Abstract
This study aimed to evaluate the thermal, chemical, and physical properties of VDW.1Seal, Fill Root ST, and ADseal sealers. Thermal properties were analyzed using Thermogravimetric analysis (TGA) and Differential thermal analysis (DTA). Attenuated total reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR) analysis was performed as a complementary test to confirm TGA/DTA analysis. The chemical composition of the set sealer material was identified using an X-ray powder diffraction (XRD) system. Other physical properties of each sealer were investigated; ten specimens were used to measure the solubility (at 24 h and 28 days), and another ten specimens were used to assess pH changes and calcium ion release (after 7 and 14 days). Film thickness was done according to ISO 6876 specs. The data were analyzed using the two-way ANOVA test. Results showed that for all sealers, TGA analysis revealed a direct relationship between sealer mass loss and temperature rise. In addition, the decomposition of the tested sealers started at 145 °C, 135 °C and 91 °C for VDW.1Seal, ADseal sealer, and Fill Root ST, respectively. XRD analysis revealed a higher degree of crystallinity for VDW.1Seal and ADseal. ADseal showed the least solubility; VDW.1Seal exhibited the highest alkalinity, calcium ion release, and the lowest film thickness.
Similar content being viewed by others
Introduction
Obturation techniques using thermo-plasticized gutta-percha are expected to fill root canal systems better than cold gutta-percha points1. Nevertheless, sealers are still essential to fill the gaps and voids between the core material and the root canal walls2. Without a sealer, both warm and cold obturation techniques will have compromised treatment outcomes3,4. Hydraulic calcium silicate-based sealers (HCSBS) have been recently developed as a substitute for epoxy resin-based sealers5. According to the source of hydration required for their setting, they are either available as powder, liquid, or premixed ready-to-use syringes6. In the former, hydration is initiated before insertion into the root canal. While in the latter, the residual moisture inside the root canal, along with dentine humidity, purportedly provides the water necessary for the hydration of the material7. Clinical studies have reported successful application of premixed HCSBS in root canal obturation8,9. Besides technique simplicity, HCSBS also showed favorable properties, including bioactivity10, biocompatibility11, and antibacterial potential12. Most are founded on the solubility of their setting reaction by-products13. The main component of HCSBS is tricalcium silicate, which, when hydrated, forms a calcium silicate hydrate matrix and calcium hydroxide that leach out to interact with the surrounding environment14,15. Recently, new premixed sealers were marketed with innovative formulations to upgrade their clinical performance. In comparison to previous HCSBS, VDW.1Seal (VDW, München, Germany) contains relatively less tricalcium silicate (5–15 wt %) and more zirconium dioxide (50–70 wt %) in addition to dimethyl sulfoxide as filler. Fill Root ST (Dental World, Molfetta, Italy) is another recently introduced premixed sealer based on calcium aluminosilicate and zirconium dioxide. Currently, there is scarce data about their physico-chemical properties in terms of solubility, alkalinity, calcium ion release, and film thickness. Likewise, it is necessary to investigate their thermal stability to determine if they can tolerate the heating temperatures generated during thermal-based obturation techniques without decomposition. Such data would give clinicians insights for better selection of the sealer type to be compatible with heat application during obturation. Therefore, this in vitro study aimed to investigate the thermal stability and some physico-chemical properties (short- and long-term solubility, pH changes, calcium ions release, and film thickness) of VDW.1Seal and Fill Root ST in comparison to an epoxy resin-based sealer, ADseal Root canal sealer (Meta BioMed, Cheongju, Korea). The null hypothesis was that there were no differences between the tested materials.
Materials and methods
All methods were carried out following relevant guidelines and regulations. The research ethics committee at the Faculty of Dentistry at the British University in Egypt approved all the experimental protocols. Since the data were evaluated retrospectively, pseudonymously, and were solely obtained for treatment purposes, a requirement of informed consent was waived by the ethics committee at the Faculty of Dentistry, The British University in Egypt (approval number 22-031, date 18/12/2022).
The root canal sealers used in the current study are represented in Table 1.
TGA/DTA analysis
Approximately 16 mg of powder for each set of sealers was obtained by grinding using an agate mortar and pestle (Nahita, Istanbul, Turkey). Sample powders were analyzed to evaluate the thermal stability of each sealer using Thermogravimetric analysis (TGA) and Differential thermal analysis (DTA) using a TGA/DTA Thermal Analyzer (Shimadzu DTG-60H Thermal Analyzer, Kyoto, Japan). Thermal measurements were performed under the flow of nitrogen atmosphere with a flow rate of 100 ml min−1 in the temperature range from ambient to 250 °C. The heating rate was 20 °C per minute. Highly sintered α-Al2O3 was used as the reference material. Thermo-analytical TGA and DTA curves were obtained simultaneously. The data were analyzed using the TGA software (CDSS, v1.1).
