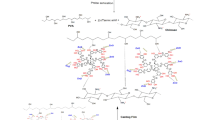
Abstract
Excess consumption of antibiotics leads to antibiotic resistance that hinders the control and cure of microbial diseases. Therefore, it is crucial to monitor the antibiotic levels in the environment. In this proposed research work, an optical nano-sensor was devised that can sense the ultra-low concentration of antibiotics, in samples like tap water using fluorescent zinc oxide quantum dots (ZnO QDs) based nano-sensor. For this, different polymers (polyvinylalcohol–PVA and polyvinylpyrrolidine–PVP) capped florescent ZnO QDs were synthesized using a modified sol–gel technique. These were used as fluorescent probes to monitor the presence of antibiotics. The optical characterizations of synthesized QDs were performed using UV–visible absorption and fluorescence spectroscopic methods while structural characteristics were analyzed by using Raman spectroscopy and X-ray diffraction spectroscopy. The formation of capped QDs was confirmed by Fourier transform infrared spectroscopy (FTIR). Charge on the synthesized QDs was obtained with the help of ZETA potential. Here ten different antibiotics were checked, Ciprofloxacin and Moxifloxacin have shown excellent sensing and specificity with PVA-ZnO QDs and PVP-ZnO QDs with LOD of 1.4 nM and 0.8 nM, and sensitivity of 36.17 units/mM and 19.33 units/mM respectively. This study also inferred the tuning of the ZnO QDs properties and specificity towards the different antibiotics can be achieved by capping QDs with different polymers.
Similar content being viewed by others
Introduction
Antibiotics, a cornerstone of the modern medicinal industry1. Antibiotics are the chemical substances produced by various microorganisms in low concentrations that destroy or kill or inhibit the growth of other microorganisms2,3. In the Global scenario, India, China, and the USA are in the top three countries for the highest antibiotics consumption. From the past data analysis, it is clear that the countries having higher per capita consumption of antibiotics have higher antibiotic resistance rates4,5,6 The antibiotic resistance mechanism is shown in Figure S17. Antibiotics have been detected in limited proportions in ground water8 as the environmental contaminant. The accurate detection of antibiotic levels in biological and clinical specimens is an urgent need due to its potential toxic effects. Till now the detection of antibiotics was performed by conventional approaches such as high-performance liquid chromatography (HPLC)9, fluorometry10, spectrophotometry11, etc. Although HPLC is widely used owing to its capability, susceptibility, and selectivity, it is highly time-consuming and needs a large amount of solvent usage. Also, these high-level devices require a higher cost of maintenance which narrows down its applicability12,13. The Electrochemical technique for the detection of analyte based on nanomaterial electrodes modified with nanomaterial is a meticulous approach used in analytical chemistry to determine drugs as well as biomolecules14. Few immunosensors for the detection of various antibiotics reported in the literature. However, the main advantage of optical sensing over the electrochemical immunosensors is that, in optical sensors, no other biomolecules like aptamer, antibodies, etc. are required15,16. The targeted molecule can be detected directly based on the specificity of the nanomaterials and hence reduces the cost and time and these sensors are fast reliable and reproducible17,18,19,20,21,22,23. A few QDs that were used as optical probes for the detection of various antibiotics are summarized in Table 1, whereas, ZnO QDs are of interest due to their stability when functionalized.
Enormous reports have been published regarding monodispersed, stable, and fluorescent ZnO QDs in several organic media24,25. ZnO QDs being highly active sometimes undergo agglomeration which leads to poor dispersion and loss of all their fluorescence properties26. Several methods and techniques are therefore, established to overcome the challenges such as modification of the surface of ZnO nanomaterials by making use of water-soluble/organic ligands, silanes, or by capping of ZnO nanomaterials with polymer. Joshi et al. have gone through the synthesis of highly fluorescent polyethyleneimine (PEI) capped ZnO27. The fluorescence spectra showed defect-related emission around 555 nm in water as a dispersion medium and no agglomeration of ZnO showing long-range degradation in luminescence. It was evident that surface modification may produce excellent binding affinity for several molecules like nucleic acid, monosaccharide, and protein, etc., which can make the ZnO QDs an excellent candidate as a fluorescence contrast agent for bio-imaging, bio-conjugation, and other applications28,29,30. Nathalia. et.al. explored the surface modification of ZnO QDs for applications in biomedicine and environmental remediation. The study discusses various functionalization strategies and their impact on the quantum dots' performance in different settings31. Ying. et.al. focused on the biomedical application of ZnO quantum dots by functionalizing them with folic acid. The study explores the potential of these functionalized quantum dots for targeted drug delivery and imaging in cancer cells32. Sobhani. et.al. explored the functionalization of ZnO quantum dots with water-soluble hyperbranched polyglycerol. This work demonstrates the potential of hyper branched polymers in improving the dispensability and stability of ZnO quantum dots in aqueous solution33.
In this study, PVA and PVP polymers were used as the capping agents for tuning the fluorescence properties of ZnO quantum dots. These polymers as a capping agent impart charges on quantum dots which enhances the specificity towards certain analytes. Polyvinyl alcohol (PVA) is a synthetic polymer soluble in water. It provides a large number of active -OH groups. It forms a polymer complex of metal ions through ligand interaction. ZnO nanomaterials synthesized without using these polymers show very low emission. But PVA functionalized ZnO QDs have Zn+ defects which play a major role in enhancing the fluorescence intensity34,35,36. Polyvinylpyrrolidone (PVP) is a water-soluble polymer, and commonly used as a viscosity modifier in nano-field. Water -dispersible ZnO QDs have been synthesized using sol–gel method as the highly fluorescent nanomaterial in which water acts as a solvent while PVP acts as a viscosity modifying agent. This helps to enhance the fluorescence intensity of the quantum dots hence, we can make the highly fluorescent quantum dots with tuned optical properties37.
