Abstract
This paper explores the complex interplay between topological indices and structural patterns in networks of iron telluride (FeTe). We want to analyses and characterize the distinct topological features of (FeTe) by utilizing an extensive set of topological indices. We investigate the relationship that these indicators have with the network’s physical characteristics by employing sophisticated statistical techniques and curve fitting models. Our results show important trends that contribute to our knowledge of the architecture of the (FeTe) network and shed light on its physiochemical properties. This study advances the area of material science by providing a solid foundation for using topological indices to predict and analyses the behavior of intricate network systems. More preciously, we study the topological indices of iron telluride networks, an artificial substance widely used with unique properties due to its crystal structure. We construct a series of topological indices for iron telluride networks with exact mathematical analysis and determine their distributions and correlations using statistical methods. Our results reveal significant patterns and trends in the network structure when the number of constituent atoms increases. These results shed new light on the fundamental factors that influence material behavior, thus offering a deeper understanding of the iron telluride network and may contribute to future research and engineering of these materials.
Similar content being viewed by others
Introduction
A graph is any graphical representation made up of lines and points. Graphs that correlate to the constitutional formulas of chemical compounds are familiar to chemists. All of the circles represent atoms, while the lines connecting the respective circles represent chemical bounds1. In graph theory, the connecting lines are referred to as edges, while the items denoted by tiny circles are known as vertices2. Two sets make up the structure known as graphs \(G=(V,E)\) in mathematics: the vertex set V and the edge set E3. The total number of edge connect with a vertex \(\zeta \) is called its degree and is denoted by \(\Upsilon (\zeta )\).
Networks of chemical compounds’ structures provide a strong foundation for comprehending and evaluating the characteristics of intricate molecular systems10. These networks offer a distinctive viewpoint on the connections between molecular structures and their attributes as they are built using the structural characteristics of chemical compounds. Chemical investigations revealed a high correlation between molecular structure’s topologies and their physical behaviors, chemical properties, and biological traits, including drug toxicity and melting and boiling points. Chen et al.4 discuss the design of multi-phase dynamic chemical networks. Avanzini et al.5 discuss the circuit theory for chemical reaction networks. The vertex-degree-based (abbreviated VDB) topological indices are a unique class of topological indices that are determined by the degree of the vertices in a (molecular) graph7. General Randic index used in chemical engineering to understand the connections between molecular structure and prospective physico-chemical properties6. Shaker et al.8 discus the superlattice via fifth zagreb topological polynomial. Zhang et al.9 derived results for multiplicative Zagreb indices of molecular graphs. Siddiqui et al.11 computed topological indices for chemical graphs.
In chemistry and related domains, the Randi\(\acute{c}\) index is a graph theory descriptor used to measure the molecular structure of chemical compounds. A molecular graph’s Randi\(\acute{c}\) index is determined by adding up the reciprocal square root of the product of the degrees of vertice pairs joined by an edge. This index is frequently used in quantitative structure-activity relationship (QSAR) investigations to predict the biological activity of chemical compounds. It offers insight into the topological arrangement of atoms in a molecule.
Then Bollobas et al.12 and Amic et al.13 worked independently and defined the generalized Randi\(\acute{c}\) index as:
It gives the connection network of atoms inside a molecular structure a numerical representation. By adding up the products of the bond orders between atom pairs in a molecule, the index is determined. This descriptor contributes to the quantification of the molecular structure’s complexity and aids in the prediction of a number of attributes, including physicochemical traits and biological activity. Estrada et al.14,15 gave a new idea of an index and named as atom bond connectivity index:
Vukicevic et al.16, defined the geometric arithmetic index as follwos:
In the subject of Graph Theory, Gutman17,18 has made significant contributions. In terms of indices, he constructed the first and second Zagreb indices theoretically in 1972, as indicated by the following equations:
Shirdal et al.19 introduced the hyper Zagreb index in 2013 as:
The first and second multiple Zagreb indices were introduced in 2012 by Ghorbani and Azimi20, who took into consideration the indices of any graph in the form of a product.
The forgotten topological index, which Furtula and Gutman21 introduced, is a new method for indices when they are raised to the power of two. It can be calculated as follows:
Vukicevic et al.22 defined the Augmented Zagreb index as:
Balaban23,24 defined the Balaban index for a graph G with order \(\acute{s}\) and size \(\acute{r}\) as:
Guo et al.25 introduced the modeling concept of genes using graph-based approach and topological indices. Liu et al.26 discuss the quality model of clouds using degree based indices. Zhang et al.27 discuss the entropy in green building projects. Zhang et al.28 computed topological indices for ceria Oxide. Furtula et al.29 explore the Augmented Zagreb index(AZI) and also examine a forgotten topological index30. Ghorbani et al.31 give a brief overview of multiple Zagreb indices. Manzoor et al.32,33 discuused the degree-based entropy measures for metal-organic superlattices and hyaluronic acid-curcumin conjugates. Rashid et al.34 discuused the eccentricity-Based topoligcal indcies measures for Hypernetworks. Imran et al.35 computed the entropy measures of dendrimers. Siddiqui et al.36 computed the entropy measures for crystal structures.