ATR-FTIR test
Attenuated total reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR) analysis (Nicolet 6700 FTIR instruments, Thermo Fisher Scientific, Waltham, USA) was performed on the set sealers as a complementary test to confirm thermal stability results. FTIR analysis was performed in the spectral range (4000–500 cm1) following the previously described procedure16.
XRD analysis
The chemical composition of the set sealer material was investigated using an X-ray powder diffraction (XRD) system (Bruker-AXS D8 X-ray diffractometer, Germany) to identify the existing crystalline phases and measure the degree of crystallinity. The crystalline structure of the test sealer was determined by passing an X-ray beam of a known wavelength into the specimen while rotating it at an angle. The intensity of X-rays from the sample was measured by a detector14, while the crystallinity was evaluated by careful evaluation of the baseline to peak separation in an extended scan range using DIFFRAC.EVA software (Bruker AXS GmbH). XRD data were collected in the 2θ range 0–60° under 30.0 mA, 40.0 kV, and a scan rate of 4°/min. The obtained XRD patterns were characterized using the Joint Committee on Powder Diffraction Standard (JCPDS) databases.
Analysis of the physical properties
Sample size calculation
Power calculation was performed using G*Power 3.1.9.7 (Heinrich Heine University, Dusseldorf, Germany). Based on the results of previous studies17,18,19 and using an alpha (α) level of 0.05 (5%) and a Beta (β) level of 0.20 (20%), i.e., power = 80%, the predicted minimum sample size (n) was nine specimens in each group. Thus, ten samples were prepared per group for evaluation of solubility, and another ten samples were prepared per group for evaluation of pH change and calcium ion release. Concerning film thickness testing, three tests were carried out according to ISO 6876 specs.
Specimen preparation
Root canal sealers were carefully injected into the specially designed molds for each test type.
Solubility
Solubility was determined according to the standards set by the International Standard Organization (ISO 6876:2012)20. Short- and long-term solubility were determined after 24 h and 28 days, respectively, following the previously described procedures20,21. Cylindrical polyethylene molds with dimensions of 1.5 mm height and 7.75 mm inner diameter were used and filled with each root canal sealer to acquire disc-shaped specimens (n = 10 per sealer), which were incubated at 37 °C and 95% relative humidity for 24 h to set. After setting, the specimens were removed from the molds and weighed three times to determine the mean mass of each specimen (M1) using a microbalance. Then, all set sealer specimens were stored separately in plastic flasks containing 7.5 mL of distilled water in an incubator at 37 °C and 95% relative humidity. The specimens were removed from the incubator, bench dried, and re-weighed (M2) after 24 h and 28 days of storage. Mass loss was expressed as a percentage of the original mass20,21. The percentage of root canal sealer solubility was calculated as follows: (M1-M2)/M1 X 100%20.
pH change
Cylindrical polytetrafluoroethylene tubes were used to shape each type of root canal sealer into disc specimens (n = 10) with a 5 mm diameter and 2 mm thickness. After the sealer setting, each specimen was soaked in 10 mL of distilled water and incubated at 37 °C and 95% relative humidity for 7 and 14 days. Then the pH change of the solution was measured immediately and after 7 and 14 days of immersion by using an electrode pH meter (Jen-way 3510 bench pH meter, UK)20. The accuracy of the pH meter was auto calibrated with calibration solutions (pH 4, 7, and 10; Merck, Darmstadt, Germany) every 48 h. After each reading, the electrode was flushed with distilled water and dried before taking another reading. The temperature of the room during measurements was adjusted to 25 °C.
Calcium ions release
The previous solutions were used to measure the release of calcium ions using inductively coupled plasma optical emission spectroscopy (ICP-OES) (Ultima 2 ICP, Horiba, USA). The cumulative amounts of the degradable calcium ions released from each sealer were measured after 7 and 14 days, respectively (mg/L)20.
Film thickness
Assessment of film thickness was evaluated according to the International Standard Organization (ISO) 6876/2012 instructions20. Two flat glass plates (5 mm in thickness, 200 ± 10 mm2 surface area) were placed over one another. Their total thickness was measured by a digital micrometer to the nearest 10 μm. Then 0.5 ml of each sealer was dispensed onto the first plate, and the second plate was placed over it. A 150 Newton load was vertically applied for 3 min on the upper glass plate. The total thickness of the plates, including the sealer, was re-measured 7 min after the time of load application. The total thickness of the plates was deducted from this amount, and the film thickness of the sealer was obtained. The mean value of the film thickness for each sealer was recorded by repeating the test three times and calculating the average value20.