In this proposed research work, the emphasis is to develop a method that is simple and selective to the detection of antibiotics in tap water using fluorescent ZnO QDs-based nano-sensors. For this, fluorescent and polymer-capped ZnO QDs using a modified sol–gel technique were synthesized. Two polymers PVA and PVP were used as capping and optical tuning agents for ZnO QDS. These were used as fluorescent probes to detect two different antibiotics selectively. The PVA-capped ZnO QDs (PVA-ZnO QDs) were used in the detection of Ciprofloxacin and PVP-capped ZnO QDs (PVP-ZnO QDs) were used for Moxifloxacin detection.
Experimental Section
Materials
Polyvinyl Alcohol PVA (Cold) was purchased from Central Drug House (P) Ltd, India. Polyvinylpyrrolidone (PVP K-30) was purchased from Sisco Research Laboratories Pvt. Ltd., India. Zinc acetate dihydrate, Moxifloxacin hydrochloride (C21H24FN3O4), Citric acid (CA) (C6H8O7), Cysteine (Cys)(C3H7NO2S) and Bovine serum albumin (BSA) were procured from Sigma-Aldrich. Uric acid (UA) (C5H4N4O3), Sodium hydroxide pellets (NaOH), Cholesterol (Cho) (C27H46O), Norfloxacin (Nor) (C16H18FN3O3), Levofloxacin (Levo) (C18H20FN3O4), Ofloxacin (Oflo) (C18H20FN3O4), Ciprofloxacin hydrochloride (Cipro) (C17H18FN3O4·HCl), Gentamicin sulfate (Genta) (C21H43N5O7·H2SO4), Florfenicol (Flor) (C12H14Cl2FNO4S), Erythromycin (Erythro) (C37H67NO13), Thiamphenicol (Thiam) (C12H15Cl2NO5S), Arginine (Arg) (C6H14N4O2), Ascorbic acid (AA) (C6H8O6), Methionine (Met) (C5H11NO2S), Casein (C81H125N22O39P), Sulfuric acid (H2SO4) and Urea (CH4N2O) were bought from Sisco Research Laboratories Pvt. Ltd., India. Methanol was procured from Emsure. Folic acid (FA) (C19H19N2O6) and Aspartic acid (Asp) (NH2CH (COOH) CH2·COOH) were purchased from CDH. Magnesium chloride (MgCll2) was acquired from Thomas Baker.), Tetracycline hydrochloride (Tet) (C22H24N2O6·HCl), Ampicillin sodium salt (Amphi) (C16H18N3O4SNa), Copper chloride (CuCl2), and Ferrous chloride (FeCl2·H2O) were obtained from Hi-media Laboratory Pvt. Ltd. Glucose (Glu) (C6H12O6) and Sodium chloride (NaCl), were bought from Fine Chem Limited (S.D.F.C.L). Calcium chloride (CaCl2) was procured from RankemAvantor India Pvt. Ltd. Potassium chloride was purchased form from Thermo Fisher Scientific Pvt. Ltd. All other chemicals and solvents used are of analytical grade and have been used directly without any further purification. All the solutions were prepared in deionized (DI) water.
Nanomaterial synthesis
ZnO QDs, PVA-ZnO QDs, and PVP-ZnO QDs were separately synthesized using a modified sol–gel approach, which involves mixing of precursor solutions (5mM solution of zinc acetate dihydrates and a 10 mM solution of sodium hydroxide prepared separately in Methanol and DI water), refluxing at desired temperature, and neutralization of pH followed by characterization. Figure 2 (Supplementary information) shows the flow chart of the reflexing sol–gel approach for the synthesis of ZnO QDs. Refluxing was performed at 65°C. For heating, a semi spherical type of electrically powered heating mantel was used with a magnetic stirrer to provide constant stirring during heating. For the condensation of vapors, chilled water was circulated in the condenser, while the temperature was measured by placing a thermometer in one of the side necks. The process continued for a certain time to ascertain the growth mechanism followed by cooling the solution at room temperature. After cooling, solution pH was neutralized by dropwise adding NaOH solution under continuous stirring until pH 7.0 was obtained. The solution was further stirred for some time to get a uniform pH. The flow chart for sol–gel synthesis has been mentioned in S2 (Supplementary information). The reaction followed during the formation of ZnO QDs are as follows:
PVA-ZnO QDs
10 ml of PVA (5 mg/ml) solution was mixed dropwise to the ZnO-NaOH reaction mixture at room temperature during continuous stirring. The pH of the solution was neutral. The QDs prepared with PVA are abbreviated as PVA -ZnO QDs.
PVP-ZnO QDs
10 ml of PVP solution (5 mg/ ml) was added as mentioned above and the QDs prepared with PVP are abbreviated as PVP-ZnO QDs.
