Abstract
A composite of Zinc oxide loaded with 5-weight % silver decorated on carbon nanotubes (Ag-loaded ZnO: CNT) was synthesized using a simple refluxed chemical method. The influence of deviation in the weight % of carbon nanotube loading on photocatalytic dye degradation (methylene blue and rose bengal) and antibiotic (antimicrobial and antifungal) performance was investigated in this study. The light capture ability of Ag-loaded ZnO:CNT in the visible region was higher in photocatalytic activity than that of Ag-loaded ZnO and ZnO:CNT. The bandgap of the Ag-loaded ZnO: CNT was tuned owing to the surface plasmon resonance effect. The photocatalytic degradation investigations were optimized by varying the wt% in CNTs, pH of dye solution, concentration of the dye solution, and amount of catalytic dose. Around 100% photocatalytic efficiency in 2 min against MB dye was observed for Ag doped ZnO with 10 wt% CNT composite at pH 9, at a rate constant 1.48 min−1. Bipolaris sorokiniana fungus was first time tested against a composite material, which demonstrated optimum fungal inhibition efficiency of 48%. They were also tested against the bacterial strains Staphylococcus aureus, Bacillus cerius, Proteus vulgaris, and Salmonella typhimurium, which showed promising antibacterial activity compared to commercially available drugs. The composite of Ag doped ZnO with 5 wt% CNT has shown competitive zone inhibition efficacy of 21.66 ± 0.57, 15.66 ± 0.57, 13.66 ± 0.57 against bacterial strains Bacillus cerius, Proteus vulgaris, and Salmonella typhimurium which were tested for the first time against Ag-loaded ZnO:CNT.
Similar content being viewed by others
Introduction
The textile industrial zone is thought to be the dominant dye sector and a source of wastewater. The unrestricted use of industrial wastewater contains a variety of chemicals that are damaging to the environment and community health. Substantial volumes of water and dyes are used in broad processing, such as printing and dyeing textiles. They discharge a large amount of sewage, and many of them contain colors that are harmful to the environment and community. Synthetic dyes and other organic pollutants are often used in various industries, including textiles, pulp, letterpress, leather, body cosmetics, plastic, and food1. As a result, eliminating them is vital and challenging. As these dyes separate into aromatic structures, they have lethal, mutagenic, and oncogenic potentials that affect their lives. Moreover, dyes can prevent light from inflowing into aquatic environments and impair aquatic life2. It is vital to confiscate the colors of sewage and discarded water to maintain the network. Methylene blue (MB), which is sometimes referred to as methyl thioninium chloride, and rose bengal (RB) are representative pollutants. They are cationic organic dyes composed of synthetic compounds with strong odors. It is poisonous to all living organisms and causes cancer. It affects the respiratory system and irritates the skin and eyes3,4. It is known that drinking water contains a substantial amount of traces of MB dye, which causes subcutaneous tissue-borne sarcoma in both humans and animals. Dyes have long-lasting effects on aquatic life, especially plants, as they reduce natural purification and photocatalysis by obstructing light diffusion. As a result, the dye must be seized before being released into freshwater5. Owing to its distinctive optical and electrical characteristics, zinc oxide (ZnO), a well-known semiconductor material with a broadband gap (3.37 eV and n-type conductivity, has gained popularity as a photocatalyst. ZnO is a viable option for several applications due to its photocatalytic activity, modest toxicity, strong stability, and affordability6,7. The narrow spectrum range of ZnO, resulting from its wide band gap and high charge carrier recombination rate, limits its photocatalytic efficacy8. As a result, the accumulation of a substantial amount of ZnO is a suitable technique. When an element is added, it can serve as a place to supply electrons or soothe catalysts to materials with a large surface area, which will help with electron transmission in the final composite9,10,11. Sher et.al developed g–C3N4–ZnO composite with Mo, Ag and Sn doping as a promising candiadate to degrade water moieties and bacterial inhibition11,12,13. In our previous study, Ag-loaded ZnO was successfully synthesized for use in clean wastewater. The amalgamation of Ag with ZnO helped in tuning the band gap and prolonged the recombination time of the charge carriers to extend the degradation rate. Since their invention, carbon nanotubes (CNTs) have drawn attention worldwide. Depending on the helicity and tube diameter, they can be semiconducting, semi-metallic, or metallic. The notable properties of multi-walled carbon nanotubes (MWCNTs) include a high aspect ratio, large surface area, strong adsorptive ability, good catalytic activity, outstanding mechanical strength, elasticity, and good electrical and thermal conductivity14. It was assumed that these were sheets of coiled graphene. Because carbon nanotubes (CNTs) are incorporated into host materials to change or improve their properties, CNT-based composites have attracted attention. Owing to their non-polar nature, CNTs are hydrophobic. The π electrons outside the carbon nanotubes create an extremely strong connection. The functionalization process can change the hydrophobic nature of CNTs. The process of attaching individual atoms or molecules to carbon nanotubes is known as the functionalization. Functionalized multi-walled carbon nanotubes (MWCNTs) are widely employed in catalyst synthesis. According to Zeo et al.15, CNT-loaded ZnO blends perform better than pure ZnO in preventing dye degradation. A ZnO:CNT composite was created using a thermal technique to break down carcinogenic acetaldehyde for 60 min while being exposed to laser radiation16. To break down methylene blue, ZnO/N-CNTs were created via a chemical precipitation method, and benzoic acid produced better outcomes17. ZnO/Co–Ni–Al layered CNT composite decomposed the acid red at pH 5.5 and demonstrated an efficiency of 96.2% when exposed to visible light for 120 min18. Hence, CNT’s are promising candidates for photocatalytic degradation of hazardous pollutants19.
In this study, the antifungal and antimicrobial characteristics of Ag-loaded ZnO:CNT were explored. Functionalized multi-walled carbon nanotubes have recently been demonstrated to reduce immunological discomfort in mice and macrophages while also improving colloidal characteristics without compromising their distinctive antibiosis efficacy20. An extensive study of antifungal and antimicrobial agents is required to elucidate the promising characteristics of Ag-loaded ZnO:CNT; hence, following the literature, it was discovered that surface changes in MWCNTs were more effective against Fusarium graminearum21. The cell membrane, which defends bacteria from the severity of the environment, undergoes physicochemical changes resulting in inactivation by the composite material22. When combined with toyocamycin, CNT demonstrated specific toxicity against Candida albicans23. Bacterial degradation is commonly associated with oxidative stress caused by ROS generated by functional materials. Spot blotch infection in wheat is triggered by the fungus, Bipolaris sorokiniana. This disease can seriously harm crops and the economy24. Therefore, CNTs are a promising option for the prevention of fungal and bacterial development19. The water quality is lowered by organic toxic waste and a few other active poisons found in wastewater. The toxins are essentially bits and pieces of bacteria, which cause the pathogenicity of water to increase25,26. With band gap energies ranging from 1.0 to 3.8 eV, numerous metal oxide semiconductor materials are engaged as heterogeneous semiconductor photocatalysts as well as antifungal and antimicrobial-resistant materials27,28. Visible-range band-gap semiconducting materials are critical photocatalysts and antibacterial agents because it is important to use sunlight efficiently to produce energy, sanitize water, and ensure ecological safety. To understand its influence on variations in color, dye concentration, catalytic dose, and pH of the dye solution, we synthesized functionalized carbon nanotubes decorated with Ag-loaded ZnO. The antibacterial effect of Ag-loaded ZnO:CNT was tested against the bacterial strains Staphylococcus aureus (NCIM-2654), Bacillus cerius (NCIM-2703), Proteus vulgaris (NCIM-2813), and Salmonella typhimurium (NCIM-2501). Additionally, we are the first to demonstrate antifungal action on a wheat spot blotch utilizing Ag-loaded ZnO:CNT composites.
