Abstract
Azole antifungal drugs are commonly used to treat vulvovaginal candidiasis (VVC). The nephrotoxicity and developmental toxicity of azole drugs have not been systematically analyzed in the real world. We used the FDA Adverse Event Reporting System (FAERS) to investigate the adverse events (AEs) associated with imidazole therapy for VVC. FAERS data (from quarter 1 2004 to quarter 3 2022) were retrieved using OpenVigil 2.1, and AEs were retrieved and standardized according to the Medical Dictionary for Regulatory Activities (MedDRA). In the top 10 System Organ Class (SOC), all four drugs have been found to have kidney and urinary system diseases and pregnancy. We found significant signals, including clotrimazole [bladder transitional cell carcinoma, (report odds ratio, ROR = 291.66)], [fetal death, (ROR = 10.28)], ketoconazole[nephrogenic anemia (ROR = 22.1)], [premature rupture of membranes (ROR = 22.91 46.45, 11, 3)], Miconazole[hematuria (ROR = 19.03)], [neonatal sepsis (ROR = 123.71)], [spontaneous abortion (ROR = 5.98)], Econazole [acute kidney injury (ROR = 4.41)], [spontaneous abortion (ROR = 19.62)]. We also discovered new adverse reactions that were not reported. Therefore, when using imidazole drugs for treatment, it is necessary to closely monitor the patient's renal function, pay attention to the developmental toxicity of the fetus during pregnancy, and be aware of potential adverse reactions that may occur.
Similar content being viewed by others
Introduction
Vulvovaginal candidiasis (VVC) is a common mucosal fungal infection among women of childbearing age, and most women experience one or two episodes in their lifetime1,2,3. A case of fetal right kidney dysplasia after fluconazole treatment of VVC during pregnancy was found in the database of Fujian Provincial Fuzhou Changle People’s District Hospital, China. Ultrasound examination of the newborn infant showed polycystic kidney disease. With the widespread use of imidazole drugs, drug related adverse reactions (AEs) have also received attention. Pregnancy has been found to be an increasingly important factor in the incidence of VVC4. With the continuous improvement of medical and social conditions, the issue of perinatal medication has received increasing attention. A recent meta-analysis showed that the overall prevalence of VVC among pregnant women was 29.2% (CI 95%: 23.4–33.0)5.
The 2015 Guidelines for the Treatment of Sexually Transmitted Diseases, released by the Centers for Disease Control and Prevention (CDC) in the United States, recommend a 7-day dose of vaginal azole medication. At present, many imidazole drugs have been approved by the FDA, and the imidazole derivatives such as clotrimazole, econazole, ketoconazole and miconazole are approved for topical treatment of genital candidiasis4,6. In a microbial community experiment of VVC patients, it was found that imidazole drugs (econazole, clotrimazole, miconazole, and ketoconazole) were sensitive to isolated strains at lower concentrations (MIC ≤ 1 μg/ml), which means that these drugs are effective in 1 μg/ml or lower, the growth of the majority of tested fungal strains can be inhibited, while other drugs have no effect on bacterial activity at this concentration7,8. The most common AEs of imidazole drugs include skin itching and allergic reactions. Due to insufficient research on the safety of azole drugs in reproductive development, we cannot completely rule out the possibility that they may lead to unknown adverse reactions related to reproductive development. Therefore, we used the FDA Adverse Event Reporting System (FAERS) database for pharmacovigilance analysis to better understand the adverse effects and relevance of imidazole drugs6,9. FAERS is one of the largest AE databases designed to support the FDA’s post-marketing safety oversight program for approved drugs and biologics10,11. Data mining techniques, such as signal detection algorithms, are increasingly being used to explore medical databases and analyze vast amounts of accumulated data to identify potential associations between drugs and AEs that may evade detection in clinical trials12.