Nanomaterial Characterization and sensing of the antibiotics
UV–Vis absorption spectroscopy of the as-synthesized QDs was carried out in the wavelength range of 190–900 nm using a double-beam Hitachi (U3900) spectrophotometer. A quartz cuvette of 10 mm path length having fixed slit width (5 nm) and a scan rate of 600 nm/minute were used for the measurements. Peak absorption wavelength was used as excitation energy for fluorescence measurement. The optical band gap of synthesized ZnO QDs were estimated using Tauc plots. Fluorescence measurements were performed using a Cary Eclipse fluorescence spectrophotometer (Agilent Technology, Model G9800A) at a constant excitation and emission slit width of 5 nm and scanning speed of 600 nm/minute. A 3.5 ml quartz cuvette with a 10 mm path length was used for measurement. To account for the changes in the functional groups, present on the surface of bio-conjugates, Fourier transform infrared spectroscopy (FTIR) in ATR mode was carried out using Perkin Elmer, Spectrum 1 US in a wavenumber range of 4000 to 400 cm−1. X-ray diffraction (XRD) patterns of ZnO QDs, PVA-ZnO QDs, and PVP-ZnO QDs were obtained using Ultima IV (Rigaku). Cu-Kα radiation (λ = 1.5415 Å) was used as an x-ray source at an applied voltage of 40 keV and current of 30 mA. The beam incident angle of 60º was kept constant. The sample was made by making a uniform film of ZnO QDs, PVP-ZnO QDs, and PVA-ZnO QDs synthesized powder on the sample holder of size 1 cm × 1 cm. The spectra were obtained for the Bragg angle ranging from 10–90° at the scan speed of 8°/min. To determine the surface charge and the zeta potential on the quantum dots’ surface somewhere in a diffuse layer in the suspension, we used the Zeta potential analyzer (ZEECOM, Microtech Co. Ltd.). Raman spectroscopy was carried out to study the structural properties of the ZnO QDs, PVA-ZnO QDs, and PVP-ZnO QDs. For this, we have used Raman-AFM Microscope alpha 300 RA (Oxford Instruments). The time-resolved fluorescence measurement of PVA-capped ZnO and PVP-capped ZnO in the absence and presence of antibiotics was recorded at 360 nm emission wavelength using a Time-Resolved Fluorescence Spectrometer (TRFS)—Edinburgh FL920 Fluorescence Life Time Spectrometer to calculate the average life-time and quenching effect (static/dynamic). The elemental analysis (EDS) was done by JSM -IT 200. The morphology and particle size of the QDs were examined using high-resolution transmission electron microscopy (HR-TEM) (JEOL-TEM-2100F).
Sensing
All the nano-conjugates were prepared by physical mixing at room temperature. For sensing of Ciprofloxacin, the concentration range of Ciprofloxacin from 1 nM to 1 mM (1 nM, 5 nM, 10 nM, 50 nM, 100 nM, 500 nM, 1 mM, 5 mM, 10 mM, 50 mM, 100 mM, 500 mM, 1 mM) every time mixed with PVA-ZnO solution and PL was measured at every concentration. For sensing of Moxifloxacin, the concentration range of Moxifloxacin from 1 nM to 1 mM every time mixed with PVP-ZnO solution, and the PL was measured at every concentration. For a spike study in tap water, both Ciprofloxacin and Moxifloxacin were taken. For interference study, different antibiotics along with other interferents were carried out with common interferents such as citric acid (10 mM), ascorbic acid (10 mM), cholesterol (10 mM), aspartic acid (10 mM), uric acid (10 mM), folic acid (10 mM), casein (10 mM), urea (10 mM), glucose (5 mM), sodium (Na+), potassium (K2+), calcium (Ca2+), copper (Cu2+), magnesium (Mg2+), manganese (Mn2+), and Zinc (Zn2+) (all ionic solutions 1mM).
Results and discussion
Figure 1A shows UV–Visible absorption spectra of synthesized ZnO QDs (a) PVA-ZnO QDs (b) and PVP-ZnO QDs(c) depicting the absorption peak at around 255 nm, 273 nm, and 265 nm, which are in good agreement with reported values for ZnO QDs obtained through sol–gel method38. The other peaks in PVA-ZnO and PVP-ZnO are due to the PVA and PVP, respectively. The Tauc plots of ZnO QDs, PVA-ZnO QDs, and PVP-ZnO QDs and shown in the respective insets of Figs. 1a–c and the optical bandgap values were obtained approximately as 3.89, 3.3 and 3.5 eV, respectively. It is clear from the absorption spectra that the polymer capping of QDs plays an important role in tuning the optical absorption and also the optical bandgap. The specific functional groups introduced by capping can further play an important role in the selectivity towards the interaction with antibiotics.
The FTIR spectra of ZnO QDs (a), PVP-ZnO QDs (b), and PVA-ZnO QDs (c) are shown in Fig. 1B. There are peaks at 582, 1020, and 1642 and a broad peak between 3000–3500 cm−1 which were assigned to Zn-O, C-O, C-O, and OH stretch bands, respectively39,40,41. The peak at 582 cm−1 in ZnO QDs is shifted slightly after capping with PVA and PVP can be observed in Fig. 1B. There are extra peaks observed at 1120 cm−1assigned to C-OH band vibration in PVA and C-N band vibration in PVP; 1293 cm−1 assigned to N–O band vibration in pyridine; 1410 cm−1 assigned to OH band vibrations; 1421 cm−1 in (b) assigned to C-N stretch in PVP; 2838 cm−1assigned to CH3-N and CH3-O in (b) and (c) respectively; and 2950 cm-1assigned to CH symmetric and anti-symmetric stretch vibrations in(b) and (c) observed. All these results indicate the successful formation of capped and uncapped ZnO QDs. Also, the polymer vibration bands show very low intensity due to their small concentrations in QDs dispersions as compared to ZnO QDS. All these results therefore confirm the formation of capped and uncapped ZnO QDs.