Experimental section
Material
In current efforts, analytical-grade compounds have been used in the due course of investigation. Zinc acetate dihydrate (Sisco Research Lab (SRL)), diethylene glycol (Sisco Research Lab (SRL)), silver nitrate (Thomas Baker (Chemicals) Pvt. Ltd.), purity 99.8%, sodium hydroxide pellets (ACROS organics), multi-walled carbon Nano Tube (Monad Nano Tech Pvt. Ltd, India), methylene blue (Sisco Research Lab (SRL)), rose bengal (Molychem), and potato dextrose agar medium (Himedia, Mumbai, India) were commercially purchased. All solutions used in this investigation were prepared using double-distilled water (DDW).
Characterization
The crystal structure of the Ag-loaded ZnO crystal structure was examined by X-ray diffraction (XRD) (D/B max-2400, Rigaku, USA) to ascertain their phase and average crystallite size. The morphology was examined using a JEOL JSM 6360-A field-emission scanning electron microscope (FESEM). Optical analysis was conducted using a UV-visible spectrophotometer (JASCO V-670, Germany), photoluminescence (PL) was observed and recorded using a PL spectrometer (Horiba Fluorolog), the X-ray photoelectron spectroscopy (XPS) was executed on PHI Versaprobe III by using Al Ka X-rays. An OSRAM 300 W halogen lamp was used as the visible light source (emission range: 400–800 nm) for the photocatalytic degradation of the MB and RB dyes.
Preparation of silver loaded zinc oxide: carbon nano tube composites
Initially, we functionalized the already purchased carbon nanotubes by adding sodium lauryl sulfate (SLS) at a ratio of 1:20. The SLS plays quite an essential role in the functionalization of CNTs, upon functionalization of CNTs, the attraction of their surfaces to several solvents and polymer increases. The imperfections on the CNTs shaped by SLS were alleviated by attachment with different functional group formations, as described in our previous work29,30. Furthermore, Ag-loaded ZnO:CNT nanoparticles with (5, 10, 15, and 20) wt% functionalized CNTs was prepared by adding the desired amount of zinc acetate and silver nitrate in double distilled water. The reflux chemical method was implemented in which 1 M zinc acetate and 5 wt% silver nitrate was added to 150 ml distilled water at 80 °C for 2 h. using 20 ml ethylene glycol. This solution was mixed with functionalized CNTs under constant stirring for 2 h at 80 °C using the refluxed chemical method. A separate 2 M sodium hydroxide (NaOH) solution was prepared in 150 ml DDW and poured slowly at a rate of 2 drops per second. to the above solution. The solution was agitated by stirring for 3 h. Consequently, a blackish solution was obtained. The blackish solution was cooled overnight, rinsed, and washed multiple times with ethyl alcohol. The precipitate was then collected and incubated at 70 °C for 24 h. This precipitate was crushed into a fine powder, which was then annealed at 300 °C for approximately 3 h. Thus, Ag-loaded ZnO/CNT nanoparticles were prepared. These Ag-loaded ZnO/CNT samples were labeled as 5CNT, 10CNT, 15CNT, and 20CNT respectively for (5, 10, 15, and 20) wt% of CNT’s.
Antifungal activity measurement
The antifungal activity of the silver-loaded zinc oxide: carbon nanotube was evaluated using the agar diffusion process, as previously described29. To perform this test, potato dextrose agar (PDA) medium was mixed into 0.1 L of double distilled water and boiled until completely dissolved. The PDA and glass Petri dishes were autoclaved for 30 min at 15 Pa of pressure to sterilize them, and the PDA medium was spread into the Petri dishes using a laminar flow chamber. Following the solidification of the medium, 20 mg of the produced material was placed on the plate. The center of the Petri plate was then injected with a fungal disc from Bipolaris sorokiniana29. The potato dextrose agar disc was removed from the center using a sterile cork borer for inoculation. A control without Ag-loaded ZnO:CNT composites was used for comparison with incubation of the plates for 72 h at 25 °C. The growth of the fungal zone was also observed. We measured the diameter of such inhibitors to determine their antifungal activity using the poisoned food approach31.
Antimicrobial activity measurement
During the water refinement process, several supplementary toxins and organic pollutants are present in sewage. These toxic materials contain bits and pieces of microorganisms, and it was previously discovered that organic metals are primarily responsible for the proliferation of dangerous bacteria in water32. Consequently, it was discovered that photocatalysts possess the potential to allow microbial pollutants to reside on them33. Examining the antibacterial activity of Ag-loaded ZnO:CNT (50 µg/mL) and commercial antibiotics (50 µg/mL), a notable zone of inhibition was found. Microbial cultures were cultivated in inocula using a sterile saline solution. Nutrient agar plates were used as substrates for the development of bacteria. Staphylococcus aureus was allowed to grow on sterile savored agar plates; similarly, Bacillus cereus, Proteus vulgaris, and Salmonella typhimurium cultures were allowed to thrive on clean nutrient agar plates. Powdered 5CNT, 10CNT, 15CNT, and 20CNT were mixed with sterile distilled water and dimethyl sulfoxide (DMSO) using a micropipette. To track the antibacterial activity, the plates were incubated for twenty-four hours at 37 °C34.
Photodegradation activity
We generated dye solutions of various concentrations in DDW to evaluate the photocatalytic degradation activity, and the sample was investigated using a UV-visible study, which yielded the absorbance of an unadulterated dye solution of a given concentration. A fixed number of catalyst samples were combined with MB and RB dye solutions. The solutions were held in a gloomy environment for 30 min with regular agitation to avoid light interactions and to sustain adsorption/desorption symmetry among the photocatalyst surfaces and dye molecules35. The solutions were then exposed to visible light to evaluate their photocatalytic degradation ability. Small samples were taken at regular intervals for UV-Vis measurements until the total disintegration of the dye solution was observed. The fading of the characteristic absorbance peak from the UV-Vis experiments confirmed dye degradation. By pulling out aliquots for UV-Vis spectroscopic measurement at time intervals of 5 min for each sample, the absorbance maxima of MB and RB were observed at 664 nm and 546 nm, respectively.
