected under different cropping systems. Interestingly, the CAc-management reduced the N2O fluxes but with slightly higher CO2 emissions, however, curtailing the net GHG-emissions. Triple cropping systems and the CA-management significantly influenced the plant biochemical entities in chickpea viz. proline content, super-oxide dismutase, ascorbate peroxidase, catalase and glutathione reductase. Least proline and higher values of superoxide dismutase, glutathione reductase, catalase and ascorbate peroxidase activity in chickpea under CAc indicate the ability of CA-management on moisture-stress tolerance under semi-arid ecologies. Overall, the system-intensification of pearlmillet-chickpea cropping system by the mungbean (PCMCS) coupled with triple zero-tillage and residue-retention (CAc) may enhance the chickpea productivity, micronutrient biofortification, moisture-stress tolerance, and soil health with reduced GHG-emissions under prevailing semi-arid conditions of south-Asia. Although, the small-holders still have to maintain a balance while adopting CAc or CAp where livestock rearing equally competes for quality fodder.
Materials and methods
Experimental site
The present experiment was conducted during 2019–2020 and 2020–2021 at research farm of ICAR–Indian Agricultural Research Institute, New Delhi [Latitude 28°4′N; Longitude 77°12′E; Altitude 228.6 m]. The region falls under semi-arid climate having severe winters and hot-dry summers. Almost 70–80% of the annual rainfall (~ 652 mm) is received during July–September and rest 20–30% during October to May2. Total rainfall received during the chickpea growing season was 336.7 mm (2019–2020) and 73.7 mm (2020–2021) (Fig. 7). The soil of the experiment was sandy loam in texture (Inceptisol), slightly alkaline in reaction, poor in soil organic carbon (SOC) and available-N and medium in available-P and available-K. Detailed initial physico-chemical properties of experimental soil are enlisted in Table 5.
Meteorological data of New Delhi, India for the chickpea growing season (Rabi season) corresponding to the year (A) 2019–20 and (B) 2020–21. [Note: Tmax. = Maximum Temperature; Tmin. = Minimum Temperature; RH = Relative humidity; RF = Rainfall; SS = Sunshine hours; EVP = Pan evaporation, WS = Wind speed].
Treatments detail and crop management
The experiment was laid out in a split-plot design with three replications. In main-plots, tillage and residue management practices were used while diverse cropping systems were allotted in sub-plots (Table 6). Chickpea variety ‘Pusa-1103’ was sown in 2nd week of October during both years, at 45 cm row spacing using 80 kg seed ha−1. Gap filling and thinning operations were done within 20 days of sowing. The crop was fertilized with 20 kg N + 40 kg P2O5 + 40 kg K2O per ha. Plant nutrients; N, P and K were applied through urea (46% N), single superphosphate (16% P2O5) and muriate of potash (60% K2O), respectively. The whole amount of NPK fertilizers were applied as basal at sowing of the chickpea. All the crops including chickpea were raised entirely under rainfed conditions on conserved soil moisture. To control weeds, pre-emergence application of pendimethalin was done using 0.75 kg a.i. ha−1 in 400 L ha−1 spray solution. Under CA plots, after harvest of preceding crop, paraquate 0.75 kg a.i. ha−1 was applied using 400 L water ha−1 as spray solution. All the crops were grown using standard package of practices except the respective treatment plans51,56.
Soil sampling and analysis
Current experimentation was done on a long-term experiment. Fresh and moist soil samples from 0 to 15 cm depth were collected immediately after completion of two years of crop rotations of the experiment conducted during 2019–2020 and 2020–2021 i.e. 3rd and 4th year of the long-term experiment. These samples were then transferred to the laboratory for microbial analysis. The total bacterial population was counted using the Pour plating method57, in which the samples were incubated on nutrient agar medium for 3 days at 32 °C. Counting of total fungi was performed after incubating the fungal culture plate at 30 °C for 5 days on rose bengal agar medium supplemented with streptomycin (30 µg ml−1)58. Likewise, total actinomycete counting was done using actinomycete isolation agar (AIA) plates with 50 mg ml−1 nalidixic acid59, where the AIA plates were incubated for 7 days at 28 °C. The results of triplicate readings were presented as CFU g−1 dry soil. The soil acid and alkaline phosphatase enzymatic activities were determined using 16 mM para (p)-nitrophenyl phosphate as substrate60 and reported as μmol p-nitrophenol g−1 h−1. Likewise, Glucosidase activity was estimated using 25 mM p-nitrophenol-β-D-glucopyranoside as substrate61 and expressed as μmol p-nitrophenol g−1 h−1. Dehydrogenase activity was determined by the rate of reduction of triphenyltetrazolium chloride to triphenylformazan51 and expressed as μg TPF g−1 24 h−1. Soil microbial activity expressed as FDA hydrolysis was determined following the method developed by Green et al.62.