Figure 2A shows the fluorescence spectra of as synthesized ZnO QDs, PVA-ZnO QDs, and PVP-ZnO QDs obtained at room temperature with an excitation energy of 230 nm to observe the effect of polymer capping. The spectra reveal four emission peaks for all three nanomaterials; for bare ZnO QDs the main peak is centered around 343 nm, the secondary emission peak is around 460 nm, a small peak is centered at 500 nm, and a broad peak between 550–700 nm in agreement with a report for sol–gel synthesized ZnO QDs42.
For PVA-ZnO QDs, the main peak is centered around 339 nm, the secondary emission peak around 445 nm, a small peak centered at 490 nm, and a broad peak between 550–700 nm. For PVP-ZnO QDs, the main peak is centered around 320 nm, the secondary emission peak around 460 nm, a small peak centered at 505 nm, and a broad peak between 550–700 nm. A slight left shift in the peak position has been obtained for the PVA-ZnO QDs and PVP-ZnO QDs by the optical bandgap variation after polymer capping. The optical parameters are summarized in Table 2. Further, the excitation wavelength-dependent fluorescence study (210–260 nm) was carried out and the results were plotted in Figure S3 and found the maximum emission was obtained at 230 nm excitation wavelength.
The XRD was performed to study the purity of the prepared samples. The three samples were drop casted on glass slides and allowed to dry in air for 24 h. The same process was repeated more times to obtain a thick layer of ZnO QDs, PVA-ZnO QDs, and PVP-ZnO QDs. No crystalline structure can be seen in the XRD spectra of each sample confirming the purity of QDs, as it is known that quantum dots do not show any sharp peaks in XRD spectra. The broad humps shown in Fig. 2B confirmed the small size of the QDs. As the QDs have sizes less than 10 nm, the SEM images did not show any specific informative results. Whereas, the energy dispersive x-ray spectroscopy (EDS) (Figure S3 in Supplementary information) was performed to find the elemental composition of the prepared samples. Raman Spectroscopy of ZnO QDs, PVA-ZnO QDs, and PVP-ZnO QDs were carried out and the spectra are shown in Fig. 2C. There are five common peaks corresponding to ZnO have been obtained at wavenumbers 369.59, 481.95, 929.35, and 1095.15 cm−1 in the Raman spectra of each sample. The peak at 369.59 cm-1 is due to the E2H peak, while the small peak at 481.95 cm-1 is due to the A1(LO) mode. The peak at 929.35 cm−1 corresponds to A1(TO) + E2L. The peak observed at 1095.15 cm−1 is due to the 2 (LO) mode. The rest of the peaks in PVA-ZnO QDs and PVP-ZnO QDs are due to the PVA and PVP, respectively. In each of the samples, carbon, oxygen, zinc, and sodium were obtained as shown in Fig. 3a–c. Carbon is due to the carbon tape used for EDS measurement; Na is due to the use of NaOH while synthesizing the samples. The presence of Zinc and Oxygen confirms the formation of ZnO quantum dots in all the samples as shown in Figure S4 (Supplementary information). Surface charge plays a critical role in the surface properties of the nanomaterials. Zeta potential study helps in studying the surface charge of the nanoparticle. Here zeta study has been performed on ZnO QDs, PVP-ZnO QDs, and PVA-ZnO QDs. A histogram has been plotted for each sample as shown in Fig. 2D. In each of the samples, positive charge is obtained varying in value. ZnO QDs have zeta potential ranging from 20–180 mV with maximum zeta potential centered at 100–110 mV. PVA-ZnO QDs has zeta potential ranging from 100–300 mV with maximum zeta potential centered at 150–250 mV. PVP-ZnO QDs have zeta potential ranging from 100–350 mV with maximum zeta potential centered at 200–300 mV. The zeta potential increased with the capping of the polymers.
The particle size of the PVA and PVP-capped ZnO QDs was studied using TEM. The TEM images at 20 nm scale and the corresponding particle size histogram are shown in Fig. 3. It is clear from Fig. 3a,c that the quantum dots were dispersed and have a range of particle size below 10 nm. The average particle size in PVA-ZnO (Fig. 3b) was 6–7 nm and in case of PVP-ZnO (Fig. 3d), it was 4 nm confirming the QDs formation.
The selectivity study of ZnO QDs, PVA-ZnO QDs, and PVP-ZnO QDs towards ten antibiotics namely: Amoxicillin, Azaeothromycin, Ciprofloxacin, Floxacin, Gentamycin, Levofloxacin, Moxifloxacin, Norfloxacin, Tetracyclin, and Thiamphenocol was carried out. In the ZnO QDs, no specificity towards any antibiotic has been obtained. The peak wavelength in the absorption study shows (Figure S5 (A)) no change with the interaction of antibiotics. There was a decrease in the fluorescence intensity of ZnO QDs when interacting with all the antibiotics. Therefore, no specificity has been achieved as shown in Figure S5 A (b).
When the same study was performed with PVA-ZnO QDs, specificity towards Ciprofloxacin was obtained. The absorption peak wavelength study shows a slight change in the wavelength with the interaction of Ciprofloxacin as shown in Figure S5 B (a). At the same time, the fluorescence intensity of both ZnO QDs and Ciprofloxacin were decreased (Figure S5 B (b)). It was observed A mixed response of PVA-ZnO QDs when interacting with all the other antibiotics in the form of both the increase and decrease in the intensity. No change in the wavelength of other antibiotics can be seen (Figure S5 B (a)) which shows the specificity towards ciprofloxacin for PVA-ZnO QDs.