Results and discussion
X-ray diffraction analysis
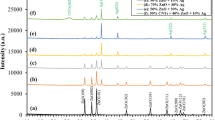
Figure 1 shows the X-ray diffraction pattern array of the 5 wt% Ag loaded ZnO composed with (5 to 20) wt% CNT’s. The major ZnO peaks have hexagonal phases, which is in good agreement with JCPDS Card No. 653411 for all samples (indicated by # mark in Fig. 1). As we have amalgamated Ag with ZnO, the (111) and (200) peaks (indicated by * mark in Fig. 1) corresponding to Ag are observed with low-intensity minor phase development, as reported in our previous work36. Silver was found in interstices as well as in substitutional Zn sites, as reported elsewhere. The existence of the (002) carbon peak was (indicated by □ mark in Fig. 1) also observed in all the samples owing to composite formation with CNTs. Other diffraction peaks show no significant change as a result of CNT integration, which is consistent with the physical interaction of CNT with the Ag-ZnO array37. The integration of CNTs into Ag-loaded ZnO precludes the secondary phase formation in the XRD configuration of the materials. The Scherrer equation was employed to calculate the microstrain, dislocation density, and average crystallite size for the (100), (002), (101), (102), (110), (103), (200), and (112) peaks of ZnO, as summarized in Table 1.
The microstrain and dislocation density were the highest for 10CNT, and the mean crystallite size was 19 nm for 10CNT. This could have been caused by the occurrence of silver ions in the ZnO array hindering the formation of crystal grains, and resistance to grain boundary growth was created, possibly as a result of the Zinner-Pinning effect38.
Optical analysis
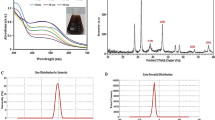
The absorption spectra of the Ag-loaded ZnO/CNT composites are shown in the inset of Fig. 2a. Owing to the relocation of charges from the valence band to the conduction band of ZnO, a strong absorption edge was observed in the UV region (200–420 nm)6,39. As shown in Fig. 2a, the Tauc plot was used to measure the bandgap energy (Eg) of the composites. The bandgap energies (Eg) of 5CNT, 10CNT, 15CNT, and 20CNT were found to be around 3.25 eV at the absorbance maxima obtained of 359 nm. The accumulation of silver ions produces substantial alterations in the absorption spectra of ZnO, causing high absorbance in the complete visible spectrum in the form of a bulge, owing to the surface plasmon band40. The increase in luminescence can be caused by surface plasmon scattering4. At the interface of Ag and ZnO in the array, the localized electric field associated with metal ions is amplified, inducing surface plasmon excitation in the Ag nanoparticles positioned at the interface, which enhances the visible absorption of light photons41. As a result, the existence of Ag nanoparticles on ZnO materials causes an increase in visible light capture due to the plasmon band on the Ag surface42.
The conduction band potential and valance band potential of Ag-ZnO were measured by using the Mulliken electronegativity theory13
where, X is the absolute electronegativity of the semiconductor and for ZnO it is 5.76 eV (vs NHE), Eg is the band gap energy of the semiconductor, EVB is the valence band edge potential of semiconductor and Ee is the energy of free electrons and it is ~ 4.5 eV (vs NHE) and ECB shows the conduction band edge potential43. The calculated values of valence band and conduction band potentials were 2.88 eV (vs NHE) and − 0.37 eV (vs NHE) respectively. Additionally, the work functions of Ag and ZnO are 4.3 and 5.2 eV, respectively; thus, the transmission of an electron from Ag to ZnO is suitable, which is in good agreement with photoluminescence spectroscopy studies44,45. The work function of functionalized MWCNT is 5.1 eV46. Also, it is demonstrated that MWCNT turns to be photosensitizer by captivating light so that they get excited to higher energy level leading to electron transfer.
Photoluminescence spectroscopy is a broad technique used to investigate the reunion of light-induced charge carriers in semiconductors47,48. The simultaneous recombination of photogenerated electron–hole pairs and radiation produces PL spectra with small PL intensity, indicating a sluggish reunion rate12 Here, we recorded the PL spectra of all composite samples under ambient thermal conditions, as shown in Fig. 2b, when excited at 320 nm. The PL spectrum showed stronger absorption at 380 nm and poorer absorption at 354 nm in the UV region49,50. The emission characteristics of all samples are comparable, with the peak at approximately 530 nm showing lowered intensities due to the transfer of electrons from the Zni level positioned beneath the conduction band to the valence band, signifying quenching of PL emission51. The plasmonic absorption of Ag nanoparticles may be responsible for the decrease in the visual emission intensity of the catalysts49,50,52,53. UV radiation due to near-band emission can be initiated by the wide band gap of ZnO. Furthermore, a couple of frail bands in the visible area near 430–440 nm could be caused by surface flaws of ZnO nanoparticles and bound pairs of charge carriers. Hence, it extends its support to the notion of integration of Ag nanoparticles and CNTs, which increases the lifetime of photogenerated charge carriers and subsequently raises the photocatalytic activity owing to lowered surface deficiencies in the Ag-loaded ZnO:CNT composite structure.
FT-IR spectra depict information about a specific substance, functional groups, molecular shape, and inter-/intramolecular interactions. We employed the room-temperature KBr pellet method to study the FT-IR spectra of the 5CNT, 10CNT, 15CNT, and 20CNT nanocomposites, as depicted in Fig. 2c. The spectra were run over the wave number range of 400–4000 cm−1. The absorption band between 2850 and 2920 cm−1 was found to be an asymmetric stretching mode for the C-H bond, which may be caused by sodium lauryl sulfate residues. Figure 2d shows the EDS analysis of 5CNT, 10CNT, 15CNT, and 20CNT composites.
The absorption band at 2350 cm−1 was discovered to correspond to CO2. In addition, the H–O–H bending vibration of water was observed at 1530 cm−1, while the band near 1030 cm−1 represented the asymmetric stretching of C–O54. The vibration modes of Zn–O correspond to the bands in the low wavenumber area of 435 cm−155. This implies that the hydroxyl and carboxylic groups present on the exterior of the catalysts improve photocatalytic activity56.
Morphological analysis
FESEM and EDS studies
FESEM images of the 5CNT, 10CNT, 15CNT, and 20CNT composites at 500 nm magnification are shown in Fig. 3. It is found that there is an agglomeration of Ag-loaded ZnO nanoparticles displaying cauliflower-like morphology. Each FESEM micrograph shows varying particle sizes ranging from 60 to 90 nm, which may be due to the change in the weight percentage contribution of CNT to the material. Larger particles occur owing to the agglomeration effect and the concentration of nucleation sites. The average particle size and diameter of the CNT calculated using the FESEM images were approximately 75 nm and 80 nm, respectively.