Yield parameters and yield estimation
Pods plant−1 were counted from 10 randomly selected chickpea plants and there average was taken. Grain yield was recorded (at 14% moisture content) from the net plot area and expressed in t ha-1 following the methodology of Rana et al.51.
Plant chemical analysis
Chickpea plant samples (grain and straw) were collected after crop harvest during both the years (2019–20 and 2020–21), and thereafter oven-dried at 60 ± 2 °C for 72 h. Subsequently, samples were grinded in Willey-Mill fitted with stainless steel parts and passed through a 1 mm sieve. Nitrogen concentrations in these samples were estimated by the Micro-Kjeldahl’s method55, and total P and K were determined using a sulfuric-nitric-perchloric acid digest51. Nutrient uptake was computed by multiplying respective nutrient concentrations by grain and straw yield and expressed in kg ha-1. Micronutrient (Fe, Zn) content in grain as well as straw was determined by a di-acid digestion method using atomic absorption spectrophotometry51.
Relative water content
Relative water content (RWC) of the chickpea leaf was determined from first fully expanded top leaf of the plants at flowering stage. Leaf fresh weight was recorded immediately and then the leaf was incubated in distilled water for at least 4 h at 40 °C in the dark, blotted dried and its turgid weight was measured. Finally, dry weight was determined after drying it at 80 °C for 48 h in the oven. The RWC was calculated with the following formula63:
Here Fw is the fresh weight, Dw is the dry weight and Tw is the turgid weight.
Soil moisture content
Soil moisture content were determined at monthly interval during the chickpea crop growing period from 0 to 15 cm soil profile during both the study years i.e. 2019–20, and 2020–21 using standard procedures51.
Plant biochemical properties
Protein, proline content, superoxide dismutase, ascorbate peroxidase, catalase and glutathione reductase activities were determined from chickpea plant samples at flowering stage using standard methodology of Lowry et al.64, Bates et al.65, Beauchamp and Fridovich66, Nakano and Asada67, Aebi68, and Foyer et al.69, respectively.
GHG-emission studies
Fluxes of greenhouse gases (GHGs) i.e. CO2 and N2O were measured during both chickpea growing seasons (October to March), using the static chamber method70,71 and for continuous 7-days after fertilization and rainfall. Acrylic chambers of 15 cm × 15 cm × 100 cm size, fitted with thermometer, battery operated fan and rubber septa on the top were used for sampling of gases. Samples were collected once in a week between 9 and 11 AM using a 20 ml syringe fitted with a 3-way stopcock, at 0, 30, and 60 min after chamber closure. For each treatment, sampling was carried out in triplicate. CO2 and N2O concentrations in the collected sample were analyzed by Gas Chromatograph (GC: Hewlett Packard 5890)70 having a stainless steel column fitted with a flame ionization and electron capture detector. The cumulative amount of CO2 and N2O emissions were determined by linear interpolation of two adjacent intervals of measurements carried out on the sampling days assuming that GHGs emissions followed a linear trend during the periods when no sample was taken72,73.
The emissions of CO2 and N2O from soil were calculated by the following equation:
Here F is the CO2/N2O flux, ρ is the gas density, \(V\) is the volume of the close chamber (m3), \(A\) is the surface area of the closed chamber (m2), \(\frac{\mathrm{\Delta c}}{\mathrm{\Delta t}}\) represents the rate of increase of CO2/N2O gas concentration in the chamber (mg/μg m−3 h−1) and T (absolute temperature) is calculated as 273 + mean temperature (°C) of the chamber. Total CO2/N2O flux for the entire cultivation period was computed by linear interpolation using the following Eq. 74:
where \({R}_{i}\) is the CO2/N2O emission flux (g m−2 d−1) on the ith sampling interval, \({D}_{i}\) represents the number of days in the ith sampling interval, and n is the number of sampling intervals.