We have shown the bibliometric analysis of the degree-based topological indices by various nations throughout the world in Fig. 1.
Using the Scopus database (https://www.scopus.com/), this bibliometric analysis employs 1243 research publications on the application of topological indices in various domains. As seen below, we displayed the results of our bibliometric study of the topological Index terms in Fig. 2.
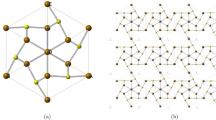
Structure of iron telluride network \((FeTe_2)\)
Iron Telluride \((FeTe_2)\) is a compound that has gained significant attention in the field of materials science due to its unique properties. It belongs to the family of transition metal dichalcogenides (TMDCs), which are layered materials with a general formula of \(MX_2\), where M is a transition metal and X is a chalcogen (sulfur, selenium, or tellurium). \(FeTe_2\) has a layered crystal structure consisting of iron atoms sandwiched between two layers of tellurium atoms. Iron Telluride Network \((FeTe_2)\) is a two dimensional network shown in Fig. 3. The synthesis of Iron ditelluride can be achieved through various methods, including chemical vapor transport, chemical vapor deposition, and hydrothermal synthesis37. Since iron ditelluride exhibits distinct electrical and magnetic properties, it has garnered a lot of interest in the field of materials research. It displays a combined state of superconductivity and magnetism, making it a type-II superconductor. Because of this characteristic, it is a desirable material for a number of uses, such as energy storage, spintronics, and quantum computing38.
The Iron Telluride \((FeTe_2)\) formulas are computed by initially using unit cell is shown in Fig. 3. In order to compute the vertices, we now use Matlab software to generalize these formulas for vertices together with Table 1. The edge partition of iron telluride \((FeTe_2)\) will be taken as follows in order to determine its topological indices: The edge partition of iron telluride \((FeTe_2)\) with (m, n) is greater or equal to 1 is displayed in Table 2. The edge set is divided into five sets, let’s say \(E_1\), \(E_2\), \(E_3\), \(E_4\), and \(E_5\), according to the degree of each edge’s end vertices. The order and size of \(FeTe_{2}\) is \(4mn+2m+n+1\) and \(6mn+m\), respectively.
The \(2\times 2\) sheet of Iron Telluride \((FeTe_2)\) network38.
Results for iron telluride network \((FeTe_2)\)
The derivation for General Randic index is:
A mathematical notion that is used to quantify the topological complexity of a graph, as shown in Figs. 4 and 5, is the Randic index. Table 3 presents a numerical comparison of the Randic indices as well.
The derivation of ABC index is:
The derivation of GA index is:
The first Zagreb index :
The second Zagreb index:
The numerical comparison and graphical depiction of the \(ABC(FeTe_2)\), \(GA(FeTe_2)\), \(M_1(FeTe_2)\), and \(M_2(FeTe_2)\), respectively, are shown in Table 4 and Figs. 6 and 7.
The Hyper Zagreb index:
The derivation of the Forgotten index:
The derivation of the Augmented Zagreb index:
The Balaban index:
The HM, F, AZI, and J, respectively, are represented graphically and numerically in Table 5 and Figs. 8 and 9.
The initial multiple Zagreb index was identified in our investigation as:
We defined the second multiple Zagreb index in our investigation as:
The \(PM_1\) and \(PM_2\) are represented graphically and numerically in Table 6 and Fig. 10.
Analysis of heat of formation via rational curve fitting
A mathematical model’s parameters are found using a statistical technique called curve fitting, which enables the model to approximately describe the observed data. In this section, we give the concept of the Information entropy and Heat of Formation(Enthalpy) of Iron Telluride \((FeTe_2)\). The standard molar enthalpy of Iron Telluride \((FeTe_2)\) or \((FeTe_{1.99})\) is \(-51.9(-65.8) kJ/mol\) at temperature 298.15K. Mathematical formula to calculate Heat of Formation (HOF) for different formula units is given as:
where, \(Avogadro's Number = 6.02214076 \times 10^{23} mol^{-1}\). A substance’s mole is equivalent to \(6.02214076 \times 10^{23} mol^{-1}\) of that material (such as atoms, molecules, or ions).Each material is in its normal state when a mole of a chemical is created by combining its constituent elements, and the amount of energy absorbed or released is measured by the heat of formation (HOF). This is measured in kilojoules per mole, or KJ/mol. It can also be described as having a normal heat of formation or a specific heat capacity.