Statistical analysis
The results were collected and analyzed statistically using a two-way ANOVA. Tukey’s post-hoc test was conducted for multiple comparisons. The significance level was set at (P ≤ 0.05). Data were managed using SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Thermal and chemical analysis results
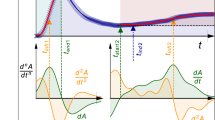
DTA/TGA results for tested sealers are represented in Fig. 1a–c. The blue-colored curve represents the chemical change in the sealer’s chemical composition (Differential Thermal Analysis, DTA). The red-colored curve shows the weight loss behavior of the sealer sample as a function of increasing the temperature (Thermal Gravimetric Analysis, TGA). The curve in turquoise gives the percentage (%) of weight loss as a function of the increasing temperature (Thermal Gravimetric Analysis, TGA).
TGA/DTA data is represented in Fig. 1. DTA data of VDW.1Seal (Fig. 1a) showed a medium, very broad endothermic band around 90 °C and two very weak two endothermic peaks at 145 °C and 170 °C, respectively. TGA data (Table 2) revealed a total weight loss of 7.9%. DTA data of Fill Root ST canal sealer (Fig. 1b) showed a strong, broad endothermic band around 91°C with a total weight loss of 2.93% starting from 60 °C and ending at 91°C as indicated from TGA results (Table 2). DTA data of ADseal Root canal sealer (Fig. 1c) showed a weak, very broad band in the range 50–135 °C with a total weight loss of about 7.2%, as revealed from TGA data (Table 2).
The ATR-FTIR analysis of the tested sealers is represented in Fig. 2. The VDW.1Seal sample revealed a very weak broad band at 3383 cm−1 and a medium-strong band at 1633 cm−1. While ATR-FTIR analysis of the Fill Root ST canal sealer sample revealed a very weak broad band at 3382 cm−1 and a medium-strong band at 1634 cm−1, Moreover, ATR-FTIR analysis of the ADseal root canal sealer sample revealed a very weak broad band at 3717 cm−1 and a medium-strong band at 1633 cm−1.
The XRD analysis of VDW.1Seal (Fig. 3a) revealed that the degree of crystallinity is about 73%, with crystalline diffraction peaks at 2θ values of 28.6°, 31.5°, and 44.3° that exhibited peaks for ZrO2 (ICDD File No. 37-1484). The XRD analysis of Fill Root canal sealer (Fig. 3b) revealed that the degree of crystallinity is about 67%, with crystalline diffraction peaks at 2θ values of 28.4°, 31.7°, and 49.5° that exhibited peaks for Hafnium oxide (ICDD File No. 43-1017), and values of 33.5°, 34.5°, and 60.0° that exhibited peaks for Germanium oxide (ICDD File No. 65-0333). The XRD analysis of ADseal root canal sealer (Fig. 3c) revealed that the degree of crystallinity is about 74%, with crystalline diffraction peaks at 2θ values of 28.29°and 31.5° that exhibited peaks for ZrO2 (ICDD File No. 37-1484).
Physical analysis results
Solubility
Solubility results are reported in Table 3. There was a significant difference in the solubility between the three types of sealers after 24 h and after 28 days (p = 0.0001*). The ADseal showed the least solubility (1 and 1.24%), while the Fill Root ST presented the highest solubility (3.32 and 4.76%). Within the same material, there was a significant increase in solubility with time (p ≤ 0.05).
pH changes results
The results are reported in Table 4. The pH recorded for all sealers increased over time, which was significant for the VDW.1Seal and Fill Root ST sealers (p < 0.05). At all observation points, the highest pH value was recorded for the VDW.1Seal sealer and the lowest was recorded for the ADseal sealer (p < 0.05).
Calcium ions release results
The results are reported in Table 5. The calcium ion release recorded for all sealers increased over time, which was significant for all sealers (p < 0.05). While for both observation points, the highest release was recorded for the VDW.1Seal sealer (17.86 and 48.62 mg/l) and the lowest for the ADseal sealer (0.22 and 1.5 mg/l) (p < 0.05).
Film thickness results
The VDW.1Seal showed the lowest film thickness of (49.4 ± 2.07) µm, followed by the ADseal sealer (80.6 ± 1.14) µm, while the thickest was the Fill Root ST sealer (90 ± 4.3) µm, and these differences were statistically significant (p < 0.001).