In the PVP-ZnO QDs, specificity towards Moxifloxacin has been obtained. The absorption peak wavelength study shows a slight change in the peak wavelength when interacting with Moxifloxacin as shown in Figure S5 C (a). On the other hand, the fluorescence intensity of ZnO QDs was decreased after the interaction with Moxi. There has been a mixed response of PVP-ZnO QDs when interacting with all the other antibiotics in the form of both the increase and decrease in the intensity. No change in the wavelength of other antibiotics can be seen which shows the high specificity towards Moxifloxacin for PVP-ZnO QDs as shown in Figure S5 C (b).
Ciprofloxacin sensing study against PVA-ZnO QDs
The sensing study of PVA-ZnO QDs towards Ciprofloxacin was performed by fluorescence study in the concentration range of 1 nM–1 mM of Ciprofloxacin at an excitation wavelength of 230 nm as shown in Fig. 4a. It is clear from Fig. 4a that, complete quenching of the fluorescence of PVA-ZnO QDs and Cipro mixture obtained which shows the systematic interaction between the Cipro and PVA-ZnO QDs. The fluorescence peak intensity at each concentration was plotted against log of the concentration of Cipro and a linear plot was obtained with two slopes as shown in the inset of Fig. 4a. The linearity ranges obtained were 1 nM to 1 µM with R2 = 0.965 and 5 µM to 1 mM with R2 = 0.983.
Moxifloxacin sensing study against PVP-ZnO QDs
The antibiotic sensing study was performed using fluorescence of PVP-ZnO QDs towards Moxifloxacin, in the concentration range of 1 nM – 1 mM of Moxifloxacinat excitation wavelength of 230 nm as shown in Fig. 5a. The fluorescence intensity was completely quenched at higher concentrations showing the interaction between the Moxifloxacin and PVP-ZnO QDs. The calibration plot between the peak intensity and log of concentration, showed two slopes in the range of 1 nM to 1 µM with R2 = 0.956 and 5 µM to 1 mM with R2 = 0.99 as shown in the inset of Fig. 5a.
Spike sample study in tap water
Tap water was spiked with Cipro and Moxi in the concentration range of 1 nM – 1 mM and performed the sensing with PVA-ZnO QDs and PVP-ZnO QDs using fluorescence spectra as shown in Figs. 4b and 5b respectively. It is observed from Figs. 4b and 5b that; complete quenching of the fluorescence has been obtained as in the case of response study of both analytes. The calibration curve was plotted in the spiked sample study also and shown in the insets of Figs. 4b and 5b. These results indicate that the present system can be applicable for the sensing of Cipro and Moxi in real samples as well as contaminated water sources, hospital wastewater, aquaculture sources, etc. The recovery percentages were tabulated in Table S1a (Supplementary information).
Interference study-ciprofloxacin
Figure 4b and 5c show the interference study performed on common interferants such as citric acid, ascorbic acid, cholesterol, aspartic acid, uric acid, folic acid, casein, urea, glucose, sodium (Na+), potassium (K2+), calcium (Ca2+), copper (Cu2+), magnesium (Mg2+), manganese (Mn2+), and Zinc (Zn2+) that present in tap water samples. The highest change in the intensity can be seen for the Cipro and Moxi, which confirm the highly selective nature of the PVA-ZnO QDs and PVP-ZnO QDs towards Cipro and Moxi respectively. The interferent concentrations were taken according to the permissible levels43,44.
Time-resolved fluorescence spectroscopic analysis
The time-resolved fluorescence spectra of PVA-ZnO were recorded using a time-correlated single-photon counting (TCSPC) instrument at 360 nm emission wavelength in the presence and absence of Ciprofloxacin. The measured data showed a nominal decrease in the lifetime (< 2 ns) of ZnO-PVA in the presence of Ciprofloxacin. These results indicated that Ciprofloxacin is quenching the ZnO-PVA QDs by the static quenching process. The decay plot of PVA-ZnO QDs in the presence of Ciprofloxacin is plotted in Fig. 6a. The lifetime was estimated and tabulated in Table 3.
On the other hand, in the case of PVP-ZnO QDs, the lifetime (from the TCSPC study) showed further small change in the presence and absence of Moxifloxacin which again indicates the quenching is static in nature. The decay plot of PVP-ZnO QDs in the presence of Moxifloxacin is plotted in Fig. 6b. It is clear from Table 3 that the lifetime change is too small in the case of PVP-ZnO QDs as compared to PVA-ZnO QDs.
Interaction mechanism of PVA-ZnO QDs with Ciprofloxacin and PVP-ZnO QDs with Moxifloxacin
Quenching of fluorescence intensity is a phenomenon that occurs due to the formation of a complex between two interacting species45. This interaction results in the formation of supramolecular structure46,47. This is the reason for the quenching of fluorescence intensity of PVA-ZnO QDs and PVP-ZnO QDs after the interaction with Cipro and Moxi, respectively48. This process is illustrated in Fig. 7a that shows the formation of the supramolecule and Fig. 7b shows quenching of fluorescence intensity49. Due to the presence of the OH group in the PVA structure, upon capping PVA with ZnO QDs, ZnO interacts with the OH group present in PVA forming zinc hydroxide. When Cipro interacts with PVA-ZnO QDs, a complex is formed between PVA-ZnO QDs and Cipro at the site of fluorine present in the Cipro structure. Being highly electronegative, fluorine dissociated zinc hydroxide present in PVA-ZnO QDs by releasing OH- ions. These OH- ions get associated at the site of fluorine in the Cipro structure. When the photon is incident on this complex an electron gets excited from the valance band of Zinc oxide and reaches the conduction band upon its return it loses the energy and falls into the LUMO and then to the HOMO of Cipro. This happens due to the formation of a complex between Cipro and PVA-ZnO QDs. This confirms the quenching of fluorescence intensity which is static in nature. This is in a complete argument with a time-resolved fluorescence study50 that states a very small decrease in the lifetime of PVA-ZnO QDs upon interaction with Cipro.