MATLAB’s Power built-in function is used to create models between Heat of Formation and each information entropy since it provides a lower RMSE value for the best fit. The mean squared error (RMSE), the sum of squared errors (SSE), and \(R^2\) are the accuracy metrics that are employed. First, calculate the values of various indices using the Shanon entropy formula. Then we use the Maple software to determine the entropy’s graphical behavior and computed numerical values. Furthermore, curves were fitted between the Heat of Formation values and a number of related entropies. The models for the relationship between indices vs. HOF that were described are shown below. Graphical behaviour is presented in Figs. 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24.
-
HOF using \(R_1(FeTe_{2})\)
\(R_1\) is standardized(normalized) by using the symbol \(\mu \) (mu) to represent the mean value of 1267 and the symbol \(\sigma \) (sigma) to represent the standard deviation of 1160. The Coefficients: \(\chi _1 = -2.389\), with \(\mathbb {C}_{b} = (-4.81, 0.03131)\), \(\chi _2= -2.559\), with \(\mathbb {C}_{b} = (-6.793, 1.674)\), \(\chi _3 = -0.07126\), with \(\mathbb {C}_{b} = (-1.813, 1.671)\), \(\chi _4 = 0.1775\), with \(\mathbb {C}_{b} = (-1.633, 1.988)\), \(\Upsilon _1 = 1.32\), with \(\mathbb {C}_{b} = (0.7449, 1.896)\), \(\Upsilon _2 = 0.3262\), with \(\mathbb {C}_{b} = (-0.08985, 0.7423)\), \(\Upsilon _3 = -0.01093\), with \(\mathbb {C}_{b} = (-0.2133, 0.1915)\). In all curve fittings, the confidence bound is \(95\%\).
SSE : 0.05051, \(R-square: 0.9989\), \(Adjusted R-square: 0.9921\), and RMSE : 0.2247
-
HOF using \(R_{-1}(FeTe_{2})\)
where \(R_{-1}\) is standardized by \(\mu =21.75\) and \(\sigma =17.32\).The Coefficients:\(\chi _1 = -2.418\), with \(\mathbb {C}_{b} = (-4.742, -0.09399)\), \(\chi _2 = -2.418\), with \(\mathbb {C}_{b} = (-6.636, 1.8)\), \(\chi _3 = 0.1319\), with \(\mathbb {C}_{b} = (-1.592, 1.856)\),\(\chi _4 = 0.1728\), with \(\mathbb {C}_{b} = (-1.749, 2.095)\), \(\Upsilon _1 = 1.26\), with \(\mathbb {C}_{b} = (0.6277, 1.892)\), \(\Upsilon _2 = 0.2355\), with \(\mathbb {C}_{b} = (-0.2099, 0.681)\). \(\Upsilon _3 = -0.02212\), with \(\mathbb {C}_{b} = (-0.2473, 0.2031)\).
SSE : 0.04895, \(R-square: 0.9989\), \(Adjusted R-square: 0.9924\), and RMSE : 0.2212.
-
HOF using \({R_{\frac{1}{2}}(FeTe_{2})}\)
In which \(R_\frac{1}{2}\) is standardized by \(\mu =442.7\) and \(\sigma =398\). The Coefficients: \(\chi _1 = -2.394\), with \(\mathbb {C}_{b} = (-4.797, 0.009614)\), \(\chi _2 = -2.536\), with \(\mathbb {C}_{b} = (-6.767, 1.696)\), \(\chi _3 = -0.03775\), with \(\mathbb {C}_{b} = (-1.776, 1.701)\), \(\chi _4= 0.1773\), with \(\mathbb {C}_{b} = (-1.651, 2.006)\), \(\Upsilon _1 = 1.31\), with \(\mathbb {C}_{b} = (0.7252, 1.896)\), \(\Upsilon _2 = 0.3113\), with \(\mathbb {C}_{b} = (-0.1102, 0.7327)\), \(\Upsilon _3 = -0.01299\), with \(\mathbb {C}_{b} = (-0.2189, 0.1929)\).
SSE : 0.05024, \(R-square: 0.9989\), \(Adjusted R-square: 0.9922\), and RMSE : 0.2241.
-
HOF using \(R_{\frac{-1}{2}}(FeTe_{2})\)
In which \(R_\frac{-1}{2}\) is standardized by \(\mu =57.48\) and \(\sigma =48.42\). The Coefficients: \(\chi _1 = -2.408\), with \(\mathbb {C}_{b} = (-4.764, -0.052) \), \(\chi _2 = -2.467\), with \(\mathbb {C}_{b} = (-6.691, 1.757)\), \(\chi _3 = 0.06116\), with \(\mathbb {C}_{b} = (-1.668, 1.791)\), \(\chi _4 = 0.1754\), with \(\mathbb {C}_{b} = (-1.707, 2.058)\), \(\Upsilon _1 = 1.281\) , with \(\mathbb {C}_{b} = (0.6679, 1.894)\), \(\Upsilon _2 = 0.2671\) , with \(\mathbb {C}_{b} = (-0.169, 0.7032)\), \(\Upsilon _3 = -0.01858\) , with \(\mathbb {C}_{b} = (-0.2354, 0.1982)\).