Discussion
Improvement in root canal shaping instruments and obturation materials is required to achieve better treatment outcomes21. The current in vitro study sought to assess a series of physicochemical properties of the newly introduced HCSBS (VDW.1 Seal and Fill Root ST) in comparison to an epoxy resin-based sealer (ADseal Root canal sealer), which was used instead of AH plus, and was less extensively investigated despite having more favorable biological properties11. Thermogravimetric analysis (TGA) and Differential thermal analysis (DTA) were chosen to investigate whether the tested sealers can withstand the temperatures generated inside the root canals during thermal-based obturation techniques without decomposition and, therefore, allow clinicians to predict their clinical behavior when subjected to heat and achieve better clinical decisions for higher treatment outcomes.
TGA measures the weight loss over a temperature range, while DTA determines the endothermic and exothermic event temperatures of the materials at the onset of melting and crystallization. The thermal analysis determines the behavior and thermal stability of the materials upon heating22,23,24. The chemical structure of the sealer and the predicted behavior could be complementary assessed by using ATR-FTIR analysis, which is an accurate and quick analytical method that requires minimal samples25,26. Moreover, non-destructive XRD analysis was done to detect the degree of crystallinity and identify the crystallographic phases of the tested materials27.
Most commercial thermal obturation devices have four temperature settings (150 °C, 180 °C, 200 °C, and 230 °C). Although it has been shown that the actual temperature generated inside the root canal is less than that on the device display2, for each TGA curve, we opted to investigate the sealer mass loss at 50 °C, 100 °C, 150 °C, 200 °C, and 250 °C to assess the thermal stability of the tested sealers before, during, and after this temperature range.
TGA/DTA thermal analysis for all tested sealers revealed a broad endothermic band below 100 °C. These losses may be attributed to physical adsorbed moisture (H2O). To further explore this finding, ATR-FTIR was conducted at ambient temperature, and it revealed stretching and scissors bands of 3383.92, 3382.45, and 3717.21 for VDW.1Seal, Fill Root ST, and ADseal, respectively. This is suggestive of the release of moisture (the OH of water). Moreover, the other peak bands of 1633, 1634, and 1633 were recorded at higher temperatures for VDW.1Seal, Fill Root ST, and ADseal, respectively, which may be due to stretching and scissors bands of free H2O.
For all sealers, TGA/DTA analysis revealed a direct relationship between sealer mass loss and temperature rise. The decomposition of the tested sealers started at 145 °C, 135 °C and 91 °C for VDW.1Seal, ADseal sealer, and Fill Root ST, respectively. This can be primarily explained based on the XRD analysis that revealed a higher degree of crystallinity (73% and 74%) for VDW.1Seal and ADseal, respectively, as well as their inclusion of the stable zirconium oxide ceramic fillers, which resist thermal decomposition up to 2000 ºC28,29, in comparison with the Fill Root ST whose crystallinity was only 67%.
Similar favorable thermal stability of HCSBS was reported by Donnermeyer et al. 202130 and 20225, Vipiana et al. 20152, and Atmeh and AlShawaimi 201731, who also reported that heating of epoxy resin sealers resulted in time-dependent irreversible structural changes, though associated with less mass loss (1.2% at 250 oC). In contrast, Donnermeyer et al. 202032 showed that heating an epoxy resin sealer up to 97 °C for 60 s did not lead to any substantial physical or chemical changes. It is worth mentioning that the actual temperatures delivered by heat pluggers are much lower than the temperatures shown on the device display2,33.
Although the crystallinity of the dental materials provides thermal stability and preservation of the structural integrity against degradation and solubility of the materials by the action of the environment34,35, still, the presence of some degree of amorphous phase permits a reasonable grade of solubility and ions leaching responsible for pH changes and precipitation, which are essential for remineralization, sealing, and tissue healing36,37,38.
The solubility of the tested sealers was investigated in the short term after 24 h and in the long term after 28 days. Regarding the short-term solubility, the least soluble was the ADseal sealer, owing to its resinous nature in addition to the highest degree of crystallinity of its composition, followed by the VDW.1Seal, with a comparable degree of crystallinity and the presence of the highly stable zirconium oxide fillers, while the most soluble was the Fill Root ST sealer, which exhibited the least degree of crystallinity. Regarding long-term solubility, there was a significant increase in the solubility of all sealers (p ≤ 0.05), which may be due to the increased interaction with the surrounding aqueous environment. It was noted that only ADseal and VDW.1Seal root canal sealers fulfilled the minimum requirements of solubility described by ISO 6876:2012, which is 3% of the initial mass by 24 h. It is worth mentioning that solubility values reported from laboratory tests may not disclose the actual clinical behavior of materials. Using water as a storage medium may overemphasize the solubility39, whereas physiological solutions could encourage mineral deposition and increase rather than decrease the net mass22, resulting in different values for the solubility of the same material when tested using different storage media40.