Methods
Data sources
The data for this study was retrieved from the publicly available FAERS database, which follows the International Conference on Harmonization (ICH E2B) guidelines for international safety reporting13. Due to the voluntary nature of the report, the reporter may not be able to provide complete patient information, medication use history, and details of adverse events. In addition, adverse reactions may be related to the underlying condition being treated, caused by taking other medications at the same time, or occur for other reasons. The information in these reports reflects only the reporter's observations and opinions. The subjective judgment and interpretation of the reporter may also have an impact on the accuracy of the data. Therefore, when using FAERS data for causal relationship assessment, it is necessary to carefully review and verify the data. Classification and Standardization of AEs in FAERS are according to the Medical Dictionary of Regulatory Activities (MedDRA)14. We used the open tool OpenVigil 2.1 (https://openvigil.sourceforge.net/) to query the FAERS database. OpenVigil 2.1 is a tool for extracting, cleaning, mining, and analyzing pharmacovigilance data in FAERS15. FAERS data from the first quarter of 2004 to the third quarter of 2022 are currently included. In the OpenVigil database, the generic names of the target drugs were clotrimazole, econazole, ketoconazole and miconazole, gender was restricted to female, and we searched for AEs related the four drugs.
Data mining
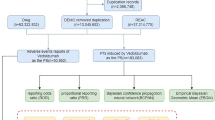
Report odds ratio (ROR)16 and Proportion report ratio (PRR)17 were used for data detection; the larger the ROR and PRR values, the stronger the signal, indicating a stronger statistical relationship between the target drug and AEs18. ROR and PRR are both frequency methods, which can estimate relative risk and reduce bias due to the selection of control groups. They are characterized by simple calculation and good consistency of results. The Bayesian Confidence Propagation Neural Network19 adopts a neural network supervised learning method, using known adverse drug reactions as a machine learning training set, and the detected adverse reactions have a strong correlation20. We used the above three methods as conditions for screening imidazole effective signal generation (Supplementary Table S1).
Informed consent
All of our authors have read the final manuscript and agree to submit it.
Results
Descriptive characteristics
In the FAERS database, each report is encoded using the preferred term (PT) in MedDRA21. A given PT can be assigned to one or more High Level Term, High Level Group Term, and System Organ Class (SOC) levels in MedDRA. We used MedDRA 25.1 to standardize the analysis of adverse drug event signals and analyze SOC and PT according to the case report in the database.
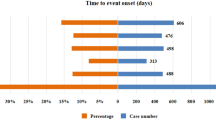
We collected a total of 23, 169 AE reports. After excluding product problems and manual exclusion of unrelated AEs, the number of AEs reported with positive signals screened according to the inclusion criteria was clotrimazole 2256, econazole 490, ketoconazole 1901 and miconazole 5338. Clotrimazole accounted for 22.59%, econazole for 4.91%, ketoconazole for 19.04%, and miconazole for 53.46% (Fig. 1). The age distribution of patients with AEs was mainly in those aged ≥ 19 years: 19–39 years (2169 cases, 21.72%), 45–59 years (2171 cases, 21.74%), and ≥ 60 years (1948 cases, 19.51%). Severe AEs accounted for 31.92% (3187 cases) of the total AEs: Clotrimazole: 1407/2256 (62%); Miconazole: 740/5338 (13%); Ketoconazole: 675/1901 (35%); Econazole: 365/490 (74%), (Table 1).
Drug safety signals
We screened developmental toxicity keywords as low level terms and obtained 312 developmental toxicity keywords (Supplementary Table S2). Renal and urinary disorders were mapped based on the classification in MedDRA. We discovered some unexpected important safety signals. Renal failure has been found as an AE for various drugs, and includes chronic kidney disease and acute kidney injury. We found that patients using clotrimazole were more likely to develop bladder transitional cell carcinoma [291.66 (613.67, 138.62)], and fetal death [10.28 (24.92, 4.24)] based on ROR signal strength. Patients using ketoconazole were more likely to develop nephrogenic anemia [22.1 (59.36, 8.23)] and premature rupture of membranes [22.91 (46.45, 11.3)]. Miconazole was more likely to cause hematuria [19.03 (30.48, 11.88)], neonatal sepsis [123.71 (274.76, 55.7)] and spontaneous abortion [5.98 (16.02, 2.24)]. Econazole was more likely to cause acute kidney injury [4.41 (9.97, 1.95)] and spontaneous abortion [19.62 (41.34, 9.31)] in clinical trials. These results indicate that these drugs can cause nephrotoxicity in clinical trials, and we should pay attention to related AEs (Table 2).