Similarly, the formation of supramolecular complex structure is the reason for quenching of PVP-ZnO QDs fluorescence intensity on interaction with Moxi with static quenching51. The ketone groups on the ortho position of PVP-ZnO QDs get oxidized by the OH group present in Moxi. Due to the oxidation of the ketone group present on the ortho position of PVP, the pie bond from the ortho position gets delocalized in the ring and formation of ether bond between PVP and Moxi. Hydrogen gets dissociated giving two electrons which when excited by the photon move from the valence band to the conduction band and fall back instantaneously into the valence band of Moxi resulting in static quenching. The static quenching was further confirmed by the TRFS study as mentioned above52.
Conclusion
In the present study, it is proved that the polymer functionalization of ZnO QDs can tune the optical absorption and bandgap when functionalized with polymers like PVP and PVA. The absorption and emission peak shift as compared to the bare ZnO QDs and FTIR studies revealed the successful functionalization of ZnO-QDs. The Zeta potential studies reveal that the surface charge increased due to the polymer functionalization, and it is observed that each of the samples carries a positive charge. It is observed that the polymer functionalized ZnO QDs interacted specifically with different antibiotics such as PVA-ZnO QDs towards Cipro and PVP-ZnO QDs towards Moxifloxacin. Whereas, the non-functionalised ZnO QDs did not show specifity. The fluorescence quenching of PVA-ZnO QDs by Cipro and PVP-ZnO QDs by Moxi was static in nature. The spike sample studies in tap water showed promising results towards the applicability of the developed sensing systems in real samples. The specificity was proved by interference studies.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request and all data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Arikekpar, E. E. & Antibiotics, I. Classification and mechanisms of action with emphasis on molecular perspectives. Int. J. Appl. Microbiol. Biotechnol. Res. 4, 90–101 (2016).
Kumar, R., Lakshmi, G. B. V. S., Dhiman, T. K., Singh, K. & Solanki, P. R. Highly sensitive amoxicillin immunosensor based on aqueous vanadium disulphide quantum dots. J. Electroanal. Chem. 892, 115266 (2021).
Alm, R. A. & Lahiri, S. D. Narrow-spectrum antibacterial agents—benefits and challenges. Antibiotics 9, 1–8 (2020).
Farooqui, H. H., Mehta, A. & Selvaraj, S. Outpatient antibiotic prescription rate and pattern in the private sector in India: Evidence from medical audit data. PLoS One 14, 1–11 (2019).
Song, Y., Han, Z., Song, K. & Zhen, T. Antibiotic consumption trends in china: evidence from six-year surveillance sales records in shandong province. Front. Pharmacol. 11, 1–8 (2020).
Cesur, S. & Demiröz, A. P. Antibiotics and the Mechanisms of Resistance to Antibiotics. Med. J. Islam. World Acad. Sci. 21, 138–142 (2013).
Zhou, R. et al. Effective control of microbial spoilage in soybeans by water-soluble ZnO nanoparticles. Food Chem. 388, 132994 (2022).
Pang, M., Hu, J. & Zeng, H. C. Synthesis, morphological control, and antibacterial properties of hollow/solid Ag2S/Ag heterodimers. J. Am. Chem. Soc. 132, 10771–10785 (2010).
Shiuan Yih, B. et al. Development of Cloud Point Extraction for Parabens Using Different Surfactants with Modifiers. Mater. Today Proc. 19, 1787–1795 (2019).
Brecher, M. E., Wong, E. C. C., Chen, S. E., Vampola, C. & Rocco, R. M. Antibiotic-labeled probes and microvolume fluorimetry for the rapid detection of bacterial contamination in platelet components: A preliminary report. Transfusion 40, 411–413 (2000).
Kochan, K. et al. Rapid approach for detection of antibiotic resistance in bacteria using vibrational spectroscopy. Anal. Chem. 92, 8235–8243 (2020).
Lakshmi, G., Kondal, S., Dhiman, T. K., Gupta, P. K. & Solanki, P. A disposable, environment-friendly, cost-effective paper based electrochemical cholesterol biosensor fabricated using screen printing technique. ECS Trans. 107, 18113 (2022).
Lakshmi, G., Poddar, M., Dhiman, T. K., Singh, A. K. & Solanki, P. R. Gold-Ceria nanocomposite based highly sensitive and selective aptasensing platform for the detection of the Chlorpyrifos in Solanum tuberosum. Coll. Surf. A Physicochem. Eng. Asp. 653, 129819 (2022).
Singh, A. K., Dhiman, T. K., Lakshmi, V. S. G. B. & Solanki, P. R. Dimanganese trioxide (Mn2O3) based label-free electrochemical biosensor for detection of Aflatoxin-B1. Bioelectrochemistry 137, 107684 (2021).
