Abstract
In this single-center study, 62 eyes of 41 patients with regular astigmatism, who received cataract surgery with TECNIS Eyhance Toric II intraocular lens (IOL) insertion between January 1, 2022, and June 30, 2023, were retrospectively investigated. At 3 months postoperatively, there were significant improvements in the mean logMAR uncorrected distance visual acuity (VA) (from 0.64 ± 0.42 to 0.11 ± 0.13, p < 0.001) and logMAR corrected distance VA (from 0.28 ± 0.30 to 0.03 ± 0.06, p < 0.001). An 81.9% reduction in astigmatism (from 1.66 ± 0.49 D corneal cylinder preoperatively to − 0.30 ± 0.39 D refractive cylinder at 3 months postoperatively, p < 0.001) was observed. In the vector analysis, the average of difference vector was 0.27 ± 0.35 D, and the average correction index was 1.09 ± 0.22, revealing a minimal overcorrection. Mean IOL misalignment at 3 months postoperatively was 0.68 ± 4.17°, and average absolute misalignment was 3.41 ± 2.44°. Defocus curves showed < 0.2 logMAR range between − 0.85 and 1.27 diopters, and distance-corrected near and intermediate VA were 0.38 ± 0.14 and 0.17 ± 0.13, respectively. These results indicate that the new enhanced monofocal toric IOL is a safe and effective treatment for astigmatic eyes with cataract.
Similar content being viewed by others
Introduction
In modern cataract surgery, effective management of corneal astigmatism is significantly important, offering the potential to substantially reduce postoperative reliance on spectacles. Approximately 36–47% of patients undergoing cataract surgery present with preexisting astigmatism of 1.00 diopter (D) or higher, which highlights the prevalence and relevance of addressing astigmatism during these procedures1,2. Various techniques, such as limbal relaxing incisions, corneal laser surgery, strategic phacoemulsification incisions, and toric intraocular lenses (IOLs), are available for intraoperative astigmatism management3. Among these, toric IOLs have gained prominence because of their predictability and safety4. However, the rotational stability of toric lenses is crucial. Prior research has indicated that a mere 1° misalignment correlates with a 3.3% loss of corrective efficacy5.
The TECNIS Eyhance Toric II IOL (Johnson & Johnson Vision, Santa Ana, CA, US) is a single-piece, aspheric refractive toric IOL based on the platform of the TECNIS toric ZCT monofocal IOL. The new lens features the same hydrophobic acrylic material, base geometry, corneal spherical aberration correction, and dimensions as the previous TECNIS toric IOL. However, it is an enhanced monofocal lens, akin to the TECNIS Eyhance IOL, featuring a higher-order polynomial aspheric anterior surface for reinforced intermediate visual acuity (VA)6.
Although previous satisfaction surveys7 and a single prospective cohort study8 have explored this IOL, a comprehensive understanding of its vector analysis, aberrations, and defocus curve data remains elusive. The present study aims to describe the near, intermediate, and distant visual outcomes, residual astigmatism, aberrations, and rotational stability after the implantation of this novel enhanced monofocal toric IOL.
Materials and methods
Study population
This retrospective, single-center study adhered to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University Health System (IRB file number: 4-2023-1662). Informed consent was waived by the Institutional Review Board of Yonsei University Health System due to the retrospective design and the use of de-identified patient data. The study enrolled patients who underwent cataract surgery with Eyhance Toric II IOL insertion between January 1, 2022, and June 30, 2023, at Yonsei University College of Medicine, Seoul, Republic of Korea. The inclusion criterion was cataract patients with preexisting regular corneal astigmatism of 1.0 D or more seeking toric IOL implantation. Preoperative astigmatism correction was guided by a systematic ‘stepladder’ approach to determine the optimal surgical strategy9. In this approach, for patients with less than 1 D of corneal astigmatism, the preferred method is to place the phacoemulsification incision on the steep corneal meridian. For astigmatism larger than or equal to 1 D, single or paired peripheral corneal-relaxing incisions or toric IOLs are indicated9. Consequently, this study enrolled patients with preoperative astigmatism of 1.0 D or more.