AE signal detection of drugs
In this study, we conducted signal detection on targeted drugs to identify specific clinical cases associated with AEs related to clotrimazole, econazole, miconazole and ketoconazole. We found 9985 AE signals, mainly involving 23 SOCs. The SOC signals of the four imidazole drugs mainly included vascular disorders; skin and subcutaneous tissue disorders; Respiratory, thoracic and mediastinal disorders; renal and urinary disorders; psychiatric disorders; pregnancy, puerperium and perinatal conditions; reproductive system and breast disorders; and so on. Among them, renal and urinary disorders; pregnancy, purperium and perinal conditions signals were found in the top ten SOC distributions, indicating that paying attention to related adverse reactions is a key issue for future research (Fig. 2).
Relationship between main AE signals detection and SOCs
According to the top ten SOC signals, AEs signals are detected according to PT, and the AEs of all detected signals are sorted by the top 50 in order of relevant intensity (lower limit of the 95% CI of the ROR estimates ). For clotrimazole, the strongest signal [ROR = 15.79 (10.12, 24.65)] and highest frequency (40 cases) were for chronic kidney disease, followed by acute kidney injury [ROR = 10.45 (6.59, 16.57)] and renal failure [ROR = 8.66 (5.40, 13.90)] (Fig. 3). Clotrimazole had a strong positive signal for kidney damage in clinical applications. A pharmacological study found that long-term administration of large amounts of clotrimazole mainly manifested as damage to the adrenal cortex and liver12. This suggests that clotrimazole may cause nephrotoxicity in clinical practice.
For econazole, there was a large signal intensity for skin and subcutaneous tissue diseases, among which scarlatiniform rash had the strongest signal [ROR = 47 496.48 (14 946.01, 150 937.63)], followed by nail poisoning [ROR = 3423.23 (1062.81, 11 025.99)] (Fig. 3). Spontaneous abortion occurred most frequently (nine cases) [ROR = 19.62 (9.31, 41.34)], suggesting that skin and reproductive toxicity were the primary concerns with use of econazole.
For ketoconazole, the AEs with the highest frequency were chronic kidney disease [ROR = 11.64 (7.0, 19.37)], followed by alopecia [ROR = 7.88 (5.52, 11.31)] (Fig. 3). This suggests that ketoconazole causes a range of skin and subcutaneous tissue diseases, and nephrotoxicity is also a possibility.
Miconazole mainly caused diseases of the reproductive system and breast and diseases of the skin and subcutaneous tissue (Fig. 3). The most severe AE was vulvar edema [ROR = 1043.36 (636.18, 1803.50)], and the most frequent was vulvovaginal burning [ROR = 842.44 (579.19, 1276.23)]. Miconazole is particularly effective against Candida, and can be used for systemic treatment16,30.
Comparing drug information leaflets
We sorted AEs signals of imidazoles according to ROR signal values by comparing drug information leaflets (Table 3) and analyzed positive signals (Supplementary Table 3).We found that cotrimoxazole causes neurological disease such as post-viral fatigue syndrome [2282.44 (10 236.93, 508.9)], and immune system diseases such as endocrine ophthalmopathy [464.68 (1114.93, 193.67)]. Econazole causes nail toxicity [3423.23 (11 025.99, 1062.81)] and adrenal insufficiency [166.33 (316.97, 87.28)]. Miconazole causes increased cortisol [625.29 (1388.52, 281.58)], hypothalamic pituitary adult axis suppression [1012.69 (2354.84, 435.5)], and antiphospholipid antibiotics [428.41 (1122.53, 163.5)]. Ketoconazole can lead to Jarisch–Herxheimer reaction [543.61 (1912.59, 154.51)].
Discussion
Mucosal fungal infections are common clinically and can usually be adequately treated with azole compounds22,23. Azoles are a class of promising antifungal drugs that have been widely used in clinical practice24.