Figure 2d. shows the EDS spectrum for 5CNT, 10CNT, 15CNT and 20CNT. The composition and distribution of Ag-loaded ZnO:CNT composite nanoparticles are indicated by EDS mapping, which shows the even distribution of CNTs in the Ag Ag-loaded ZnO array. The spreading of the Zn and oxygen peaks in the EDS mapping confirms the purity of ZnO. The presence of Ag and carbon peaks in the Ag-loaded ZnO:CNT arrangement supplements the composite development in the matrix57.
X-ray photoelectron spectroscopy (XPS) studies
Elemental composition and oxidation states of Ag doped ZnO/(10%)CNT were studied with the help of x-ray photoelectron spectroscopy (Fig. 4). As shown in Fig. 4a, the C 1s spectrum shows two peaks observed at binding energies of 284.4, and 285.4 eV that can link to C=C, and C–C respectively58,59. The peak at 284.4 is due to sp2 hybridized carbon atoms whereas different functional groups such as carboxyl and hydroxyl are attached to the surface of CNT are responsible for the peak at higher binding energy of 285.4 correspond to the sp3 hybridized carbon atoms60. Moreover, Ag 3d spin–orbit coupling is apparent from Fig. 4b, where two discrete peaks observed at binding energies 368.3 and 374.3 eV accredited to Ag 3d5/2 and Ag 3d3/2, respectively. Both peaks have 6 eV difference in their binding energies approves the presence of the Ag in the metallic nature over the surface of the ZnO61. Zn with 2+ valance state in ZnO is confirmed with Fig. 4c displaying the two binding energy peaks of Zn-2p3/2 and Zn-2p1/2 with energy difference of 23.1 eV corresponding to spin–orbit doublet. Also the O1s spectrum shown two peaks at 530.2 and 531.7 eV (Fig. 4d). The peak at 530.2 eV was due to O2- ions attached with zinc in ZnO crystal structure, and the peak at 531.7 can be attributed to oxygen deficiencies or presence of hydroxyl and carboxyl group due to surface defects62.
Photocatalytic studies
The photocatalytic activity of the synthesized nanohybrid catalysts was initially studied in the presence of visible light with 10 ppm methylene blue (MB) and 10 ppm rose bengal (RB) as symbolic pollutants, as shown in Figs. 5 and 6. In the current study, we prepared an array of stock solutions of MB and RB dyes in 1000 ml DDW and stored them in the dark. Catalysts 5CNT, 10CNT, 15CNT, and 20CNT have shown dye disintegration efficiencies of 78%, 99%, 98%, and 93% within 20 min of visible light irradiation against 10 ppm MB dye solution, as shown in Fig. 7a and c. Similarly, 80%, 99%, 91%, and 74% dye degradation efficiency within 20 min of visible light irradiation against 10 ppm RB dye solution, respectively, as shown in Fig. 7b and d. The photocatalytic degradation efficiency was calculated using Eq. (3)63.
where C0 is the absorbance at time’ t = 0 min and Ct is the absorbance at time ‘t’ min.
Thus, the 10CNT catalysts showed optimized results for the disintegration of all dyes. The stability of 10CNT photocatalysts was studied by repeating the degradation studies, which have prolifically shown 91% efficiency even after four reusability cycles. The variation in the pH value of the dye solution was also explored during this study for the 10 ppm MB dye solution, and the photocatalytic degradation experiment was performed with pH values ranging from 6 to 10. We also carried out variations in catalytic doses from 0.6 g/liter, 0.8 g/liter, 1 g/liter, and 1.2 g/liter loading of photocatalysts against 10 ppm MB dye solutions at an optimum pH value-9 in the presence of visible-light irradiation. A spectroscopic study of fixed time interval samples was carried out using UV-Vis spectroscopy, which revealed that the preliminary absorption peak at 664 nm gradually declined with time. This indicates that the MB dye solution crumbled into constituents such as CO2, H2O, and other bland organic waste products64 (Table 2).
The proposed mechanism of the electron–hole pair generation and photocatalytic degradation by Ag doped ZnO:CNT is as shown below29,30.
Parameter variation in photocatalytic studies
In this study, we explored aspects such as variations in dye concentration, pH, and catalytic dose to optimize these parameters. The dye concentration was optimized by studying 10, 30, and 50 ppm solutions of MB dye against 5CNT, 10CNT, 15CNT, and 20CNT catalysts. It was observed that with an increase in the concentration of the dye, there was an upsurge in the time required for photocatalytic disintegration, as shown in Fig. 8a,b, with significant variances as matched with 30 ppm and 50 ppm solutions. Thus, the dye concentration is inversely proportional to the dye degradation efficiency of the catalyst. The catalyst concentration inhibited dye degradation. The 10CNT catalysts have shown 99% degradation efficiency at an MB dye concentration of 10 ppm. Based on this information, we tried varying the pH and catalytic dosage of the 10 ppm MB dye solution against 10CNT catalysts.
The consequence of the variation in the pH of 10 ppm MB dye solution on the disintegration performance was calculated using 100 mg of 10CNT catalyst, as displayed in Fig. 8c. The initial pH of the MB solution was ~ 6.9. With an increase in pH, there was considerable reduction in photocatalytic degradation time Here, the pH for MB was varied from 6 to 10, showing the best result at pH 9, with 100% degradation within 2 min of visible light irradiation65.
Hence, we performed photocatalytic degradation of MB dyes using four different weight ratios of the 10CNT catalysts, that is, 0.6, 0.8, 1, and 1.2 g g/L at a modified pH of 936. As shown in Fig. 8d, the MB dye gave the best results at 1 g/liter for 10CNT. In this study, we observed that an increased catalytic dose was responsible for the improved degradation efficiency because it provided additional reaction locations for the dye molecules66. This effect may be caused by the snooping of the radiation flowing into the reaction mixture, which is attributed to the upsurge in the catalyst dose67. This might also be due to the inflated quantity of hydroxyl and superoxide radicals produced by increasing the catalyst mass. Beyond the 1 g/L catalytic dose, no significant change in the photocatalytic dye degradation performance was observed.
Repeated use of the 10CNT catalysts was tested by accumulating the catalyst mass after the dye disintegration reaction was completed. The samples were cleaned and filtered multiple times using ethanol and DDW. To verify the reusability of the collected samples, they were dried and used again under the same experimental conditions. Thus the 10CNT sample sustained up to four cycles, showing 91%efficiency against MB dye even at the end of the fourth cycle.