SSE : 0.04947, \(R-square: 0.9989\), \(Adjusted R-square: 0.9923\), and RMSE : 0.2224.
The Randi\(\acute{c}\) index for a specific molecule corresponds to its value, and the coefficients control how the Randi\(\acute{c}\) index and the heat of formation are related.
-
HOF using \(M_{1} (FeTe_{2})\)
where \(M_1\) is standardized by \(\mu =891\) and \(\sigma =798.7\). The Coefficients: \(\chi _1\) = -2.394, with \(\mathbb {C}_{b}\) = (-4.796, 0.007124), \(\chi _2\) = -2.533 , with \(\mathbb {C}_{b}\) = (-6.764, 1.698), \(\chi _3\) = -0.03386 , with \(\mathbb {C}_{b}\) = (-1.772, 1.704), \(\chi _4\)= 0.1773, with \(\mathbb {C}_{b}\) = (-1.653, 2.008), \(\Upsilon _1\) = 1.309, with \(\mathbb {C}_{b}\) = (0.7229, 1.896), \(\Upsilon _2\) = 0.3095, with \(\mathbb {C}_{b}\) = (-0.1125, 0.7316), \(\Upsilon _3\) = -0.01322, with \(\mathbb {C}_{b}\) = (-0.2195, 0.1931).
SSE : 0.0502, \(R-square: 0.9989\), \( Adjusted R-square: 0.9922\), and RMSE : 0.2241.
-
HOF using \(M_{2}(FeTe_{2})\)
where \(M_2\) is standardized by \(\mu = 1267\) and \(\sigma = 1160\). The Coefficients: \(\chi _1\) = -2.389, with \(\mathbb {C}_{b}\) = (− 4.81, 0.03131), \(\chi _2\) = − 2.559, with \(\mathbb {C}_{b}\) = (− 6.793, 1.674), \(\chi _3\) = − 0.07126, with \(\mathbb {C}_{b}\) = (− 1.813, 1.671), \(\chi _4\)= 0.1775, with \(\mathbb {C}_{b}\) = (− 1.633, 1.988), \(\Upsilon _1\) = 1.322, with \(\mathbb {C}_{b}\) = (0.7449, 1.896), \(\Upsilon _2\) = 0.3262, with \(\mathbb {C}_{b}\) = (− 0.08985, 0.7423), \(\Upsilon _3\) = − 0.01093, with \(\mathbb {C}_{b}\) = (− 0.2133, 0.1915).
SSE : 0.05051, \(R-square: 0.9989\), \(Adjusted R-square: 0.9921\), and RMSE : 0.2247.
The objective is to create a mathematical formula or model that can accurately predict the heat of formation using the Zagreb index.
-
HOF using \(ABC(FeTe_{2})\)
In which ABC is standardized by \(\mu = 112.7\) and \(\sigma = 94.31\). The Coefficients: \(\chi _1 = -2.278\), with \(\mathbb {C}_{b} = (-4.245, -0.3114)\), \(\chi _2 = -1.632\), with \(\mathbb {C}_{b} = (-5.356, 2.092)\), \(\chi _3 = 0.7934\), with \(\mathbb {C}_{b} = (-1.128, 2.715)\), \(\chi _4= 0.09387\), with \(\mathbb {C}_{b} = (-1.229, 1.417)\), \(\Upsilon _1 = 0.9576\), with \(\mathbb {C}_{b}= (0.2465, 1.669)\), \(\Upsilon _2 = -0.2132\), with \(\mathbb {C}_{b}= (-0.5772, 0.1507)\), \(\Upsilon _3 = -0.1344\), with \(\mathbb {C}_{b}= (-0.3936, 0.1248)\).
SSE : 0.07398, \(R-square: 0.9984\), \(Adjusted R-square: 0.9885\), and RMSE : 0.2720.
The potential correlation or relationship between the atom bond connectivity index and the heat of formation is investigated by using a curve fitting model.
-
HOF using \(GA(FeTe_{2})\)
where GA is standardized by \(\mu = 156.2\) and \(\sigma = 137.3\).
The Coefficients: \(\chi _1\) = − 2.399 with \(\mathbb {C}_{b}\) = (− 4.784, − 0.01472), \(\chi _2\) = − 2.509, with \(\mathbb {C}_{b}\) = (− 6.737, 1.719), \(\chi _3\) = 0.000643, with \(\mathbb {C}_{b}\) = (− 1.734, 1.735), \(\chi _4\)= 0.1768, with \(\mathbb {C}_{b}\) = (− 1.673, 2.026), \(\Upsilon _1\) = 1.299, with \(\mathbb {C}_{b}\) = (0.7028, 1.895), \(\Upsilon _2\) = 0.2941, with \(\mathbb {C}_{b}\) = (− 0.1332, 0.7215), \(\Upsilon _3\) = − 0.01524, with \(\mathbb {C}_{b}\) = (− 0.2253, 0.19948).