At present, there is no research indicating that exposure to imidazole drugs can lead to fetal developmental toxicity and renal toxicity. To investigate potential safety issues, we conducted a two-step analysis of the data. First, we screened and obtained 312 developmental toxicity keywords (Supplementary Table S2). And classify kidney and urinary system diseases based on MedDRA. Next, we conducted SOC distribution on the 9985 AE signals discovered, and found that they mainly involve 23 SOC. Our Renal and urinary disorders; pregnancy, purperium and perinal conditions signals. Were found in the SOC of all four drugs. Next, we analyzed the top 50 PTs from the top 10 SOCs. Then, we analyzed the top 50 PTs for each drug data ranking and segmented the data by age group: 0–18, 19–39, 40–59 and ≥ 60 years. We screened the Lowest Level Clotrimazole AEs included bladder transitional cell carcinoma [291.66 (613.67, 138.62)] and fetal death [10.28 (24.92, 4.24)]. Ketoconazole AEs included nephrogenic anemia [22.1 (59.36, 8.23)] and premature rupture of membranes [22.91 (46.45, 11.3). Miconazole AEs included hematuria [19.03 (30.48, 11.88)], neonatal sepsis [123.71 (274.76, 55.7)] and spontaneous abortion [5.98 (16.02, 2.24)]. Econazole AEs included acute kidney injury [4.41 (9.97, 1.95)] and spontaneous abortion [19.62 (41.34, 9.31)]. The imidazole drugs were analyzed by screening the top 50 PTs based on ROR intensity. Clotrimazole was associated with neurological disorders, such as laryngotracheal edema [2282.44 (10, 236.93, 508.9)], and immune system diseases, such as endocrine ophthalmopathy [464.68 (1114.93, 193.67)]. Econazole can cause nail toxicity [3423.23 (11, 025.99, 1062.81)] and adrenal insufficiency [166.33 (316.97, 87.28)]. Miconazole can cause increased cortisol [625.29 (1388.52, 281.58)], hypothalamic pituitary adrenal axis suppression [1012.69 (2354.84, 435.5)] and antiphospholipid antibodies [428.41 (1122.53, 163.5)]. Ketoconazole can cause Jarisch–Herxheimer reaction [543.61 (1912.59, 154.51)].
In comparison with our study, it was found that the frequency of adverse drug reactions was partially consistent with the adverse events observed for various drugs25. In this study, we conducted a retrospective study of azole drugs in the FAERS database, and detected the major AEs, with particular monitoring of their associated reproductive toxicity. Continued attention should be given to exploring how to use the clinical characteristics of AEs to provide early warning for post-marketing of drugs.
Limitations
This study had several limitations that must be acknowledged. First, we have selectively selected the first four imidazole drugs that combine safety and efficacy, and may have missed the adverse reactions associated with other imidazole drugs. Second, FAERS is a passive and spontaneous AE database that receives voluntary reports from undefined populations9,26. Some studies claim that only 5% of serious AEs are actually submitted27. Case reports are often incomplete, missing, or duplicated12. At the same time, some information that would have allowed more detailed analysis of each study is missing28,29,30,31. Finally, the AEs may have been related to the underlying disease being treated, or caused by some other drug being taken concurrently, or occurred for other reasons. The subjective judgment and interpretation of the reporters may also have an impact on the accuracy of the data. Therefore, when using FAERS data for causal relationship assessment, it is necessary to carefully review and verify the data.
Conclusions
This study utilized the FAERS database to analyze potential AEs of representative drugs used for the treatment of VVC, and collected renal and developmental toxicity of imidazole drugs in clinical practice. These findings emphasize the importance of monitoring rare but severe AEs associated with imidazole drug use. At the same time, by comparing the instructions, it was found that AEs were not recorded in the instructions. Although the overall incidence of these events may be low, their clinical significance deserves careful consideration, especially in pregnant women or vulnerable populations with existing renal dysfunction. Healthcare providers must always be aware of the latest safety information about these drugs and adjust treatment decisions accordingly to minimize potential risks to patients. Long-term observation of these AEs is required to ensure the health and safety of patients treated with these drugs32.
Data availability
This study utilized the FDA Adverse Event Reporting System (FAERS) database provided by the FDA. The dataset generated and analyzed during this study is available from the corresponding author upon reasonable request.
References
Fidel, P. L. Jr. Vaginal candidiasis: Review and role of local mucosal immunity. AIDS Patient Care STDS 12, 359–366. https://doi.org/10.1089/apc.1998.12.359 (1998).