Statistical analysis
The data related to each parameter were analyzed as per the procedure of analysis of variance (ANOVA) to determine treatment effects through Tukey’s honestly significant difference test as a post hoc mean separation test (p < 0.05) by using SAS 9.1 software (SAS Institute, Cary, NC). Tukey’s procedure was used where ANOVA was found significant.
Research involving plants
It is stated that the current experimental research on the plants comply with the relevant institutional, national, and international guidelines and legislation. It is also stated that the appropriate permissions has been taken wherever necessary, for collection of plant specimens. It is also stated that the authors comply with the ‘IUCN Policy Statement on Research Involving Species at Risk of Extinction’ and the ‘Convention on the Trade in Endangered Species of Wild Fauna and Flora’.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Choudhary, G. L., Rana, K. S., Bana, R. S. & Prajapat, K. Moisture conservation and zinc fertilization impacts on quality, profitability and moisture use indices of chickpea (Cicer arietinum L.) under limited moisture conditions. Legume Res. 39, 734–740 (2016).
Bana, R. S., Pooniya, V., Choudhary, A. K., Rana, K. S. & Tyagi, V. K. Influence of organic nutrient sources and moisture management on productivity, biofortification and soil health in pearl millet (Pennisetum glaucum) + clusterbean (Cyamopsis tetragonaloba) intercropping system of semi-arid India. Indian J. Agric. Sci. 86, 1418–1425 (2016).
Kumar, A. & Verma, J. P. The role of microbes to improve crop productivity and soil health. In Ecological Wisdom Inspired Restoration Engineering 249–265 (Springer, 2019).
Choudhary, A. K. et al. Integrated crop management technology for enhanced productivity, resource-use efficiency and soil health in legumes—A review. Indian J. Agric. Sci. 90, 1839–1849 (2020).
Bana, R. S. et al. A Manual on Dryland Farming and Watershed Management 114 (IARI, 2013).
Bana, R. S., Pooniya, V., Choudhary, A. K. & Rana, K. S. Agronomic Interventions for Sustainability of Major Cropping Systems of India 34 (IARI, 2014).
Palm, C., Blanco-Canqui, H., DeClerck, F., Gatere, L. & Grace, P. Conservation agriculture and ecosystem services: An overview. Agric. Ecosyst. Environ. 1, 87–105 (2014).
Farooq, M. & Siddique, K. H. Conservation agriculture: Concepts, brief history, and impacts on agricultural systems. In Conservation Agriculture 3–17 (Springer, 2015).
Harish, M. N. et al. Double zero-tillage and foliar-p fertilization coupled with microbial-inoculants lead to improved maize productivity and quality in a maize-wheat rotation of semi-arid agro-ecology. Sci. Rep. 12, 3161 (2022).
Harish, M. N. et al. Double zero-tillage and foliar-P nutrition coupled with bio-inoculants enhance physiological photosynthetic characteristics and resilience to nutritional and environmental stresses in maize–wheat rotation. Front. Plant Sci. 13, 959541 (2022).
Rusinamhodzi, L. et al. A meta-analysis of long-term effects of conservation agriculture on maize grain yield under rainfed conditions. Agron. Sustain. Dev. 31, 657 (2011).
Corsi, S. et al. Conservation agriculture as a driving force to accumulate carbon in soils: An analysis of RDP in Lombardy. In Agricultural Cooperative Management and Policy 281–296 (Springer, 2014).
Kumar, A. et al. Energy budgeting and carbon footprints of zero-tilled pigeonpea–wheat cropping system under sole or dual crop basis residue mulching and Zn-fertilization in a semi-arid agro-ecology. Energy 231, 120862 (2021).