Exclusion criteria included (i) loss of follow-up before postoperative 3 months, (ii) a history of ocular surgery or trauma, (iii) ocular comorbidities impacting visual acuity, such as diabetic retinopathy, retinal detachment, glaucoma, pseudoexfoliation syndrome, uveitis, and wet age-related macular degeneration, and (iv) patients requiring additional astigmatism correction through femtolaser-assisted arcuate incision due to calculated residual astigmatism exceeding 0.50 D after toric IOL implantation.
Preoperative assessment
A comprehensive preoperative evaluation comprised of subjective refraction, uncorrected and best-corrected VA measurements, slit-lamp examination, noncontact tonometry, and fundoscopy under mydriasis. Eyes were categorized based on anterior keratometric readings as follows: with-the-rule (WTR) when the steep corneal cylinder axis was within 30 degrees of vertical (60–120 degrees), against-the-rule (ATR) when the cylinder axis was within 30 degrees of the horizontal axis (0–30 degrees and 150–180 degrees), and oblique if it was neither WTR nor ATR (30–60 degrees and 120–150 degrees), consistent with previous literature10.
Biometric assessments were performed using a swept-source optical coherence tomography (OCT)-based optical biometer (IOL Master-700, Carl Zeiss Meditec, Germany). The IOL power and target diopters were calculated using the IOL-Master 700, with an optical A-constant of 119.3 and a lens factor of 2.04 utilized for the SRK/T and Barrett Universal II formulas, respectively. Toric IOL cylinder power and axis placement were determined using the online TECNIS® Toric calculator (www.tecnistoriccalc.com) provided by the manufacturer (Johnson & Johnson Surgical Vision®), taking posterior corneal astigmatism into account. The aberrometer iTrace® (Tracey Technologies Corp., Houston, TX) was used for the wavefront analysis11. Total ocular aberrations, encompassing the root mean square (RMS) of total high-order aberration (HOA), trefoil, coma, and spherical aberration, were assessed at a 4-mm pupil diameter in a dark room using the iTrace® aberrometer.
Surgical technique
Prior to surgery, with the patient seated upright, reference markings at 0° and 180° were established on the corneal epithelium inside the limbus of the operated eye. Intraoperatively, a Mendez ring facilitated localization of the incision site and determination of the IOL placement axis. To optimize outcomes with this lens, three modifications were made to the standard cataract surgery procedure: First, all eyes underwent capsulorrhexis using the CATALYS™ Precision Laser System (OptiMedica Corporation, Sunnyvale, CA, USA) femtosecond laser to ensure a round, central, and adequately sized capsulorrhexis. Second, only cohesive ophthalmic viscosurgical device (OVD) was used during the operation, as dispersive OVD is known to be difficult to remove and tend to remain behind the IOL, resulting in early rotation of the IOL12. Third, we ensured thorough cortical removal and polishing of the anterior margin of the capsulorrhexis, as YAG capsulotomy for posterior capsular opacity is known to result in gross IOL tilt and astigmatic error13.
All operations were conducted by a single experienced surgeon (T. I. Kim) using the Centurion® Vision system (Alcon Surgical, Texas, USA). Femtosecond laser-assisted pretreatment included lens fragmentation and a 5.3-mm diameter capsulorhexis. The corneal incision was consistently made at 180° regardless of the laterality of the operative eye. The alignment of toric IOLs was intraoperatively confirmed using the intrastromal corneal marks obtained with the 3-dimensional spectral-domain OCT-controlled laser assistance of the CATALYS™ Precision Laser System14. Following surgery, all eyes received a standardized postoperative regimen, including the topical application of antibiotics, steroids, and nonsteroidal anti-inflammatory drugs.