Antifungal activity
In this study, we evaluated the antifungal activity of the phytopathogenic fungus Bipolaris sorokiniana, which causes spot blotch infections in wheat crops. Potato dextrose agar (PDA) is a regularly used medium for fungal culture, making it an appropriate substrate for testing the antifungal activity of catalysts. PDA is composed of potato infusion and dextrose, both of which supply nutrients for fungal development. The 5CNT, 10CNT, 15CNT, and 20CNT catalysts were added to the media at a specified concentration to test the antifungal activity. The medium was subsequently injected with fungal spores and fungal development was tracked over time. The antifungal activity of the substance under test is determined by the suppression of fungal growth, which manifests as a lack of apparent growth or a reduction in the number of fungal colonies. These findings suggest that Ag-loaded ZnO has substantial potential as a substitute for synthetic fungicides in the management of plant diseases36. The photo-induced generation of ROS and the poisoning effect due to Zn2+ release are the two key contributors to the antifungal performance of ZnO nanoparticles. The mechanism of action of Ag-loaded ZnO:CNT against fungi involves the creation and accumulation of ROS and free radicals, which primarily disturb the cell wall, surface proteins, and nucleic acids of the fungi. In addition, they block proton pumps. This suggests that Ag-loaded ZnO:CNT has the potential to be an effective treatment for fungal infections68. The antifungal activity of Ag-loaded ZnO:CNT was evaluated using the poisoned food method, as shown in Fig. 969. The diameter was measured using the Kirby-Bauer method, as shown in Eq. (12).
where Dc is the diameter of growth in the control plate and Ds is the diameter of the plate containing Ag-loaded ZnO:CNT nanoparticles. The % antifungal activities of the 5CNT, 10CNT, 15CNT, and 20CNT were 48%, 46%, 43%, and 40%, respectively.
Antibacterial activity
The Ag-loaded ZnO:CNT composites were evaluated for their antimicrobial activity against Staphylococcus aureus (NCIM-2654), Bacillus cerius (NCIM-2703), Proteus vulgaris (NCIM-2813), and Salmonella typhimurium (NCIM-2501). The inoculum for microbial cultures was produced in sterile saline water. Bacterial growth was conducted on nutrient agar plates. Cultures of S. aureus, B. cerius, P. vulgaris, and S. typhimurium were spread out on a sterile nutrient agar plate. Sterile corkborers measuring 5 mm were used to create wells on these plates. Using a micropipette, 100 µg/ml of the synthesized substance was distributed in sterile dimethyl sulfoxide (DMSO). To evaluate antibacterial activity, the plates were incubated for 24 h at 370 °C. The antibacterial activity of the composite material was examined in conjunction with a blank dimethyl sulfoxide (DMSO) negative control. Using streptomycin as the reference medication, the antibacterial abilities of the composite materials were investigated using the agar well gel diffusion method. This study demonstrates the considerable antibacterial performance of the prepared composites because they curtail the growth of the given bacterial strains. Initially, DMSO medium was prepared using a liquid auger suspension. Bacterial strains were prepared separately for each sample and control, and the bacterial control suspensions were incubated for 12 h. at 37 °C70,71.
The Ag-loaded ZnO:CNT composite samples were prepared at 2.5 mg/mL, and 10 µL drops of the sample were mixed with the above bacterial suspensions. Figure 10 shows the antibacterial activity of Ag-loaded ZnO:CNT catalysts against the bacterial strains, using streptomycin as a positive control and dimethyl sulfoxide (DMSO) and distilled water as a negative control. In the present study, the zone of inhibition (ZOI) values revealed that the 5CNT composite was more effective in constraining the development of the bacterial strain B. cerius, as the maximum ZOI was 21.66 ± 0.57 mm. The ZOIs of 5CNT composite against S. aureus, P. vulgaris, and S. typhimurium bacterial strains were 17.68 ± 0.57 mm 15.66 ± 0.57 mm, and 13.66 ± 0.57 mm, respectively, as illustrated in Table 3. The commercial drug streptomycin, which is exclusively effective against specific bacterial strains, was used to compare the outcomes. The ZOIs were 17.66 ± 0.57 mm, 25.66 ± 0.57 mm, 20.33 ± 0.57 mm, and 19.00 ± 1.00 mm for S. aureus, B. cerius, P. vulgaris, and S. typhimurium, respectively72. The aforementioned information shows the average ± standard error of three replicates (Table 3).
Conclusions
In this study, Ag-loaded ZnO:CNT nanocomposites were prepared using a refluxed chemical method. XRD analysis confirmed the structural clarity of the Ag-loaded ZnO:CNT nanocomposites. XRD investigation revealed the presence of silver and carbon peaks, and the average crystallite size ranged from 19 to 25 nm. The microstrain and dislocation density for Ag-loaded ZnO:CNT changed slightly with a change in the CNT content. The lattice parameters, crystal structure, and band gap energy of the Ag-loaded ZnO:CNT crystal structures for 5CNT, 10CNT, 15CNT, and 20CNT were found to be very similar. FE-SEM and EDS analyses confirmed the presence of Ag-loaded ZnO on the exterior of the CNT. The FESEM micrograph shows an agglomerated nano-flower-like morphology. The photocatalytic activities of MB and RB dyes were higher for the 10CNT. There was an increase in the photocatalytic efficiency with an increase in the pH of the dye solution up to the optimum value. The optimum result for MB dye was observed for 10CNT at pH 9, showing 100% photocatalytic efficiency in 2 min at a rate constant 1.48 min−1. While The RB dye exhibited 99% photocatalytic efficiency in 20 min at pH 5 for 10CNT at a rate of 0.20 min−1. The reuse of the 5CNT composite has shown excellent sustainability, it has shown 91% efficiency after 4th cycle. The variation in the MB dye concentration from 10 to 50 ppm revealed that the time required to degrade the dye solution increased with increasing dye concentration. In addition, we investigated the use of Ag-loaded ZnO:CNT as an antifungal agent against Bipolaris sorokiniana. It was observed that 5CNT showed better inhibition of Bipolaris sorokiniana fungus showing 48% efficiency. The 5CNT samples were tested against bacterial strains Staphylococcus aureus, Bacillus cerius, Proteus vulgaris, and Salmonella typhimurium and showed promising antibacterial activity in comparison with commercially available drugs.
In future research, we plan to incorporate advanced characterization techniques such as Electrochemical Impedance Spectroscopy (EIS) and Transmission Electron Microscopy (TEM) to gain deeper insights into the charge transfer dynamics and detailed structural properties of our silver stacked zinc oxide garnished on carbon nanotube composites. These analyses will complement our current Field Emission Scanning Electron Microscopy (FESEM) studies and provide a more comprehensive understanding of the materials' photocatalytic, antibacterial, and antifungal activities.
Data availability
The datasets generated or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Bagheri, M., Najafabadi, N. R. & Borna, E. Removal of reactive blue 203 dye photocatalytic using ZnO nanoparticles stabilized on functionalized MWCNTs. J. King Saud Univ. - Sci. 32, 799–804. https://doi.org/10.1016/j.jksus.2019.02.012 (2020).
Kim, S. P., Choi, M. Y. & Choi, H. C. Characterization and photocatalytic performance of SnO2-CNT nanocomposites. Appl. Surf. Sci. 357, 302–308. https://doi.org/10.1016/j.apsusc.2015.09.044 (2015).