Hashmi, S. Z. H. et al. Levofloxacin detection using l-cysteine capped mgs quantum dots via the photoinduced electron transfer process. Front. Nanotechnol. https://doi.org/10.3389/fnano.2021.616186 (2021).
Sajwan, R. K. et al. Enhanced fluorescence of mercaptopropionic acid-capped zinc sulfide quantum dots with moxifloxacin in food and water samples via reductive photoinduced electron transfer. Environ. Sci. Nano 8, 2693–2705 (2021).
Bhatt, G. & Bhattacharya, S. Biosensors on chip: A critical review from an aspect of micro/nanoscales. J. Micromanufact. 2, 198–219 (2019).
Watts, H. J., Lowe, C. R. & Pollard-Knight, D. V. Optical biosensor for monitoring microbial cells. Anal. Chem. 66, 2465–2470 (1994).
Myszka, D. G. Improving biosensor analysis. J. Mol. Recognit. 12, 279–284 (1999).
Ma, F., ChenLi, C. & YangZhang, C. Development of quantum dot-based biosensors: Principles and applications. J. Mater. Chem. B 6, 6173–6190 (2018).
Milićević, D. & Hlaváč, J. Fluorescence-based on-resin detection of three model proteases. Chemosensors 9, 11 (2021).
Verma, A. K., Dhiman, T. K., Lakshmi, G. & Solanki, P. Optical tuning of polymer functionalized zinc oxide quantum dots as a selective probe for specific detection of antibiotics. Biorxiv 4, 90 (2022).
Kim, D. & Yoo, S. Aptamer-conjugated quantum dot optical biosensors: Strategies and applications. Chemosensors 9, 1–20 (2021).
Verma, A. K. & Ansari, Z. A. Fluorescent ZnO quantum dot probe to study glucose-glucose oxidase interaction via fluorescence resonance energy transfer. Sens. Lett. 18, 351–365 (2020).
Kumar Verma, A. Fluorescent quantum dots based FRET: The spectroscopic ruler in nano-biotechnology; Theories, principle, applications and advanced perspectives. Biomed. J. Sci. Tech. Res. 47, 37987–37995 (2022).
Garimella, L. B. V. S., Dhiman, T. K., Kumar, R., Singh, A. K. & Solanki, P. R. One-step synthesized ZnO np-based optical sensors for detection of aldicarb via a photoinduced electron transfer route. ACS Omega 5, 2552–2560 (2020).
Haranath, D., Sahai, S. & Joshi, P. Tuning of emission colors in zinc oxide quantum dots. Appl. Phys. Lett. https://doi.org/10.1063/1.2944142 (2008).
Wu, Y. L. et al. Surface modifications of ZnO quantum dots for bio-imaging. Nanotechnology 18, 215604 (2007).
ChunXiang, X. U. et al. Nanostructured ZnO for biosensing applications. Chinese Sci. Bull. 58, 2563–2566 (2013).
Verma, A. K. ZnO quantum dots a novel nanomaterial for various applications: Recent advances and challenges. Indian J. Biochem. Biophys. 59, 1190–1198 (2022).
Rissi, N. C., Hammer, P. & Chiavacci, L. A. Surface modification of ZnO quantum dots by organosilanes and oleic acid with enhanced luminescence for potential biological application. Mater. Res. Express https://doi.org/10.1088/2053-1591/aa58fc (2017).
Ma, Y. Y., Ding, H. & Xiong, H. M. Folic acid functionalized ZnO quantum dots for targeted cancer cell imaging. Nanotechnology 26, 305702 (2015).
Sobhani, Z., Khalifeh, R., Banizamani, M. & Rajabzadeh, M. Water-soluble ZnO quantum dots modified by polyglycerol: The pH-sensitive and targeted fluorescent probe for delivery of an anticancer drug. J. Drug Deliv. Sci. Technol. 76, 103452 (2022).
Tang, X. & Alavi, S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydr. Polym. 85, 7–16 (2011).
Ahlawat, A., Dhiman, T. K., Solanki, P. R. & RANA, P. S. Enhanced Photocatalytic Degradation of p-Nitrophenol and Phenol Red Through Synergistic Effects of a CeO2-TiO2 Nanocomposite. Catal. Res. 02, 1–13 (2022).
Baker, M. I., Walsh, S. P., Schwartz, Z. & Boyan, B. D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 100, 1451–1457 (2012).
Dang, H., Huang, L.-K., Zhang, Y., Wang, C.-F. & Chen, S. Large-Scale Ultrasonic Fabrication of White Fluorescent Carbon Dots. Ind. Eng. Chem. Res. 55, 5335–5341 (2016).
Spanhel, L. & Anderson, M. A. Semiconductor clusters in the sol-gel process: quantized aggregation, gelation, and crystal growth in concentrated zinc oxide colloids. J. Am. Chem. Soc. 113, 2826–2833 (1991).
Rajabi, H. R. et al. Microwave assisted extraction as an efficient approach for biosynthesis of zinc oxide nanoparticles: Synthesis, characterization, and biological properties. Mater. Sci. Eng. C 78, 1109–1118 (2017).
Moradi Alvand, Z., Rajabi, H. R., Mirzaei, A., Masoumiasl, A. & Sadatfaraji, H. Rapid and green synthesis of cadmium telluride quantum dots with low toxicity based on a plant-mediated approach after microwave and ultrasonic assisted extraction: Synthesis, characterization, biological potentials and comparison study. Mater. Sci. Eng. C 98, 535–544 (2019).