SSE : 0.04993, \(R-square: 0.9989\), \(Adjusted R-square: 0.9922\), and RMSE : 0.2235.
The heat of formation for new values of the geometric arithmetic index that lie within the range of the data used to fit the curve can be predicted using the curve fitting model once its parameters have been established.
-
HOF using \(PM_{1}(FeTe_{2})\)
In which \(PM_1\) is standardized by \(\mu = 1.334e+09\) and \(\sigma = 1.913e+09\).
The Coefficients: \(\chi _1\) = 36.73, with \(\mathbb {C}_{b}\) = (− 572.5, 646), \(\chi _2\) = − 65.91, with \(\mathbb {C}_{b}\) = (− 1156, 1024), \(\chi _3\) = − 31.2, with \(\mathbb {C}_{b}\) = (− 551.4, 489), \(\chi _4\) = 27.52, with \(\mathbb {C}_{b}\) = (− 457.4, 512.5), \(\chi _5\) = 1.853, with \(\mathbb {C}_{b}\) =(− 44.13, 47.84), \(\Upsilon _1\) = 1.565, with \(\mathbb {C}_{b}\) = (− 16.02, 19.15).
SSE : 0.4302, \(R-square: 0.9904\), \(Adjusted R-square: 0.9425\), and RMSE : 0.6559.
-
HOF using \(PM_{2}(FeTe_{2})\)
where \(PM_2\) is standardized by \(\mu = 1.2e+09\) and \(\sigma = 1.721e+09\).
The Coefficients: \(\chi _1\) =\(-2.216e+05\), with \(\mathbb {C}_{b}\) =\((-3.415e+10, 3.415e+10)\), \(\chi _2\) = \(3.967e+05\), with \(\mathbb {C}_{b}\) = \((-6.114e+10, 6.114e+10)\), \(\chi _3\) =\(1.883e+05\), with \(\mathbb {C}_{b}\) = \((-2.902e+10, 2.902e+10)\), \(\chi _4\) =\(-1.767e+05\), with \(\mathbb {C}_{b}\) = \((-2.724e+10, 2.724e+10)\), \(\chi _5\) =\(-1.554e+04\), with \(\mathbb {C}_{b}\) = \((-2.394e+09, 2.394e+09)\), \(\Upsilon _1\)= \(-6498\), with \(\mathbb {C}_{b}\) = \((-1.002e+09, 1.002e+09)\).
SSE : 0.6218, \(R-square: 0.9861\), \(Adjusted R-square: 0.9169\), and RMSE : 0.7886.
By applying a curve fitting model, it is possible to produce a mathematical representation that can help understand and forecast the relationship between the various Zagreb indices and the heat of formation. The features of molecule structure that influence energy stability may become clearer as a result.
-
HOF using \(HM(FeTe_{2})\)
In which HM is standardized by \(\mu = 5113\) and \(\sigma = 4663\).
The Coefficients: \(\chi _1\) = -2.39, with \(\mathbb {C}_{b}\) = (-4.808, 0.02739), \(\chi _2\) = -2.555, with \(\mathbb {C}_{b}\) = (-6.788, 1.678), \(\chi _3\) = -0.06526, with \(\mathbb {C}_{b}\) = (-1.807, 1.676), \(\chi _4\)= 0.1775, with \(\mathbb {C}_{b}\) = (-1.636, 1.991), \(\Upsilon _1\) = 1.319, with \(\mathbb {C}_{b}\) = (0.7414, 1.896), \(\Upsilon _2\) = 0.3235, with \(\mathbb {C}_{b}\) = (-0.09351, 0.7406), \(\Upsilon _3\) = -0.0113, with \(\mathbb {C}_{b}\) = (-0.2143, 0.1917).
SSE : 0.05046, \(R-square: 0.9989\), \(Adjusted R-square: 0.9921\), and RMSE : 0.2246.
-
HOF using \(F(FeTe_{2})\)
where F is standardized by \(\mu = 2580\) and \(\sigma = 2343\).