Tan, Y. et al. Facile solvothermal synthesis of Cu2SnS3 architectures and their visible-light-driven photocatalytic properties. Mater. Lett. 89, 240–242. https://doi.org/10.1016/j.matlet.2012.08.117 (2012).
Rohilla, S. et al. Excellent Uv-light triggered photocatalytic performance of ZnO.SiO2 nanocomposite for water pollutant compound methyl orange dye. Nanomaterials 11, 2548. https://doi.org/10.3390/nano11102548 (2021).
Vallejo, W., DÍaz-Uribe, C. & Rios, K. Methylene blue photocatalytic degradation under visible irradiation on In2S3 synthesized by chemical bath deposition. Adv. Phys. Chem. 2017, 1–5. https://doi.org/10.1155/2017/6358601 (2017).
Patole, S., Islam, M., Aiyer, R. C. & Mahamuni, S. Optical studies of ZnO/Ag nanojunctions. J. Mater. Sci. 41, 5602–5607. https://doi.org/10.1007/s10853-006-0296-0 (2006).
Xu, J., Pan, Q., Shun, Y. & Tian, Z. Grain size control and gas sensing properties of ZnO gas sensor. Sens. Actuators B Chem. 66, 277–279. https://doi.org/10.1016/S0925-4005(00)00381-6 (2000).
Selvam, N. C. S., Narayanan, S., Kennedy, L. J. & Vijaya, J. J. Pure and Mg-doped self-assembled ZnO nano-particles for the enhanced photocatalytic degradation of 4-chlorophenol. J. Environ. Sci. 25, 2157–2167. https://doi.org/10.1016/S1001-0742(12)60277-0 (2013).
Machale, A. R., Shelke, H. D., Phaltane, S. A. & Kadam, L. D. Influence of hydrothermal temperature on the structural, morphological, optical and photocatalytic properties of ternary Cu2SnS3 nanoparticles. Chem. Phys. Lett. 808, 140097. https://doi.org/10.1016/j.cplett.2022.140097 (2022).
Han, Z. et al. Ag/ZnO flower heterostructures as a visible-light driven photocatalyst via surface plasmon resonance. Appl. Catal. B Environ. 126, 298–305. https://doi.org/10.1016/j.apcatb.2012.07.002 (2012).
Sher, M. et al. Designing of highly active G-C3N4/Sn doped ZnO heterostructure as a photocatalyst for the disinfection and degradation of the organic pollutants under visible light irradiation. J. Photochem. Photobiol. A Chem. 418, 113393. https://doi.org/10.1016/j.jphotochem.2021.113393 (2021).
Sher, M. et al. Synthesis of novel ternary hybrid G-C3N4@Ag-ZnO nanocomposite with Z-scheme enhanced solar light-driven methylene blue degradation and antibacterial activities. J. Environ. Chem. Eng. 9, 105366. https://doi.org/10.1016/j.jece.2021.105366 (2021).
Sher, M., Shahid, S. & Javed, M. Synthesis of a novel ternary (g-C3N4 nanosheets loaded with Mo Doped ZnOnanoparticles) nanocomposite for superior photocatalytic and antibacterial applications. J. Photochem. Photobiol. B Biol. 219, 112202. https://doi.org/10.1016/j.jphotobiol.2021.112202 (2021).
Nasser, A., Sina, B. & Abuali Maryam, J. S. A new method for preparing ZnO/CNT nanocomposites with enhanced photocatalytic degradation of malachite green under visible light. J. Photochem. Photobiol. A Chem. 36, 100061. https://doi.org/10.1016/j.cogsc.2022.100630 (2022).
Zhu, G. et al. A facile synthesis of ZnO/CNT hierarchical microsphere composites with enhanced photocatalytic degradation of methylene blue. RSC Adv. 5, 72476–72481. https://doi.org/10.1039/c5ra11873e (2015).
Saleh, T. A., Gondal, M. A., Drmosh, Q. A., Yamani, Z. H. & AL-yamani, A. Enhancement in photocatalytic activity for acetaldehyde removal by embedding ZnO nano particles on multiwall carbon nanotubes. Chem. Eng. J. 166, 407–412. https://doi.org/10.1016/j.cej.2010.10.070 (2011).
Yu, C., Wang, Y., Liu, Y., Guo, C. & Hu, Y. Facile growth of ZnO nanocrystals on nitrogen-doped carbon nanotubes for visible-light photodegradation of dyes. Mater. Lett. 100, 278–281. https://doi.org/10.1016/j.matlet.2013.03.041 (2013).
Khodam, F., Rezvani, Z. & Amani-Ghadim, A. R. Fabrication of a novel ZnO/MMO/CNT nanohybrid derived from multi-cationic layered double hydroxide for photocatalytic degradation of azo dye under visible light. RSC Adv. 5, 19675–19685. https://doi.org/10.1039/c4ra17001f (2015).
Ahmad, M., Ahmad, I., Ahmed, E., Akhtar, M. S. & Khalid, N. R. Facile and inexpensive synthesis of Ag doped ZnO/CNTs composite: Study on the efficient photocatalytic activity and photocatalytic mechanism. J. Mol. Liq. 311, 113326. https://doi.org/10.1016/j.molliq.2020.113326 (2020).
Gao, N. et al. Steering carbon nanotubes to scavenger receptor recognition by nanotube surface chemistry modification partially alleviates NFκB activation and reduces its immunotoxicity. ACS Nano 5, 4581–4591. https://doi.org/10.1021/nn200283g.Steering (2012).
Wang, X., Zhou, Z. & Chen, F. Surface modification of carbon nanotubes with an enhanced antifungal activity for the control of plant fungal pathogen. Materials 10, 1375. https://doi.org/10.3390/ma10121375 (2017).
Vatansever, F. et al. Antimicrobial strategies centered around reactive oxygen species - bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 37, 955–989. https://doi.org/10.1111/1574-6976.12026 (2013).
Ojima, Y., Yokota, N., Tanibata, Y., Nerome, S. & Azuma, M. Concentrative nucleoside transporter, CNT, results in selective toxicity of toyocamycin against Candida albicans. Microbiol. Spectr. https://doi.org/10.1128/spectrum.01138-22 (2022).
Acharya, K., Dutta, A. K. & Pradhan, P. Bipolaris sorokiniana (Sacc.) Shoem.: The most destructive wheat fungal pathogen in the warmer areas. Aust. J. Crop Sci. 5, 1064–1071 (2011).
Muzammil, K. et al. Decoration of MoO3-x on clay mineral matrix with great phosphorescence properties for oxygen activation, photochemical properties, bactericidal and oxidase-like mimics for prompt detection of pesticide. Mater. Sci. Semicond. Process. 168, 107847. https://doi.org/10.1016/j.mssp.2023.107847 (2023).
Dizaj, S. M., Lotfipour, F., Barzegar-Jalali, M., Zarrintan, M. H. & Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 44, 278–284. https://doi.org/10.1016/j.msec.2014.08.031 (2014).