To predict their biological behavior, pH changes and calcium ion release for the tested sealers were investigated at 7 and 14 days, representing the maximum peaks for calcium ion release and a subsequent increase in pH value19,41,42. The alkalinity of the hydraulic calcium silicate-based sealers is regarded as one of their chief advantages, as it leads to the formation of apatite deposits on the sealer surface after contact with body fluids43. This interaction enhances bioactivity and promotes a strong chemical bond44. Moreover, the alkaline pH provides bacteriostatic effects45 and can promote apical healing and tissue mineralization36. Whereas the release of calcium ions, which are strong extracellular signals for undifferentiated cells46, is important for healing of apical periodontitis associated with bone resorption. Results of our study showed that VDW.1Seal exhibited the highest alkalinity and the highest cumulative calcium ion release compared to the other sealers at the end of the observation period (p ≤ 0.05). Despite the relatively low amount of calcium silicate (5–15%) present in its composition47, a high open pore volume was reported for a sealer with a similar composition that promoted higher water absorption and ion release. This does not essentially predict poor clinical performance of the sealer, as nucleation of apatite and carbonate may recompense the behavior of the sealer48. The significantly higher calcium and hydroxyl ion release of hydraulic calcium silicate sealers in comparison with epoxy resin-based sealers was also reported by previous studies5,17,19,49,50. The film thickness of the root canal sealers is essential because thin film thicknesses enhance the wettability of the materials on the canal walls, providing appropriate sealing51. According to the results of this study, all tested sealers fulfilled the ISO recommendation for sealer film thickness (50 μm)5,20,52,53. The differences between the sealers were statistically significant (p ≤ 0.05), with VDW.1 Seal having the lowest film thickness, mostly due to its smaller particle size.
Limitations of this study include no assessment of the amorphous phases of the sealers was performed. Moreover, the study does not monitor the chemical changes before moisture adsorption. Furthermore, it is recommended to perform a further study to investigate the chemical elemental analysis using X-ray fluorescence (XRF). In addition, it is recommended to investigate the chemical changes before, during, and after heating by FTIR, considering the time of heat application, and compare the physicochemical properties of freshly mixed and set root canal sealers, preferably with enhanced testing methodologies that can allow better clinical translation of the results.
Conclusions
According to the findings of this study, the null hypothesis is rejected as significant differences existed among the tested sealers. It could be concluded that the chemical composition and the degree of crystallinity greatly influence the thermal stability and solubility of the root canal sealers. Moreover, the thermal stability could be increased by the incorporation of stable zirconium oxide. Furthermore, VDW.1Seal exhibited the most favorable physicochemical properties and can be compatible with thermal-based obturation techniques.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Schilder, H. & Hargreaves, K. M. Filling root canals in three dimensions. J. Endod. 32, 281–290. https://doi.org/10.1016/j.joen.2006.02.007 (2006).
Viapiana, R., Baluci, C. A., Tanomaru-Filho, M. & Camilleri, J. Investigation of chemical changes in sealers during application of the warm vertical compaction technique. Int. Endod. J. 48, 16–27. https://doi.org/10.1111/iej.12271 (2015).
Schäfer, E. & Olthoff, G. Effect of three different sealers on the sealing ability of both thermafil obturators and cold laterally compacted gutta-percha. J. Endod. 28, 638–642. https://doi.org/10.1097/00004770-200209000-00003 (2002).
Wu, M. K., Van Der Sluis, L. W. & Wesselink, P. R. Fluid transport along gutta-percha backfills with and without sealer. Oral Surg. Oral Med. Oral Pathol. 97, 257–262. https://doi.org/10.1016/j.tripleo.2003.07.008 (2004).
Donnermeyer, D., Schemkämper, P., Bürklein, S. & Schäfer, E. Short and long-term solubility, alkalizing effect, and thermal persistence of premixed calcium silicate-based sealers: AH plus bioceramic sealer vs. total fill BC sealer. Materials 15, 7320. https://doi.org/10.3390/ma15207320 (2022).
Atmeh, A. R., Hadis, M. & Camilleri, J. Real-time chemical analysis of root filling materials with heating: Guidelines for safe temperature levels. Int. Endod. J. 53, 698–708. https://doi.org/10.1111/iej.13269 (2020).