Postoperative assessment
Follow-up assessments were performed 1 day, 1 week, 1 month, and 3 months postoperatively, including uncorrected distance visual acuity (UDVA), intraocular pressure measurements, slit-lamp examinations, and auto-keratometry refractions. Manual refraction was conducted at 1-month and 3-month postoperative visits, during which the UDVA and best-corrected distance visual acuity (CDVA) were determined at a distance of 4 m.
At the 3-month follow-up, the distance-corrected near visual acuity (DCNVA) at 33 cm and distance-corrected intermediate visual acuity (DCIVA) at 66 cm were assessed using Jaeger charts. A monocular, distance-corrected defocus curve was constructed, ranging from + 2.00 to − 4.00 D with 0.50 D power increments. Postoperative corneal topography was assessed using the iTrace® system to measure high-order aberrations. As described in previous literature, an anterior segment-OCT system (Casia SS-1000, Tomey Corp, Nagoya, Japan) was used to simultaneously evaluate the postoperative position of the toric IOL axis in a fully dilated pupil15. Unlike identifying the IOL axis under slit-lamp examinations, the toric IOL software of the AS-OCT simultaneously analyses corneal topography and acquires an image of the anterior segment; any changes in the patient’s head position will result in identical rotation of the topographic axis and IOL axis. Acquisition and evaluation processes of the image are automatic, independent of the operator’s skills, and have a resolution power of 1 degree, providing high interobservation and interobserver repeatability15.
Vector analysis of refractive astigmatism
Changes in astigmatism were analyzed using the Alpins method16,17. This method involves determining and calculating the following vectors: targeted induced astigmatism (TIA), which represents the intended magnitude and axis of astigmatic correction; surgically induced astigmatism (SIA), which represents the actual astigmatic change induced by surgery; difference vector (DV), which represents the difference in astigmatism between the achieved and target astigmatism; magnitude of error (ME), indicating the arithmetic difference in the magnitudes of the SIA and TIA; correction index (CI), calculated by determining the ratio of the SIA to the TIA, obtained by dividing SIA by TIA; angle of error, indicating the axis angle difference between the SIA and TIA, with a positive or negative value depending on whether the achieved correction was counterclockwise or clockwise to the intended axis, respectively; and index of success, calculated by dividing the DV by the TIA. All calculations were performed using Excel (Microsoft Office version 2007, Microsoft Corporation, Redmond, WA)18.
Statistical analysis
Baseline characteristics and comorbidities were analyzed using descriptive statistics. Continuous variables were presented as mean ± standard deviation (SD), while categorical variables were reported as frequency (percentage). Group differences were assessed using Student’s t-test or the Mann–Whitney U test based on Levene’s test. All tests were two-tailed, with the significance level set at p < 0.05. Statistical analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA) and MedCalc Statistical Software version 14.8.1 (MedCalc, Ostend, Belgium).
Results
This study included a total of 62 eyes from 41 patients with an average age of 68.07 ± 9.61 years and cylindrical values ranging from 1.00 to 2.75 D. Misalignment from intended implantation axis was not assessed in 18 eyes either because patient refused to receive anterior segment OCT (which is not covered by the national health insurance in Korea) or the position of the toric IOL could not be identified due to poor pupil dilation in the postoperative period. Consequently, the IOL misalignment at 3 months postoperatively was analyzed in 44 eyes. The overall analysis of postoperative visual and refractive outcomes incorporated data from all 62 eyes. Baseline characteristics, preoperative refractive error, and type of toric IOL model used are shown in Table 1. Patients had an average refractive cylinder of − 1.59 ± 0.91 D and the average corneal astigmatism measured with autokeratometry was 1.66 ± 0.49 D (Table 1).