Rajabi, H. R., Sajadiasl, F., Karimi, H. & Alvand, Z. M. Green synthesis of zinc sulfide nanophotocatalysts using aqueous extract of Ficus Johannis plant for efficient photodegradation of some pollutants. J. Mater. Res. Technol. 9, 15638–15647 (2020).
Singh FN: J., et al. Role of alkali metal hydroxide in controlling the size of ZnO nanoparticles in non-aqueous medium. Int. J. Fundam. Appl. Sci. 1, 91–93 (2012).
Rajabi, H. R., Shamsipur, M., Khosravi, A. A., Khani, O. & Yousefi, M. H. Selective spectrofluorimetric determination of sulfide ion using manganese doped ZnS quantum dots as luminescent probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 107, 256–262 (2013).
Shamsipur, M. & Rajabi, H. R. Pure zinc sulfide quantum dot as highly selective luminescent probe for determination of hazardous cyanide ion. Mater. Sci. Eng. C 36, 139–145 (2014).
Lakowicz, J. R. Quenching of fluorescence. In Principles of Fluorescence Spectroscopy (ed. Lakowicz, J. R.) (Springer, 2006).
Whitten, D. G., Lopp, I. G. & Wildes, P. D. Fluorescence of zinc and magnesium etioporphyrin. I. Quenching and wavelength shifts due to complex formation. J. Am. Chem. Soc. 90, 7196–7200 (1968).
Guo, C., Sedgwick, A. C., Hirao, T. & Sessler, J. L. Supramolecular fluorescent sensors: An historical overview and update. Coord. Chem. Rev. 427, 213560 (2021).
Verma, A. K., Noumani, A. & Solanki, P. R. FRET Based Biosensor : Principle applications recent advances and challenges. Diagnostics https://doi.org/10.3390/diagnostics13081375 (2023).
Czarnik, A. W. Supramolecular chemistry, fluorescence, and sensing (The Royal Society of Chemistry, 2023).
Neelakandan, P. P., Hariharan, M. & Ramaiah, D. A supramolecular ON−OFF−ON fluorescence assay for selective recognition of GTP. J. Am. Chem. Soc. 128, 11334–11335 (2006).
Veglia, A. V. & Bracamonte, A. G. Metal-enhanced fluorescence emission and quenching protection effect with a host–guest nanophotonic-supramolecular structure. J. Nanophotonics 12, 33004 (2018).
Kumar, S. et al. Correlation of antibacterial and time resolved photoluminescence studies using bio-reduced silver nanoparticles conjugated with fluorescent quantum dots as a biomarker. J. Mater. Sci. Mater. Electron. 30, 6977–6983 (2019).
Sajwan, R. K. & Solanki, P. R. Gold@Carbon quantum dots nanocomposites based two-in-one sensor: A novel approach for sensitive detection of aminoglycosides antibiotics in food samples. Food Chem. 415, 135590 (2023).
Sajwan, R. K. & Solanki, P. R. A hybrid optical strategy based on graphene quantum dots and gold nanoparticles for selective determination of gentamicin in the milk and egg samples. Food Chem. 370, 131312 (2022).
Sajwan, R. K. et al. Enhanced fluorescence of mercaptopropionic acid-capped zinc sulfide quantum dots with moxifloxacin in food and water samplesviareductive photoinduced electron transfer. Environ. Sci. Nano 8, 2693–2705 (2021).
Sajwan, R. K., Kumar Himanshu, J. & Solanki, P. R. Polyvinyl alcohol-derived-carbon quantum dots based fluorometric “On-Off” probe for moxifloxacin detection in milk and egg samples. Food Chem. 439, 138038 (2024).
Al-Hashimi, B., Omer, K. M. & Rahman, H. S. Inner filter effect (IFE) as a simple and selective sensing platform for detection of tetracycline using milk-based nitrogen-doped carbon nanodots as fluorescence probe. Arab. J. Chem. 13, 5151–5159 (2020).
Long, D. et al. A quadruple-channel fluorescent sensor array based on label-free carbon dots for sensitive detection of tetracyclines. Analyst 144, 3307–3313 (2019).
Liu, M. L. et al. One-pot carbonization synthesis of europium-doped carbon quantum dots for highly selective detection of tetracycline. Methods Appl. Fluoresc. 5, 15003 (2017).
Chaudhary, N., Verma, D., Gopal Sharma, J. & Solanki, P. R. A novel bioinspired carbon quantum dots based optical sensor for ciprofloxacin detection. Mater. Lett. 308, 131090 (2022).
Acknowledgements
The authors would like to thank the Department of Biotechnology (DBT), India Indo-Russia project (DBT/IC-2/Indo-Russia/2017-19/02), ICMR, India (34/13/2019-TF Nano/BMS) and core funding from National Institute of Immunology, India for financial support.
Author information
Authors and Affiliations
Contributions
AKV performed all the experimental, data plotting, analyzed data and wrote the entire draft. TKD helped in the experiment and data plotting. ZH helped in the mechanism of interaction. GBVS helped in the analysis, editing, and reviewing of the draft. PS and AK designed the research, provided the facility, and edited the paper for the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Verma, A.K., Lakshmi, G.B.V.S., Dhiman, T.K. et al. Optical tuning of polymer functionalized zinc oxide quantum dots as a selective probe for the detection of antibiotics. Sci Rep 15, 1648 (2025). https://doi.org/10.1038/s41598-024-62827-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-62827-0