Al-Haddad, A. & Aziz, Z. A. C. A. Bioceramic-based root canal sealers: A review. Int. J. Biomater. https://doi.org/10.1155/2016/9753210 (2016).
Chybowski, E. A. et al. Clinical outcome of non-surgical root canal treatment using a single-cone technique with endosequence bioceramic sealer: A retrospective analysis. J. Endod. 44, 941–945. https://doi.org/10.1016/j.joen.2018.02.019 (2018).
Zavattini, A., Knight, A., Foschi, F. & Mannocci, F. Outcome of root canal treatments using a new calcium silicate root canal sealer: A non-randomized clinical trial. J. Clin. Med. 13, 782. https://doi.org/10.3390/jcm9030782 (2020).
Hamdy, T. M. Polymers and ceramics biomaterials in orthopedics and dentistry: A review article. Egypt. J. Chem. 61, 723–730. https://doi.org/10.21608/ejchem.2018.3187.1273 (2018).
Saber, S., Raafat, S., Elashirt, M., El-Banna, A. & Schafer, E. Effect of different sealers on the cytocompatibility and osteogenic potential of human periodontal ligament stem cells: An in vitro study. J. Clin. Med. 12, 2344. https://doi.org/10.3390/jcm12062344 (2023).
Zordan-Bronzel, C. L. et al. Cytocompatibility, bioactive potential and antimicrobial activity of an experimental calcium silicate-based endodontic sealer. Int. Endod. J. 52, 979–986. https://doi.org/10.1111/iej.13086 (2019).
Urban, K., Neuhaus, J., Donnermeyer, D., Schäfer, E. & Dammaschke, T. Solubility and pH value of 3 different root canal sealers: A long-term investigation. J. Endod. 44, 1736–1740. https://doi.org/10.1016/j.joen.2018.07.026 (2018).
Camilleri, J. Characterization of hydration products of mineral trioxide aggregate. Int. Endod. J. 41, 408–417. https://doi.org/10.1111/j.1365-2591.2007.01370.x (2008).
Aminoshariae, A., Primus, C. & Kulild, J. C. Tricalcium silicate cement sealers: Do the potential benefits of bioactivity justify the drawbacks?. J. Am. Dent. Assoc. 153, 750–760. https://doi.org/10.1016/j.adaj.2022.01.004 (2022).
Khan, A. S. et al. Vibrational spectroscopy of selective dental restorative materials. Appl. Spectrosc. Rev. 52, 507–540. https://doi.org/10.1080/05704928.2016.1244069 (2017).
de Miranda Candeiro, G. T., Correia, F. C., Duarte, M. A., Ribeiro-Siqueira, D. C. & Gavini, G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J. Endod. 38, 842–845. https://doi.org/10.1016/j.joen.2012.02.029 (2012).
Mohamed, A. I. A., El Far, H. M., Saeed, F. M. & Abou Raya, N. L. Evaluation of setting time and solubility of bio-ceramic sealer before and after mixing with prednisolone powder: A comparative in vitro study. Acta Sci. Dent. Sci. 5, 115–121 (2021).
Zordan-Bronzel, C. L. et al. Evaluation of physicochemical properties of a new calcium silicate-based sealer, bio-C sealer. J. Endod. 45, 1248–1252. https://doi.org/10.1016/j.joen.2019.07.006 (2019).
ISO—ISO 6876:2012-Dentistry—Root canal sealing materials. Accessed October 8, 2022. https://www.iso.org/standard/45117.html
Hamdy, T. M., Galal, M., Ismail, A. G. & Abdelraouf, R. M. Evaluation of flexibility, microstructure and elemental analysis of some contemporary nickel-titanium rotary instruments. Open Access Maced. J. Med. Sci. 7, 3647–3654. https://doi.org/10.3889/oamjms.2019.811 (2019).
Torres, F. F. E., Guerreiro-Tanomaru, J. M., Bosso-Martelo, R., Chavez-Andrade, G. M. & Tanomaru Filho, M. Solubility, porosity and fluid uptake of calcium silicate-based cements. J. Appl. Oral Sci. 26, e20170465. https://doi.org/10.1590/1678-7757-2017-0465 (2018).
Mansa, R. & Zou, S. Thermogravimetric analysis of microplastics: A mini review. Environ. Adv. 5, 100117. https://doi.org/10.1016/j.envadv.2021.100117 (2021).
Tzaneva, D. et al. Synthesis of carboxymethyl chitosan and its rheological behaviour in pharmaceutical and cosmetic emulsions. J. Appl. Pharm. Sci. 7, 70–78. https://doi.org/10.7324/JAPS.2017.71010 (2017).