Visual acuity, efficacy, and safety
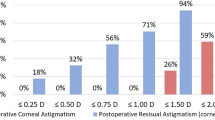
At 3 months postoperatively, significant improvements were observed in mean logMAR UDVA (from 0.64 ± 0.42 to 0.11 ± 0.13, p < 0.001) and logMAR CDVA (from 0.28 ± 0.30 to 0.03 ± 0.06, p < 0.001) (Table 2). Furthermore, UDVA and CDVA at 3 months postoperatively were 20/20 or better in 38.7% and 75.8% of the eyes, 20/32 or better in 77.4% and 95.2% of the eyes, and 20/40 or better in 95.2% and 100% of the eyes, respectively (Fig. 1A). A histogram illustrating the differences between the postoperative UDVA and preoperative CDVA is depicted in Fig. 1B, reflecting surgical efficacy. The mean efficacy index (ratio of the postoperative decimal UDVA to preoperative decimal CDVA) was 5.74 ± 7.00, and the mean safety index (ratio of the postoperative to preoperative decimal CDVA) was 2.64 ± 4.01. The refractive cylinder at 3 months postoperatively is displayed in Fig. 1C; notably, 52 (83.9%) eyes were ≤ 0.50 D and 59 (95.2%) eyes were ≤ 1.00 D.
Refractive and visual outcomes after Eyhance Toric II IOL implantation. (A) Comparison of UDVA and CDVA at 3 months postoperatively. (B) Difference of Snellen lines between UDVA at 3 months postoperatively and preoperative CDVA. (C) Refractive cylinder in diopters at postoperative 3 months. (D) Accuracy of SEQ at postoperative 3 months compared with intended goal diopter calculated with Barrett Universal II formula. IOL, intraocular lens; CDVA, corrected distance visual acuity; UDVA, uncorrected distance visual acuity; VA, visual acuity; postop, 3 months postoperatively; SEQ, spherical equivalent.
A histogram comparing the spherical equivalent refraction at 3 months postoperatively to the intended target diopter calculated using the Barrett Universal II formula is displayed in Fig. 1D, illustrating surgical predictability. The mean prediction error was − 0.14 ± 0.53 and 0.03 ± 0.49 for target diopters calculated with SRK/T and Barrett formulas, respectively (p < 0.001). The mean absolute error was 0.41 ± 0.35 and 0.38 ± 0.30 for target diopters calculated with SRK/T and Barrett formulas, respectively (p = 0.413). Remarkably, 72.6% of eyes remained within ± 0.50 D, and 95.2% remained within ± 1.00 D concerning the Barrett target diopter, indicative of good predictability.
Table 2 presents a comprehensive comparison of the pre-and postoperative outcomes in the current series. A statistically significant improvement in the UDVA of approximately 0.5 logMAR lines was found postoperatively (p < 0.001). This visual enhancement was consistent with a significant postoperative reduction in astigmatism of 81.9% (from 1.66 ± 0.49 D corneal cylinder preoperatively to − 0.30 ± 0.39 D refractive cylinder at 3 months postoperatively, p < 0.001). A significant reduction in additional diopters required for a working distance of 33 cm was also noted (2.36 ± 0.81 vs 1.77 ± 0.25, p = 0.001). Three months postoperatively, the mean logMAR DCNVA and DCIVA was 0.38 ± 0.14 and 0.17 ± 0.13, respectively.
Vector analysis
Vector analysis using the Alpins method was conducted at the 3-month follow-up (Fig. 2, Table 3). The average SIA at 3 months postoperatively was 1.57 ± 0.58 D (range, 0.27–3.34 D), with a centroid of 0.61 D @ 99° (Fig. 2B, Table 3). The average DV was 0.27 ± 0.35 D (range, 0.00–1.48 D), and the centroid was 0.23 D @ 94° (Fig. 2C, Table 3). The average index of success was 0.19 ± 0.24 (range, 0.00–0.93) and average ME was 0.13 ± 0.30. The average CI was 1.09 ± 0.22 (range, 0.30–1.66), indicating a minimal overcorrection in astigmatism correction (Fig. 2D, Table 3). In the vector analysis, the angle of error at postoperative day 1 was − 0.28 ± 7.42 degrees, exhibiting no significant difference compared to the angle of error of − 0.84 ± 7.16 at postoperative 3 months (p = 0.602) (Table 3).