Aldhalmi, A. K. et al. A novel fabricate of iron and nickel-introduced bimetallic MOFs for quickly catalytic degradation via the peroxymonosulfate, antibacterial efficiency, and cytotoxicity assay. Inorg. Chem. Commun. 153, 110823. https://doi.org/10.1016/j.inoche.2023.110823 (2023).
Yao, S., Zhou, S., Zhou, X., Wang, J. & Pu, X. TiO2-coated copper zinc tin sulfide photocatalyst for efficient photocatalytic decolourization of dye-containing wastewater. Mater. Chem. Phys. 256, 123559. https://doi.org/10.1016/j.matchemphys.2020.123559 (2020).
Wagh, S. S., Salunkhe, D. B., Patole, S. P., Jadkar, S. & Patil, R. S. Zinc oxide decorated carbon nanotubes composites for photocatalysis and antifungal application. ES Energy Environ. 21, 1–11. https://doi.org/10.30919/esee945 (2023).
Chougale, A. S. et al. Photocatalytic and antioxidant activity of ZnO/Cu/Ag/CNT nanocomposite. J. Mater. Sci. Mater. Electron. 35, 1–15. https://doi.org/10.1007/s10854-024-12620-6 (2024).
Balouiri, M., Sadiki, M. & Ibnsouda, S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 6, 71–79. https://doi.org/10.1016/j.jpha.2015.11.005 (2016).
Ali, A. et al. Mutual contaminants relational realization and photocatalytic treatment using Cu2MgSnS4 Decorated BaTiO3. Appl. Mater. Today 18, 100534. https://doi.org/10.1016/j.apmt.2019.100534 (2020).
Jadhav, P. et al. Green AgNPs decorated ZnO nanocomposites for dye degradation and antimicrobial applications. Eng. Sci. 12, 79–94. https://doi.org/10.30919/es8d1138 (2020).
Chen, Y. et al. A facile preparation method for efficiency a novel LaNiO3/SrCeO3 (p-n Type) heterojunction catalyst in photocatalytic activities, bactericidal assessment and dopamine detection. Surf. Interfaces 38, 102830. https://doi.org/10.1016/j.surfin.2023.102830 (2023).
Siva Kumar, S., Ranga Rao, V. & Nageswara Rao, G. Effect of silver impregnation on efficiency of ZnO for photocatalytic degradation of Eosin Y. Proc. Natl. Acad. Sci. India Sect. A - Phys. Sci. 83, 7–14. https://doi.org/10.1007/s40010-012-0063-3 (2013).
Wagh, S. S. et al. Comparative studies on synthesis, characterization and photocatalytic activity of Ag doped ZnO nanoparticles. ACS Omega 8, 7779–7790. https://doi.org/10.1021/acsomega.2c07499 (2023).
Wenhu, Y. et al. ZnO-decorated CNT hybrids as fillers leading to reversible nonlinear I-V behavior of polymer composites for device protection. ACS Appl. Mater. Interfaces 8, 35545–35551. https://doi.org/10.1021/acsami.6b11492 (2016).
Kelsall, R. W., Hamley, I. W. & Geoghegan, M. Nanoscale science and technology (Wiley Online Library, 2005) (ISBN 9780470850862).
Singh, R., Barman, P. B. & Sharma, D. Synthesis, structural and optical properties of Ag Doped ZnO nanoparticles with enhanced photocatalytic properties by photo degradation of organic dyes. J. Mater. Sci. Mater. Electron. 28, 5705–5717. https://doi.org/10.1007/s10854-016-6242-2 (2017).
Jana, J., Ganguly, M. & Pal, T. Enlightening surface plasmon resonance effect of metal nanoparticles for practical spectroscopic application. RSC Adv. 6, 86174–86211. https://doi.org/10.1039/c6ra14173k (2016).
Guidelli, E. J., Baffa, O. & Clarke, D. R. Enhanced UV emission from silver/ZnO and Gold/ZnO Core-shell nanoparticles: Photoluminescence, radioluminescence, and optically stimulated luminescence. Sci. Rep. 5, 1–11. https://doi.org/10.1038/srep14004 (2015).
Messih, M. F. A., Ahmed, M. A., Soltan, A. & Anis, S. S. Journal of physics and chemistry of solids synthesis and characterization of novel Ag/ZnO nanoparticles for photocatalytic degradation of methylene blue under UV and solar irradiation. J. Phys. Chem. Solids 135, 109086. https://doi.org/10.1016/j.jpcs.2019.109086 (2019).
Desai, M. A. et al. Photoelectrochemical performance of MWCNT–Ag–ZnO ternary hybrid: A study of Ag loading and MWCNT garnishing. J. Mater. Sci. 56, 8627–8642. https://doi.org/10.1007/s10853-021-05821-5 (2021).
Wu, Z. et al. ZnO Nanorods/Ag nanoparticles heterostructures with tunable Ag contents: A facile solution-phase synthesis and applications in photocatalysis. CrystEngComm 15, 5994–6002. https://doi.org/10.1039/c3ce40753e (2013).
Zhang, H., Chen, G. & Bahnemann, D. W. Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 19, 5089–5121. https://doi.org/10.1039/b821991e (2009).
Ago, H. et al. Work functions and surface functional groups of multiwall carbon nanotubes. J. Phys. Chem. B 103, 8116–8121. https://doi.org/10.1021/jp991659y (1999).
Yang, Y. et al. Polypyrrole-decorated Ag-TiO2 nanofibers exhibiting enhanced photocatalytic activity under visible-light illumination. ACS Appl. Mater. Interfaces 5, 6201–6207. https://doi.org/10.1021/am401167y (2013).
Redl, F. X., Cho, K. S., Murray, C. B. & O’Brien, S. Three-dimensional binary superlattices of magnetic nanocrystals and semiconductor quantum dots. Nature 423, 968–971. https://doi.org/10.1038/nature01702 (2003).
Özgür, Ü. et al. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 98, 1–103. https://doi.org/10.1063/1.1992666 (2005).
Studenikin, S. A., Golego, N. & Cocivera, M. Fabrication of green and orange photoluminescent, undoped ZnO films using spray pyrolysis. J. Appl. Phys. 84, 2287–2294. https://doi.org/10.1063/1.368295 (1998).
Lakowicz, J. R. Radiative decay engineering 5: Metal-enhanced fluorescence and plasmon emission. Anal. Biochem. 337, 171–194. https://doi.org/10.1016/j.ab.2004.11.026 (2005).
Zeng, H. et al. Blue luminescence of ZnO nanoparticles based on non-equilibrium processes: Defect origins and emission controls. Adv. Funct. Mater. 20, 561–572. https://doi.org/10.1002/adfm.200901884 (2010).
Fatolahi, L. et al. Optical detection of fat and adulterants concentration milk using TMDC (WS2 and MoS2)-surface plasmon resonance sensor via high sensitivity and detection accuracy. Opt. Mater. 147, 114723. https://doi.org/10.1016/j.optmat.2023.114723 (2024).