Andrew Chan, K. L. & Kazarian, S. G. Attenuated total reflection Fourier-transform infrared (ATR-FTIR) imaging of tissues and live cells. Chem. Soc. Rev. 45, 1850–1864. https://doi.org/10.1039/c5cs00515a (2016).
Dendisová, M. et al. The use of infrared spectroscopic techniques to characterize nanomaterials and nanostructures: A review. Anal. Chim. Acta 1031, 1–14. https://doi.org/10.1016/j.aca.2018.05.046 (2018).
Bunaciu, A. A., Udriştioiu, E. G. & Aboul-Enein, H. Y. X-ray diffraction: Instrumentation and applications. Crit. Rev. Anal. Chem. 45, 289–299. https://doi.org/10.1080/10408347.2014.949616 (2015).
Xue, C., Qin, Y., Fu, H. & Fan, J. Thermal stability, mechanical properties and ceramization mechanism of epoxy resin/kaolin/quartz fiber ceramifiable composites. Polymers 14, 3372. https://doi.org/10.3390/polym14163372 (2022).
Fedorov, P. P. & Yarotskaya, E. G. Zirconium dioxide. Review. Kondens Sredy Mezhfaznye Granitsy 23, 169–187. https://doi.org/10.17308/kcmf.2021.23/3427 (2021).
Donnermeyer, D. et al. Physico-chemical investigation of endodontic sealers exposed to simulated intracanal heat application: Hydraulic calcium silicate-based sealers. Materials (Basel). 14, 1–11. https://doi.org/10.3390/ma14040728 (2021).
Atmeh, A. R. & AlShwaimi, E. The effect of heating time and temperature on epoxy resin and calcium silicate-based endodontic sealers. J. Endod. 43, 2112–2118. https://doi.org/10.1016/j.joen.2017.08.008 (2017).
Donnermeyer, D., Urban, K., Burklein, S. & Schafer, E. Physico-chemical investigation of endodontic sealers exposed to simulated intracanal heat application: epoxy resins and zinc oxide–eugenols. Int. Endod. J. 53, 690–697. https://doi.org/10.1111/iej.13267 (2020).
Silver, G. K., Love, R. M. & Purton, D. G. Comparison of two vertical condensation obturation techniques: Touch ’n Heat modified and System B. Int. Endod. J. 32, 287–295. https://doi.org/10.1046/j.1365-2591.1999.00215.x (1999).
Ratih, D. N., Enggardipta, R. A., Kusumo, A. N. H. & Hadriyanto, W. Setting time, flowability, and solubility of epoxy resin-based sealer mixed with chitosan nanoparticles. Int. J. Appl. Pharm. 13, 122–126. https://doi.org/10.22159/ijap.2021v13i2.40270 (2021).
Poggio, C. et al. Solubility and pH of bioceramic root canal sealers: A comparative study. J. Clin. Exp. Dent. 9, e1189–e1194. https://doi.org/10.4317/jced.54040 (2017).
Zaki, D. Y., Zaazou, M. H., Khallaf, M. E. & Hamdy, T. M. In vivo comparative evaluation of periapical healing in response to a calcium silicate and calcium hydroxide based endodontic sealers. Open Access Maced. J. Med. Sci. 6, 1475–1479. https://doi.org/10.3889/oamjms.2018.293 (2018).
Duarte, M. A. H. et al. Evaluation of pH and calcium ion release of three root canal sealers. J. Endod. 26, 389–390. https://doi.org/10.1097/00004770-200007000-00002 (2000).
Allegrini, S. et al. Amorphous calcium phosphate (ACP) in tissue repair process. Microsc. Res. Tech. 81, 579–589. https://doi.org/10.1002/jemt.23013 (2018).
Ha, W. N., Nicholson, T. M., Kahler, B. & Walsh, L. J. Rheological characterization as an alternative method to indentation for determining the setting time of restorative and endodontic cements. Materials 10, 1415. https://doi.org/10.3390/ma10121451 (2017).
Kebudi Benezra, M., Schembri Wismayer, P. & Camilleri, J. Influence of environment on testing of hydraulic sealers. Sci. Rep. 7, 17927. https://doi.org/10.1038/s41598-017-17280-7 (2017).
Petta, T. D. M. et al. The effect of three different pulp capping cements on mineralization of dental pulp stem cells. Dent. Mater. J. 39, 222–228. https://doi.org/10.4012/dmj.2018-349 (2020).