Kumar, A. et al. Sole- or dual-crop basis residue-mulching and Zn-fertilization lead to improved productivity, rhizo-modulation and soil health in zero-tilled pigeonpea–wheat cropping system. J. Soil Sci. Plant Nutr. 22, 1193–1214 (2022).
Singh, U., Choudhary, A. K. & Sharma, S. Comparative performance of conservation agriculture vis-a-vis organic and conventional farming in enhancing plant attributes and rhizospheric bacterial diversity in Cajanus cajan: A field Study. Eur J. Soil Biol. 99, 103197 (2020).
Singh, U., Choudhary, A. K. & Sharma, S. Agricultural practices modulate the bacterial communities, and nitrogen cycling bacterial guild in rhizosphere: Field experiment with soybean. J. Sci. Food Agric. 101, 2687–2695 (2021).
Singh, U., Choudhary, A. K. & Sharma, S. A 3-year field study reveals that agri-management practices drive the dynamics of dominant bacterial taxa in the rhizosphere of Cajanus cajan. Symbiosis 86, 215–227 (2022).
Varatharajan, T. et al. Integrated management enhances crop physiology and final yield in maize intercropped with blackgram in semi-arid south-Asia. Front. Plant Sci. 13, 975569. https://doi.org/10.3389/fpls.2022.975569 (2022).
Varatharajan, T. et al. Effect of Integrated crop management and blackgram (Vigna radiata) intercropping in maize (Zea mays). Ind. J. Agric. Sci. 92(10), 1208–1213 (2022).
Bhupenchandra, I. et al. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 13, 967665 (2022).
Singh, G. et al. Crop rotation and residue management effects on soil enzyme activities, glomalin and aggregate stability under zero tillage in the Indo-Gangetic plains. Soil Tillage Res. 1, 291–300 (2018).
Choudhary, A. K. Rice productivity, Zn-biofortification and nutrient-use efficiency as influenced by Zn-fertilization under conventional transplanted-rice and the system of rice intensification. Front. Environ. Sci. 10, 869194 (2022).
Singh, U., Choudhary, A. K., Varatharajan, T. & Sharma, S. Agri-management practices affect the abundance of markers of phosphorus cycle in soil: Case study with pigeonpea and soybean. J. Soil Sci. Plant Nutr. https://doi.org/10.1007/s42729-022-00863-3 (2022).
Singh, Y. P., Tomar, S. S. & Singh, S. Effect of precise levelling, tillage and seed sowing methods of pearlmillet based cropping systems on productivity and soil quality in dryland area. Soil Tillage Res. 212, 105069 (2021).
Faiz, M. A. et al. Zero tillage, residue retention and system-intensification with legumes for enhanced pearl millet productivity and mineral biofortification in the semi-arid north Indian plains: Technology development and dissemination. Sustainability 14, 543 (2022).
Bhupenchandra, I. et al. Elucidating the impact of boron fertilization on soil physico-chemical and biological entities under cauliflower-cowpea-okra cropping system in an eastern Himalayan acidic Inceptisol. Front. Microbiol. 13, 996220 (2022).
Rajpoot, S. K., Rana, D. S. & Choudhary, A. K. Crop and water productivity, energy auditing, carbon footprints and soil health indicators of Bt-cotton transplanting led system intensification. J. Environ. Manage. 300, 113732 (2021).
Manjaiah, K. M. & Singh, D. Soil organic matter and biological properties after 26 years of maize-wheat-cowpea cropping as affected by manure and fertilization in a Cambisol in semiarid region of India. Agric. Ecosyst. Environ. 86, 155–162 (2001).
Hartman, W. H. & Richardson, C. J. Differential nutrient limitation of soil microbial biomass and metabolic quotients (Qco2): Is there a biological stoichiometry of soil microbes?. PLoS ONE 8, e5712 (2013).
Choudhary, A. K. & Rahi, S. Organic cultivation of high yielding turmeric (Curcuma longa L.) cultivars: A viable alternative to enhance rhizome productivity, profitability, quality and resource-use efficiency in monkey–menace areas of north-western Himalayas. Indus. Crops Prod. 124, 495–504 (2018).