The Coefficients: \(\chi _1\) = \(-2.391\), with \(\mathbb {C}_{b}\) = \((-4.805, 0.02353)\), \(\chi _2\) = \(-2.551\), with \(\mathbb {C}_{b}\) = \((-6.783, 1.682)\), \(\chi _3\) = \(-0.05932\), with \(\mathbb {C}_{b}\) = \((-1.8, 1.681)\), \(\chi _4\)= 0.1775, with \(\mathbb {C}_{b}\) = \((-1.639, 1.994)\), \(\Upsilon _1\) = 1.317, with \(\mathbb {C}_{b}\) = (0.7379, 1.896), \(\Upsilon _2\) = 0.3209, with \(\mathbb {C}_{b}\) = \((-0.09713, 0.7389)\), \(\Upsilon _3\) = \(-0.01167\), with \(\mathbb {C}_{b}\) = \((-0.2153, 0.1919)\).
SSE : 0.05041, \(R-square: 0.9989\), \(Adjusted R-square: 0.9921\), and RMSE : 0.2245.
A curve fitting model is used to establish a relationship between the forgetting index and heat of creation. This investigation improves knowledge of the molecule’s energy properties.
-
HOF using \(J(FeTe_{2})\)
where J is standardized by \(\mu = 213.4\) and \(\sigma = 162.1\).
The Coefficients: \(\chi _1 = -2.427\), with \(\mathbb {C}_{b}=(-4.716, -0.1388)\), \(\chi _2= -2.371\), with \(\mathbb {C}_{b}=(-6.566, 1.824)\), \(\chi _3= 0.1789\) , with \(\mathbb {C}_{b} = (-1.531, 1.889)\), \(\chi _4= 0.1658\), with \(\mathbb {C}_{b} = (-1.764, 2.096)\), \(\Upsilon _1 = 1.241\), with \(\mathbb {C}_{b} = (0.5953, 1.888)\), \(\Upsilon _2 = 0.214\), with \(\mathbb {C}_{b} = (-0.236, 0.6641)\), \(\Upsilon _3 = -0.02336\), with \(\mathbb {C}_{b} = (-0.2519, 0.2052)\).
SSE : 0.04838, \(R-square: 0.9989\), \(Adjusted R-square: 0.9925\), and RMSE : 0.22.
The topological complexity or shape of a molecule is measured by the Balaban index, a molecular graph descriptor. The heat of formation is a measurement of the energy change that takes place during the production of a compound.
-
HOF using \(AZI(FeTe_{2})\)
where AZI is standardized by \(\mu = 1641\) and \(\sigma = 1487\).
The Coefficients: \(\chi _1\) = \(-2.391\), with \(\mathbb {C}_{b}\) = \((-4.804, 0.02164)\), \(\chi _2\) = \(-2.549\), with \(\mathbb {C}_{b}\) = \((-6.781, 1.684)\), \(\chi _3\) = \(-0.0564\), with \(\mathbb {C}_{b}\) = \((-1.797, 1.684)\), \(\chi _4\) = 0.1775, with \(\mathbb {C}_{b}\) = \((-1.641, 1.996)\), \(\Upsilon _1\) = 1.316 , with \(\mathbb {C}_{b}\) = (0.7362, 1.896), \(\Upsilon _2\) = 0.3196 , with \(\mathbb {C}_{b}\) = \((-0.0989, 0.7381)\), \(\Upsilon _3\) = \(-0.01185\) , with \(\mathbb {C}_{b}\) = \((-0.2158, 0.1921)\).
SSE : 0.05039, \(R-square: 0.9989\), \(Adjusted R-square: 0.9921\), and RMSE : 0.2245.
The steps in the curve fitting process include selecting an appropriate model, calculating its parameters using the available data, and assessing the model’s goodness of fit. Thus, the fitted curve may be used to forecast the link between the improved Zagreb index and heat of formation and can also be used for analysis.
Discussion
In this work, we primarily characterised a number of topological indices for an Iron Telluride network, including as the Balaban index, the Forgotten index, the Zagreb type indices, and the generic Randic index. Chemical graph theory makes extensive use of these indices to measure molecular structural properties by looking at patterns of connectedness. Our objective was to have a better understanding of the structural characteristics and behaviour of the Iron Telluride network through the statistical study of these variables.
The universal Randic index is a topological metric that sums the reciprocal square roots of the bond degrees of each vertex in the molecular graph to describe the connectivity and branching of a molecule. In contrast, the Zagreb type indices measure the sum of the squares of the degrees of every vertex in a molecular network. They are based on the vertex degree. These indices have been utilised in QSAR/QSPR investigations to predict the biological and physicochemical features of compounds. By adding up the products of the bond degrees of each pair of vertices in the molecular graph, the Balaban index is a topological metric that assesses the branching and chemical complexity of a molecule. It is a measurement of a molecule’s topological symmetry and has been used to predict different characteristics of compounds in QSAR/QSPR investigations.
We computed these topological indices for the Iron Telluride network in our study and performed statistical analysis to comprehend their correlations and importance in forecasting the network’s chemical and structural characteristics. We examined the connections between these indices and other characteristics of the Iron Telluride network, such as its Gibbs energy and thermodynamic features, using curve fitting techniques like linear regression.