Single-angle polar plots for the (A) target induced astigmatism vector, (B) surgically induced astigmatism vector, (C) difference vector, and (D) correction index at the axis of target induced astigmatism vector at 3 months postoperatively are shown. The mean absolute of vectors and the centroid of vectors calculated in double-angle vector space are displayed in the call-out box. Cyl, cylinder; Arith, arithmetic; D, diopters; Ax, axis.
Higher-order aberrations
Figure 3 presents the ocular aberration data before surgery and 3 months after implantation of the Eyhance Toric II IOLs. The ocular root mean square high order aberrations (0.91 ± 0.77 µm vs. 0.57 ± 0.86 µm before and 3 months after surgery, respectively; p = 0.037) and spherical aberration (0.12 ± 0.42 µm vs. − 0.07 ± 0.26 µm before and 3 months after surgery, respectively; p = 0.003) were significantly decreased after surgery. Ocular coma (0.47 ± 0.64 µm vs. 0.27 ± 0.44 µm before and 3 months after surgery, respectively; p = 0.077) and trefoil aberration (0.41 ± 0.38 µm vs. 0.26 ± 0.39 µm before and 3 months after surgery, respectively; p = 0.060) showed trend of decrease at 3 months postoperatively without statistical significance (Fig. 3).
Misalignment from intended implantation axis
The current study evaluated toric IOL alignment under a fully dilated status, 3 months postoperatively. The average misalignment of the intraocular lens from the target position showed a slight counterclockwise rotation of 0.68 ± 4.17° (range, − 9–10°). The average absolute misalignment was 3.41 ± 2.44° (range, 0°–10°) at postoperative 3 months. IOL misalignment was within ± 5° in 37 of 44 eyes (84.1%) and within ± 10° in all 44 eyes (100%). In an unadjusted linear regression analysis, a trend of 2.2% increase in the absolute refractive cylinder was observed with every 1° increase in absolute IOL misalignment (95% confidence interval − 2.5–6.9%, p = 0.345), although this trend was not statistically significant.
Toric IOL alignment was also assessed using the iTrace® Toric Alignment software at 3 months postoperatively19. The toric alignment axis measured with iTrace® aberrometers exhibited a strong correlation with, though not identical to, the anterior segment-OCT-checked axis misalignment under full dilation (Pearson correlation coefficient r = 0.46; p = 0.004). On average, there was a mean − 2.45 ± 9.72 error compared to the actual IOL alignment axis measured with the anterior segment-OCT under full dilation.
Defocus curves
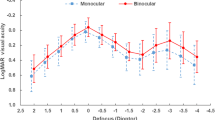
Figure 4 shows the mean distance-corrected monocular defocus curve for the Eyhance Toric II IOL. The visual acuity was 0.03 logMAR at 0 defocus, corresponding to CDVA. The mean visual acuity at − 1.50 D defocus, which corresponds to intermediate vision, was 0.25 logMAR. The mean visual acuity at − 2.50 D defocus, corresponding to near vision, was 0.46 logMAR. The depth of focus, defined by the American National Standards Institute as the dioptric power from the best peak of vision up to the defocus point for which the visual acuity is 0.2 logMAR, ranged from − 0.85 to 1.27 diopters (Fig. 4).
Subgroup analysis
Subgroup analysis was performed to assess preoperative factors associated with successful astigmatism correction (Table 4). Patients aged 70 years or older exhibited worse postoperative refractive cylinder (− 0.21 ± 0.32 vs. − 0.45 ± 0.45 in patients aged < 70 and ≥ 70 years, respectively; p = 0.014) and logMAR UDVA (0.07 ± 0.09 vs. 0.18 ± 0.15 in patients aged < 70 and ≥ 70 years, respectively; p = 0.003). Neither sex, axial length, nor the magnitude of preoperative astigmatism showed a significant association with postoperative refractive or visual outcomes.