Fong, I. W. Clinical and cost considerations in the pharmacotherapy of vulvovaginal candidiasis. PharmacoEconomics 9, 497–505. https://doi.org/10.2165/00019053-199609060-00004 (1996).
Osman Mohamed, A. et al. Prevalence of vulvovaginal candidiasis among pregnant women in Africa: A systematic review and meta-analysis. J. Infect. Dev. Ctries. 16, 1243–1251. https://doi.org/10.3855/jidc.15536 (2022).
Farr, A. et al. Guideline: Vulvovaginal candidosis (AWMF 015/072, level S2k). Mycoses 64, 583–602. https://doi.org/10.1111/myc.13248 (2021).
Richter, S. S. et al. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J. Clin. Microbiol. 43, 2155–2162. https://doi.org/10.1128/jcm.43.5.2155-2162.2005 (2005).
Zhang, L., Peng, X. M., Damu, G. L., Geng, R. X. & Zhou, C. H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 34, 340–437. https://doi.org/10.1002/med.21290 (2014).
Tian, X. et al. Adverse event profiles of PARP inhibitors: Analysis of spontaneous reports submitted to FAERS. Front. Pharmacol. 13, 851246. https://doi.org/10.3389/fphar.2022.851246 (2022).
Fromtling, R. A. Overview of medically important antifungal azole derivatives. Clin. Microbiol. Rev. 1, 187–217. https://doi.org/10.1128/cmr.1.2.187 (1988).
Lee, Y. K. et al. Antidepressants-related cardiovascular adverse events using the adverse event reporting system. Psychiatry Res. 268, 441–446. https://doi.org/10.1016/j.psychres.2018.07.044 (2018).
Goldman, S. A. Limitations and strengths of spontaneous reports data. Clin. Ther. 20 (Suppl C), C40-44. https://doi.org/10.1016/s0149-2918(98)80007-6 (1998).
Kashoki, M., Lee, C. & Stein, P. FDA oversight of postmarketing studies. N. Engl. J. Med. 377, 1201–1202. https://doi.org/10.1056/NEJMc1709185 (2017).
Wilson, A. M., Thabane, L. & Holbrook, A. Application of data mining techniques in pharmacovigilance. Br. J. Clin. Pharmacol. 57, 127–134. https://doi.org/10.1046/j.1365-2125.2003.01968.x (2004).
Savarirayan, R. et al. Infigratinib in children with achondroplasia: The PROPEL and PROPEL 2 studies. Ther. Adv. Musculoskelet. Dis. https://doi.org/10.1177/1759720x221084848 (2022).
Simon, T. A., Simon, J. H., Heaning, E. G., Gomez-Caminero, A. & Marcu, J. P. Delta-8, a cannabis-derived tetrahydrocannabinol isomer: Evaluating case report data in the Food and Drug Administration Adverse Event Reporting System (FAERS) Database. Drug Healthc. Patient Saf. 15, 25–38. https://doi.org/10.2147/dhps.S391857 (2023).
Böhm, R., Höcker, J., Cascorbi, I. & Herdegen, T. OpenVigil-free eyeballs on AERS pharmacovigilance data. Nat. Biotechnol. 30, 137–138. https://doi.org/10.1038/nbt.2113 (2012).
Noguchi, Y., Tachi, T. & Teramachi, H. Comparison of signal detection algorithms based on frequency statistical model for drug–drug interaction using spontaneous reporting systems. Pharmaceut. Res. 37, 86. https://doi.org/10.1007/s11095-020-02801-3 (2020).
Evans, S. J., Waller, P. C. & Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 10, 483–486. https://doi.org/10.1002/pds.677 (2001).
van Puijenbroek, E., Diemont, W. & van Grootheest, K. Application of quantitative signal detection in the Dutch spontaneous reporting system for adverse drug reactions. Drug Saf. 26, 293–301. https://doi.org/10.2165/00002018-200326050-00001 (2003).
Robert, M., Jouanjus, E., Khouri, C., Fouilhé Sam-Laï, N. & Revol, B. The opioid epidemic: A worldwide exploratory study using the WHO pharmacovigilance database. Addiction (Abingdon, England) 118, 771–775. https://doi.org/10.1111/add.16081 (2023).