Zhao, M., Chen, T. & Deng, C. Porous anatase TiO2 derived from a titanium metal-organic framework as a multifunctional phospho-oriented nanoreactor integrating accelerated digestion of proteins and in situ enrichment. RSC Adv. 6, 51670–51674. https://doi.org/10.1039/c6ra03837a (2016).
Kayani, Z. N., Iqbal, M., Riaz, S., Zia, R. & Naseem, S. Fabrication and properties of zinc oxide thin film prepared by sol-gel dip coating method. Mater. Sci. Pol. 33, 515–520. https://doi.org/10.1515/msp-2015-0085 (2015).
Moradi, M., Haghighi, M. & Allahyari, S. Precipitation dispersion of Ag–ZnO nanocatalyst over functionalized multiwall carbon nanotube used in degradation of acid orange from wastewater. Process Saf. Environ. Prot. 107, 414–427. https://doi.org/10.1016/j.psep.2017.03.010 (2017).
Wagh, S. S. et al. Silver doped ZnO nanoparticles synthesized for photocatalysis application. ES Energy Environ. 17, 94–105. https://doi.org/10.30919/esee8e720 (2022).
Kam, C. S. et al. Lead removal from water-dependence on the form of carbon and surface functionalization. RSC Adv. 8, 18355–18362. https://doi.org/10.1039/c8ra02264j (2018).
Kang, E. T., Ti, H. C., Neoh, K. G. & Tan, T. C. ESCA analysis of polymer-acceptor interactions in chemically synthesized polypyrrole-halogen complexes. Polym. J. 20, 399–406. https://doi.org/10.1295/polymj.20.399 (1988).
Moraes, R. A. et al. The effect of different chemical treatments on the structure and stability of aqueous dispersion of iron- and iron oxide-filled multi-walled carbon nanotubes. J. Braz. Chem. Soc. 22, 2191–2201. https://doi.org/10.1590/S0103-50532011001100024 (2011).
Chen, C. et al. Enhanced Raman scattering and photocatalytic activity of Ag/ZnO heterojunction nanocrystals. Dalt. Trans. 40, 9566–9570. https://doi.org/10.1039/c1dt10799b (2011).
Bazant, P., Kuritka, I., Munster, L. & Kalina, L. Microwave solvothermal decoration of the cellulose surface by nanostructured hybrid Ag/ZnO particles: A joint XPS, XRD and SEM study. Cellulose 22, 1275–1293. https://doi.org/10.1007/s10570-015-0561-y (2015).
Shelke, H. D. et al. Multifunctional Cu2SnS3 nanoparticles with enhanced photocatalytic dye degradation and antibacterial activity. Materials 15, 1–15. https://doi.org/10.3390/ma15093126 (2022).
Tehreem, R. et al. Synthesis of efficient light harvesting Cr, N Co - doped TiO2 nanoparticles for enhanced visible light photocatalytic degradation of xanthene dyes; eosin yellow and rose Bengal. Environ. Sci. Pollut. Res. https://doi.org/10.1007/s11356-023-28701-8 (2023).
Kunwar, S. et al. Bio-fabrication of Cu/Ag/Zn nanoparticles and their antioxidant and dye degradation activities. Catalysts 13, 1–19. https://doi.org/10.3390/catal13050891 (2023).
Arslan, S., Eyvaz, M., Gürbulak, E. & Yüksel, E. A review of state-of-the-art technologies in dye-containing wastewater treatment–the textile industry case. Text. Wastewater Treat. 1–28 (2016). https://doi.org/10.5772/64140.
Song, H. et al. Spinel-structured ZnCr2O4 with excess Zn is the active ZnO/Cr2O3 catalyst for high-temperature methanol synthesis. ACS Catal. 7, 7610–7622. https://doi.org/10.1021/acscatal.7b01822 (2017).
Mansoor, S. et al. Fabrication of silver nanoparticles against fungal pathogens. Front. Nanotechnol. 3, 1–12. https://doi.org/10.3389/fnano.2021.679358 (2021).
Article, O. & Access, O. Influence of particle size on antibacterial and antifungal strains of titanium dioxide (TiO2) nanoparticles synthesized by SILAR method. 274–282 (2023).
Syed, A. et al. Gold nanoparticles supported on chitosan and the ameliorative response on raman scattering sensing, catalytic reduction, antibacterial and cytotoxicity activities. Eur. Phys. J. Plus https://doi.org/10.1140/epjp/s13360-024-05022-4 (2024).
Syed, A. et al. Highly-impressive performances of novel NiCo2O4/Bi2O3/Ag2ZrO3 nanocomposites in photocatalysis system, removal pathway, toxicity estimation, and antibacterial activities. J. Taiwan Inst. Chem. Eng. 149, 105004. https://doi.org/10.1016/j.jtice.2023.105004 (2023).
Zhang, Q. et al. Sterilization efficiency of a novel electrochemical disinfectant against Staphylococcus aureus. Environ. Sci. Technol. 50, 3184–3192. https://doi.org/10.1021/acs.est.5b05108 (2016).
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. Mu Naushad is grateful to the Researchers Supporting Project number (RSP2024R8), King Saud University, Riyadh, Saudi Arabia for the financial support for the study.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.S.W., A.S.C., A.A.S., R.S.P., and H.M.P.. Methodology: S.S.W., A.S.C., A.A.S., R.S.P., and H.M.P.. Writing—Original Draft Preparation: S.S.W., A.S.C., A.A.S., and R.S.P.; Writing—Review and Editing: N.N., M.N., and H.M.P. Supervision: M.N., and H.M.P. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wagh, S.S., Chougale, A.S., Survase, A.A. et al. Rapid photocatalytic dye degradation, enhanced antibacterial and antifungal activities of silver stacked zinc oxide garnished on carbon nanotubes. Sci Rep 14, 14045 (2024). https://doi.org/10.1038/s41598-024-64746-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-64746-6
Keywords
This article is cited by
-
Photocatalytic degradation of reactive black 5 from synthetic and real wastewater under visible light with TiO2 coated PET photocatalysts
Scientific Reports (2025)
-
Eggshell-derived calcium oxide nanoparticles as promising anti-cancer agent: synthesis, characterization and in-vitro evaluation
Interactions (2025)
-
Facile Green Synthesis of Cerium Oxide Nanoparticles for UV-Activated Photocatalytic Treatment of Rhodamine B Dye
Journal of Inorganic and Organometallic Polymers and Materials (2025)
-
Hydrothermally Synthesized Ag Decorated ZnO/MWCNT Nanocomposites: Characterization and Enhanced Antibacterial Efficacy
Journal of Cluster Science (2025)
-
Eco-friendly synthesis of TiO2 nanoparticles using citrus peel extract for enhanced photocatalytic degradation of textile dyes
Interactions (2025)