Conclusion
Our study’s findings on the characterization of the Iron Telluride network’s topological indices and statistical analysis using curve fitting models offer insightful information that might be used for the creation of new materials. The vertex partition method, edge partition method, graph theoretical tools, analytic approaches, degree counting method, sum of degrees of neighbors method, and combinatorial computing method are the methods we employ to compute our results for \(FeTe_2\). In addition, we plot graphical representation of these mathematical results using Maple and do mathematical computations using MATLAB. Investigating topological indices offers a thorough comprehension of the network’s connectivity patterns, illuminating its distinct characteristics and possible uses. The statistical analyses that are carried out provide a quantitative viewpoint on the observed properties and lay the groundwork for further research, which adds to the larger scientific conversation. This article’s results not only improve our understanding of the Iron Telluride network but also highlight the importance of topological indices in materials science investigations. The methods and insights presented here open up new avenues for advancements in the field as we continue to decipher the molecular details of various materials.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Zhang, X., Rauf, A., Ishtiaq, M., Siddiqui, M. K. & Muhammad, M. H. On Degree Based Topological Properties of Two Carbon Nanotubes. Polycyclic Aromat. Compd. 10, 22–35 (2020).
Zhang, X. et al. Physical analysis of heat for formation and entropy of Ceria Oiotade using topological indices. Combin. Chem. High Throughput Screen. 25(3), 441–450 (2022).
Bonchev D., & Rouvray D. H. Chemical Graph Theory: Introduction and Fundamentals, ISBN 0-85626-454-7, (1991).
Chen, C. et al. Design of multi-phase dynamic chemical networks. Nat. Chem. 9(8), 799–804 (2017).
Avanzini, F., Freitas, N. & Esposito, M. Circuit theory for chemical reaction networks. Phys. Rev. X 13(2), 021041 (2023).
Gao, W., Wang, W. F. & Farahani, M. R. Topological indices study of molecular structure in anticancer drugs. J. Chem. 12, 14–24 (2016).
Rada, J. Exponential Vertex-degree-Based Topological Indices and Discrimination. MATCH Commun. Math. Comput. Chem 82(1), 29–41 (2019).
Shaker, H., Javaid, S., Babar, U., Siddiqui, M. K. & Naseem, A. Characterizing superlattice topologies via fifth M-Zagreb polynomials and structural indices. Eur. Phys. J. Plus 138, 1–12 (2023).
Zhang, X., Awais, H. M., Javaid, M. & Siddiqui, M. K. Multiplicative Zagreb indices of molecular graphs. J. Chem. 2019, 1–19 (2019).
Siddiqui, M. K., Naeem, M., Rahman, N. A., & Imran, M. Computing topological indices of certain networks. J. Optoelectron. Adv. Mater., 16 (September-October 2016), 664–692 (2016).
Siddiqui, M. K., Imran, M. & Ahmad, A. On Zagreb indices, Zagreb polynomials of some nanostar dendrimers. Appl. Math. Comput. 260, 132–139 (2016).
Bollobas, B. & Erdos, P. Graphs of extremal weights. Ars Combin. 50, 225–233 (1996).
Amic, D., Bešlo, D., Lucic, B., Nikolic, S. & Trinajstic, N. The vertex-connectivity index revisited. J. Chem. Inf. Comput. Sci. 36(5), 619–622 (1996).
Estrada, E., Torres, L., Rodriguez, L. & Gutman, I. An atom-bond connectivity index: modelling the enthalpy of formation of alkanes. Indian J. Chem. 37A, 649–655 (1996).
Gao, W., Wang, W. F., Jamil, M. K., Farooq, R. & Farahani, M. R. Generalized atom-bond connectivity analysis of several chemical molecular graphs. Bul. Chem. Commun. 46(3), 543–549 (2016).
Vukicevic, D. & Furtula, B. Topological index based on the ratios of geometrical and arithmetical means of end-vertex degrees of edges. J. Math. Chem. 46(4), 1369–1376 (2009).
Gutman, I. & Trinajstic, N. Graph theory and molecular orbitals. Total electron energy of alternant hydrocarbons. Chem. Phys. Lett. 17(4), 535–536 (1972).
Gutman, I. & Das, K. C. The first Zagreb index 30 years after. MATCH Commun. Math. Comput. Chem 50(1), 63–92 (2004).
Shirdel, G. H., Rezapour, H. & Sayadi, A. M. The hyper Zagreb index of graph operations. Iran. J. Math. Chem. 4(2), 213–220 (2013).
Ghorbani, M. & Azimi, N. Note on multiple Zagreb indices. Iran. J. Math. Chem. 3(2), 137–143 (2012).
Furtula, B. & Gutman, I. A forgotten topological index. J. Math. Chem. 53, 1184–1190 (2015).