Szarfman, A., Machado, S. G. & O’Neill, R. T. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 25, 381–392. https://doi.org/10.2165/00002018-200225060-00001 (2002).
Novitt-Moreno, A. et al. Tafenoquine for malaria prophylaxis in adults: An integrated safety analysis. Travel Med. Infect. Dis. 17, 19–27. https://doi.org/10.1016/j.tmaid.2017.05.008 (2017).
Chopra, V. et al. Prophylactic strategies in recurrent vulvovaginal candidiasis: A 2-year study testing a phytonutrient vs itraconazole. J. Biol. Regul. Homeostat. Agents 27, 875–882 (2013).
Harriott, M. M. & Noverr, M. C. Importance of candida-bacterial polymicrobial biofilms in disease. Trends Microbiol. 19, 557–563. https://doi.org/10.1016/j.tim.2011.07.004 (2011).
Pérez-Cantero, A., López-Fernández, L., Guarro, J. & Capilla, J. Azole resistance mechanisms in Aspergillus: Update and recent advances. Int. J. Antimicrob. Agents 55, 105807. https://doi.org/10.1016/j.ijantimicag.2019.09.011 (2020).
Patel, V. M., Schwartz, R. A. & Lambert, W. C. Topical antiviral and antifungal medications in pregnancy: A review of safety profiles. J. Eur. Acad. Dermatol. Venereol. JEADV 31, 1440–1446. https://doi.org/10.1111/jdv.14297 (2017).
Woods, R. H. Potential cerebrovascular accident signal for risankizumab: A disproportionality analysis of the FDA Adverse Event Reporting System (FAERS). Br. J. Clin. Pharmacol. 89, 2386–2395. https://doi.org/10.1111/bcp.15581 (2023).
Bégaud, B., Martin, K., Haramburu, F. & Moore, N. Rates of spontaneous reporting of adverse drug reactions in France. Jama 288, 1588. https://doi.org/10.1001/jama.288.13.1588 (2002).
Qian, J., Truong, C. B. & Tanni, K. A. Author's Reply to Joerg Putzke et al. Comment on: "Safety of marketed cancer supportive care biosimilars in the US: A disproportionality analysis using the Food and Drug Administration Adverse Event Reporting System (FAERS) database. BioDrugs Clin. Immunother. Biopharmaceut. Gene Therapy 35, 375–377 https://doi.org/10.1007/s40259-021-00474-x (2021).
Zhang, K. W. & Guha, A. Cardiovascular events in men with prostate cancer receiving hormone therapy: An analysis of the FDA Adverse Event Reporting System (FAERS). Reply. J. Urol. 207, 243. https://doi.org/10.1097/ju.0000000000002289 (2022).
Mazhar, F. et al. Association of hyponatraemia and antidepressant drugs: A pharmacovigilance-pharmacodynamic assessment through an analysis of the US Food and Drug Administration Adverse Event Reporting System (FAERS) database. CNS Drugs 33, 581–592. https://doi.org/10.1007/s40263-019-00631-5 (2019).
He, L. et al. Characteristics and spectrum of cardiotoxicity induced by various antipsychotics: A real-world study from 2015 to 2020 based on FAERS. Front. Pharmacol. 12, 815151. https://doi.org/10.3389/fphar.2021.815151 (2021).
Akapo, O. O. et al. In silico structural modeling and analysis of interactions of tremellomycetes cytochrome P450 monooxygenases CYP51s with substrates and azoles. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22157811 (2021).
Funding
This paper was funded in part by Clinical Toxicology of Chinese Society of Toxicology [number CST2021CT101].
Author information
Authors and Affiliations
Contributions
Conception and design: TY Z. and CZ C. Data analysis: XW C. and TY Z. Interpretation of the data and drafting the paper: TY Z. and CZ C. Critical revision of the paper for intellectual content: CZ C. F S. HT J. and D L. Writing review and editing work: B W. and WF L. The final manuscript was read, checked and approved by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, T., Chen, C., Chen, X. et al. Possible adverse events of imidazole antifungal drugs during treatment of vulvovaginal candidiasis: analysis of the FDA Adverse Event Reporting System. Sci Rep 14, 14560 (2024). https://doi.org/10.1038/s41598-024-63315-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-63315-1