Tetarwal, J. P. & Rana, K. S. Impact of cropping system, fertility level and moisture-conservation practice on productivity, nutrient uptake, water use and profitability of pearl millet (Pennisetum glaucum) under rainfed conditions. Indian J. Agron. 51, 263–266 (2006).
Choudhary, G. L., Rana, K. S., Rana, D. S. & Bana, R. S. Performance of chickpea (Cicer arietinum) as influenced by moisture management and zinc fortification in pearl millet–chickpea cropping system under limited moisture conditions. Indian J. Agron. 59, 61–67 (2014).
Ankit, R. S. B. et al. No-tillage with residue retention and foliar sulphur nutrition enhances productivity, mineral biofortification and crude protein in rainfed pearl millet under Typic Haplustepts: Elucidating the responses imposed on an eight-year long-term experiment. Plants 11, 943 (2022).
Abdullah, A. S. Minimum tillage and residue management increase soil water content, soil organic matter and canola seed yield and seed oil content in the semiarid areas of Northern Iraq. Soil Till. Res. 144, 150–155 (2014).
Varatharajan, T., Choudhary, A. K., Pooniya, V., Dass, A. & Harish, M. N. Integrated crop management practices for enhancing productivity, profitability, production-efficiency and monetary-efficiency of pigeonpea (Cajanus cajan) in Indo-Gangetic plains region. Indian J. Agric. Sci. 89, 559–563 (2019).
Chen, X., Wang, X., Liebman, M., Cavigelli, M. & Wander, M. Influence of residue and nitrogen fertilizer additions on carbon mineralization in soils with different texture and cropping histories. PLoS ONE 9, e103720 (2014).
Yadav, R. K., Purakayastha, T. J., Khan, M. A. & Kaushik, S. C. Long-term impact of manuring and fertilization on enrichment, stability and quality of organic carbon in Inceptisol under two potato-based cropping systems. Sci. Total Environ. 609, 1535–1543 (2017).
Heba, M. N., Rana, D. S., Choudhary, A. K., Rajanna, G. A. & Pande, P. Influence of sulphur and zinc nutrition on productivity, quality and biofortification in groundnut (Arachis hypogea L.) in south-Asian alluvial soil. J. Plant Nutr. 44, 1151–1157 (2021).
Bana, R. C. et al. Zinc-coated urea for enhanced zinc biofortification, nitrogen use efficiency and yield of basmati rice under Typic Fluvents. Sustainability 14, 104 (2022).
Sarker, U. & Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci Rep. 8(1), 16496 (2018).
Asada, K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Ann. Rev. Pl. Biol. 50, 601–639 (1999).
Kar, R. K. Plant responses to water stress. Role of reactive oxygen species. Plant Signal. Behav. 6, 1741–1745 (2011).
Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Pl. Sci. 7, 405–410 (2002).
Yanai, J. et al. Spatial variability of nitrous oxide emissions and their soil-related determining factors in an agricultural field. J. Environ. Qual. 32, 1965–1977 (2003).
Lee, J., Six, J., King, A. P., Kessel, C. V. & Rolston, E. D. Tillage and field scale controls on greenhouse gas emissions. J. Environ. Qual. 35, 714–725 (2006).
Bhatia, A., Pathak, H., Jain, N., Singh, P. K. & Singh, A. K. Global warming potential of manure amended soils under rice–wheat system in the Indo-Gangetic plains. Atmos. Environ. 39, 6976–6984 (2005).
Tian, S. et al. Greenhouse gas flux and crop productivity after 10 years of reduced and no tillage in a wheat–maize cropping system. PLoS ONE 8, e73450 (2013).
Bouyoucos, G. J. A recalibration of the hydrometer method for making mechanical analysis of soils. Agron. J. 43, 434–438 (1951).
Richards, L. A. Diagnosis and improvement of saline alkaline soils, In USDA Handbook No. 60. 111–112 (USDA, 1954).
Piper, C. S. Soil and Plant Analysis 200–210 (Asia Hans Publisher, 1966).
Rana, K. S., Choudhary, A. K., Sepat, S., Bana, R. S. & Dass, A. Methodological and Analytical Agronomy. 276+ xii (IARI, 2014a).
Jackson, M. L. Soil Chemical Analysis. 498 (Prentice Hall of India Pvt. Ltd., 1973).