Wang, D., Huang, Y. & Liu, B. Bounds on augmented zagreb index, MATCH Commun. Math. Comput. Chem. 66, 209–216 (2011).
Balaban, A. T. Highly discriminating distance-based topological index. Chem. Phys. Lett. 69(5), 399–404 (1962).
Balaban, A. T. & Quintas, L. V. The smallest graphs, trees, and 4-trees with degenerate topological index. J. Math. Chem. 14, 213–233 (1963).
Guo, Y. et al. Integrated modeling for retired mechanical product genes in remanufacturing: A knowledge graph-based approach. Adv. Eng. Inform. 59(102254), 1–12 (2024).
Liu, Q. et al. Reduced reference perceptual quality model with application to rate control for video-based point cloud compression. IEEE Trans. Image Process. 30, 6623–6636 (2021).
Zhang, R., Yin, L., Jia, J., Yin, Y. & Li, C. Application of ATS-GWIFBM operator based on improved time entropy in green building projects. Adv. Civ. Eng. 2019(3519195), 45–55 (2019).
Zhang, X. et al. Physical analysis of heat for formation and entropy of Ceria Oxide using topological indices. Combin. Chem. High Throughput Screen. 25(3), 441–450 (2022).
Furtula, B., Graovac, A. & Vukicevic, D. Augmented Zagreb index. J. Math. Chem. 46(2), 370–360 (2010).
Furtula, B. & Gutman, I. A forgotten topological index. J. Math. Chem. 53(4), 1164–1190 (2015).
Ghorbani, M. & Azimi, N. Note on multiple Zagreb indices. Iran. J. Math. Chem. 3(2), 137–143 (2012).
Manzoor, S., Siddiqui, M. K. & Ahmad, S. On physical analysis of degree-based entropy measures for metal-organic superlattices. Eur. Phys. J. Plus 136(3), 1–22 (2021).
Manzoor, S., Siddiqui, M. K. & Ahmad, S. Degree-based entropy of molecular structure of hyaluronic acid-curcumin conjugates. Eur. Phys. J. Plus 136(1), 1–21 (2021).
Rashid, M. A., Ahmad, S., Siddiqui, M. K., Manzoor, S. & Dhlamini, M. An Analysis of Eccentricity-Based Invariants for Biochemical Hypernetworks. Complexity 2021, 1–15 (2021).
Imran, M., Manzoor, S., Siddiqui, M. K., Ahmad, S., & Muhammad, M. H. On physical analysis of synthesis strategies and entropy measures of dendrimers. Arab. J. Chem. 15(2), 103574, 1–18, (2022).
Siddiqui, M. K., Manzoor, S., Ahmad, S. & Kaabar, M. K. On computation and analysis of entropy measures for crystal structures. Math. Probl. Eng. 2021, 1–19 (2021).
Chudnovskiy, F., Luryi, S. & Spivak, B. Switching device based on first-order metal-insulator transition induced by external electric field. Future trends in microelectronics: the nano millennium 148, 24–44 (2002).
Zhang, C. et al. Charge mediated reversible metal-insulator transition in monolayer \(MoTe2\) and \(W x Mo1-xTe2\) alloy. ACS Nano 10(8), 7370–7375 (2016).
Luo, H. & Chen, X. Investigation on Spark Plasma Sintering of Iron Telluride Nanocrystals: Densification Behavior, Grain Growth, and Mechanical Properties. J. Am. Ceram. Soc. 101(1), 184–193 (2018).
Jenkins, H. D. B. Thermodynamics of the relationship between lattice energy and lattice enthalpy. J. Chem. Educ. 62(6), 950–960 (2005).
Author information
Authors and Affiliations
Contributions
Hong Yang contributed to Investigation, analyzing the data curation, and designing the experiments. Muhammad Farhan Hanif contributed to data analysis, computation, funding resources, calculation verifications. Muhammad Kamran Siddiqui contributed to supervision, conceptualization, Methodology, project administration, and resources, and wrote the initial draft of the paper. Muhammad Faisal Hanif contributed for computation, and investigated and approved the final draft of the paper. Hira Ahmed contributes to Matlab calculations, Maple graphs improvement. Samuel Asefa Fufa contributes to formal analyzing experiments, software, validation and funding. All authors read and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, H., Hanif, M.F., Siddiqui, M.K. et al. Topological indices and patterns in iron telluride networks. Sci Rep 14, 14297 (2024). https://doi.org/10.1038/s41598-024-65205-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-65205-y
Keywords
This article is cited by
-
Exploring topological indices and Gibbs energy for terephthalic tetragonal network via curve-fitting model
Chemical Papers (2025)
-
Predicting Skin Permeability of Compounds with Elasticnet, Ridge and Decision Tree Regression Methods
Journal of Pharmaceutical Innovation (2025)
-
On exploring the statistical behavior of Sombor and Revan indices in chemical networks
Chemical Papers (2025)