Galal, M., Zaki, D. Y., Rabie, M. I., El-Shereif, S. M. & Hamdy, T. M. Solubility, pH change, and calcium ion release of low solubility endodontic mineral trioxide aggregate. Bull. Natl. Res. Cent. 44, 42. https://doi.org/10.1186/s42269-020-00303-1 (2020).
Shalabi, M., Saber, S. & Elswify, T. Influence of blood contamination on the bond strength and biointeractivity of Biodentine used as root-end filling. Saudi Dent. J. 32, 373–381. https://doi.org/10.1016/j.sdentj.2019.11.005 (2020).
Camilleri, L., Atmeh, A., Li, X. & Meschi, N. Present satus and future directions: Hydraulic materials for endodontic use. Int. Endod. J. 55(Suppl 3), 710–777. https://doi.org/10.1111/iej.13709 (2022).
Padan, E., Bibi, E., Ito, M. & Krulwich, T. A. Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta 1717, 67–88. https://doi.org/10.1016/j.bbamem.2005.09.010 (2005).
Matsumoto, S. et al. Calcium ions released from mineral trioxide aggregate are taken up by C2C12 cells via the L-type voltage-dependent calcium channel. J. Hard Tissue Biol. 22, 13–18. https://doi.org/10.2485/jhtb.22.13 (2013).
Su U. Safety data sheet according to 1907/2006/EC, Article 31 SECTION 1 : Identification of the substance/mixture and of the company/undertaking SECTION 2 : Hazards identification SECTION 3 : Composition/information on ingredients SECTION 4 : First a. Published online 2015:2–14
Zamparini, F. et al. Chemical-physical properties and bioactivity of new premixed calcium silicate-bioceramic root canal sealers. Int. J. Mol. Sci. 23, 13914. https://doi.org/10.3390/ijms232213914 (2022).
Siboni, F., Taddei, P., Zamparini, F., Prati, C. & Gandolfi, M. G. Properties of bioroot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int. Endod. J. 50(Suppl 2), e120–e136. https://doi.org/10.1111/iej.12856 (2017).
Lee, J. K., Kwak, S. W., Ha, J. H., Lee, W. C. & Kim, H. C. Physicochemical properties of epoxy resin-based and bioceramic-based root canal sealers. Bioinorg. Chem. Appl. 2017, 2582849. https://doi.org/10.1155/2017/2582849 (2017).
Katakidis, A., Sidiropoulos, K., Koulaouzidou, E., Gogos, C. & Economides, N. Flow characteristics and alkalinity of novel bioceramic root canal sealers. Restor. Dent. Endod. 45, e42. https://doi.org/10.5395/rde.2020.45.e42 (2020).
Zhou, H. M. et al. Physical properties of 5 root canal sealers. J. Endod. 39, 1281–1286. https://doi.org/10.1016/j.joen.2013.06.012 (2013).
Wuersching, S. N., Diegritz, C., Hickel, R., Huth, K. C. & Kollmuss, M. A comprehensive in vitro comparison of the biological and physicochemical properties of bioactive root canal sealers. Clin. Oral Investig. 26, 6209–6222. https://doi.org/10.1007/s00784-022-04570-2 (2022).
Acknowledgements
The authors are grateful to Professor Sabry A. El-Korashy, Professor of Inorganic Chemistry, Department of Chemistry, Faculty of Science, Suez Canal University, Ismailia, Egypt, for his support during the interpretation of the chemical and thermal analysis.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
S.S.: design, writing and revision. M.G: design, testing, data analysis & interpretation, writing and revision. A.G.: design, testing, data analysis & interpretation. T.H: testing, data analysis & interpretation, writing and revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saber, S., Galal, M.M., Ismail, A.G. et al. Thermal, chemical and physical analysis of VDW.1Seal, Fill Root ST, and ADseal root canal sealers. Sci Rep 13, 14829 (2023). https://doi.org/10.1038/s41598-023-41798-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-41798-8
This article is cited by
-
Investigation of physicochemical properties of endodontic bioceramic sealers
Journal of the Australian Ceramic Society (2025)
-
Quality of different obturation techniques to fill perforating internal root resorption: a micro-computed tomographic study
BMC Oral Health (2024)
-
Physicochemical properties of AH plus bioceramic sealer, Bio-C Sealer, and ADseal root canal sealer
Head & Face Medicine (2024)
-
Ex-vivo evaluation of clinically-set hydraulic sealers used with different canal dryness protocols and obturation techniques: a randomized clinical trial
Clinical Oral Investigations (2024)
-
Impact of low-fusion gutta-percha cones used in variations of the continuous wave condensation technique with filling sealers based on bioceramic compounds on the quality of root canal filling
Odontology (2024)