Jackson, M. L. Soil Chemical Analysis. 498 (Prentice Hall, 1958).
Olsen, S. R., Cole, C. L., Watanabe, F. S. & Dean, L. A. Estimation of available phosphorus in soil by extraction with sodium bicarbonate in USDA Circular No. 939. 72–75 (USDA, 1954).
Piper, C.S. Soil and Plant Analysis. 286–287 (University of Adelaide, 1950).
Rana, K. S., Choudhary, A. K., Sepat, S. & Bana, R.S. Advances in Field Crop Production. 475 (IARI, 2014b).
Zuberer, D. A. Recovery and enumeration of viable bacteria in Methods of Soil Analysis: Part 2- Microbiological and Biochemical Properties (ed. R.W. Weaver, S. Angle, P. Bottomley & S.H. Mickelson) 119–144 (SSSA, 1994).
Martin, J. P. Use of acid, rose Bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 69, 215–232 (1950).
Himedia. The HiMedia Manual for Microbiology Laboratory Practice. (HiMedia Laboratories Pvt. Ltd, 2009).
Tabatabai, M. A. & Bremner, J. M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1(4), 301–307 (1969).
Eivazi, F. & Tabatabai, M. A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 20, 601–606 (1988).
Green, V. S., Stott, D. E. & Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 38, 693–701 (2006).
Jones, H. G. Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 58, 119–130 (2007).
Lowry, O. H., Rosenbrough, N. J., Farr, A. L. & Randall, R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water stress studies. Plant Soil 39, 205–207 (1973).
Beauchamp, C. & Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287 (1971).
Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880 (1981).
Aebi, H. Catalase. In Methods of Enzymatic Analysis (ed. Bergmeyer, H. U.) 673–680 (Academic Press Inc., 1974).
Foyer, C. H. et al. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photo-inhibition in poplar trees. Plant Physiol. 109, 1047–1057 (1995).
Bhatia, A. et al. Effect of elevated tropospheric ozone on methane and nitrous oxide emission from rice soil in north India. Agric. Ecosyst. Environ. 144, 21–28 (2011).
Sapkota, T. B. et al. Tillage, residue and nitrogen management effects on methane and nitrous oxide emission from rice–wheat system of Indian Northwest Indo-Gangetic Plains. J. Integr. Environ. 12, 31–46 (2015).
Pathak, H., Sankhyan, S., Dubey, D. S., Bhatia, A. & Jain, N. Dry direct–seeding of rice for mitigating greenhouse gas emission: Field experimentation and simulation. Paddy Water Environ. 11, 593–601 (2013).
Parihar, C. M., Rana, K. S. & Parihar, M. D. Crop productivity, quality and nutrient uptake of pearl millet (Pennisetum Glaucum)–Indian mustard (Brassica Juncea) cropping system as influenced by land configuration and direct and residual effect of nutrient management. Indian J. Agric. Sci. 79, 927–930 (2009).
Malyan, S. K. et al. Mitigation of greenhouse gas intensity by supplementing with azolla and moderating the dose of nitrogen fertilizer. Biocatal. Agric. Biotechnol. 20, 101266 (2019).
Acknowledgements
The authors are thankful to ICAR–Indian Agricultural Research Institute, New Delhi for providing necessary facilities during the conduct of the study for bringing out this valuable publication.
Author information
Authors and Affiliations
Contributions
R.S.B. and A.K.C. conceptualized, designed, and conducted the research study, performed biochemical analysis, and wrote, reviewed, and edited the manuscript. M.A.F. conducted the research study and performed soil analysis. S.S. performed microbial analysis, and wrote, reviewed, and edited the manuscript. S.D.B., S.G. and R.C.N. assembled the data, prepared figures and tables, editing and results validation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bana, R.S., Faiz, M.A., Sangwan, S. et al. Triple-zero tillage and system intensification lead to enhanced productivity, micronutrient biofortification and moisture-stress tolerance ability in chickpea in a pearlmillet-chickpea cropping system of semi-arid climate. Sci Rep 13, 10226 (2023). https://doi.org/10.1038/s41598-023-36044-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36044-0