Further subgroup analysis based on the degree of preoperative astigmatism revealed that eyes with ATR astigmatism were significantly undercorrected compared to those with WTR astigmatism (correction index 1.00 ± 0.11 vs. 0.85 ± 0.19 in the WTR and ATR groups, respectively; p = 0.003). Although the ATR group exhibited a trend towards higher refractive cylinder (− 0.21 ± 0.32 vs. − 0.39 ± 0.43 in the WTR and ATR groups, respectively; p = 0.078), this trend did not reach statistical significance. Eyes with oblique astigmatism showed the poorest postoperative outcomes in terms of mean logMAR UDVA, refractive cylinder, and correction index, although these differences were not statistically significant due to the small sample size in this group (n = 3).
Discussion
The postoperative findings of our study underscore the efficacy and safety of this newly developed monofocal toric IOL. This novel toric IOL, designed to address moderate-to-high astigmatism, exhibited outcomes comparable to those of previously established toric IOLs, with proven efficacy and safety in preliminary studies20,21,22,23. At 3 months postoperatively, significant enhancements were observed in mean logMAR UDVA and logMAR CDVA.
Mean prediction error was small for target diopters calculated with SRK/T and Barrett formulas, showing good predictability. Notably, preoperative astigmatism showed reduction by 81.9% at 3 months postoperatively and a large majority of eyes demonstrated residual refractive cylinder values of ≤ 0.50 D (83.9%) at the same interval, aligning with outcomes observed in established toric IOLs20,21,22,23. Remarkably, the enhanced monofocal toric IOL also revealed a significant reduction in additional diopters needed for a 33 cm working distance.
Vector analysis assesses astigmatic outcomes post-toric IOL implantation by considering both the magnitude and axis of astigmatic correction achieved. The TIA value, representing the predicted corneal plane equivalent cylinder power values of the IOL provided by the manufacturer, was 1.43 ± 0.47 D. The mean SIA, indicating the actual astigmatic change induced by the surgical procedure, was 1.55 ± 0.65 D. The mean angle of error between the SIA and the TIA was − 0.84 ± 7.16 degrees, demonstrating accurate placement of the IOLs at their intended axis of implantation at 3 months postoperatively. The negative angle of error possibly suggests that the surgically induced astigmatism due to corneal incision, predicted to be 0.50 @ 180° for left eyes and 0.30 @ 180° for right eyes preoperatively in our medical center, was actually smaller than expected. The standard deviation indicates a variable axis of effect of the incision, consistent with findings in previous studies24. The correction index of 1.09 and the mean positive magnitude of error of 0.13 ± 0.30 reflect slight overcorrection of the achieved astigmatic treatment. This overcorrection could potentially stem from underestimation of the toric IOL power at the corneal plane by the manufacturer25 or minor measurement errors using autokeratometry values26,27, possibly compounded by IOL misalignment. Therefore, considering this overcorrection preoperatively and reducing cylindrical power of the implanted IOL may further reduce residual refractive error and improve outcomes in surgeries using these toric IOL.
In the subgroup analysis, patients aged 70 years or older were more likely to result in worse postoperative UDVA a higher refractive astigmatism. Furthermore, eyes with preoperative ATR astigmatism were significantly more undercorrected compared to those with WTR astigmatism. The impact of the degree of preoperative astigmatism on postoperative refractive outcomes following toric IOL implantation varies among studies. A previous retrospective analysis investigating the effect of preoperative corneal astigmatism orientation on outcomes of low-cylinder-power toric IOLs found that the axis of preoperative corneal astigmatism was not a significant factor in the toric IOL group.28 However, another study indicated that, based solely on anterior corneal curvature, toric IOLs undercorrected ATR and oblique astigmatism, with the magnitude of error and postoperative residual refractive astigmatism being significantly smaller in the WTR group10. Consistent with these findings, our study demonstrated that eyes with ATR astigmatism prior to the implantation of Eyhance toric II IOLs were significantly undercorrected compared to those with WTR astigmatism, and there was a trend towards higher refractive cylinder in the ATR group, even after accounting for posterior corneal astigmatism in the toric IOL power calculation. These results emphasize that surgeons should be aware of the potential undercorrection, especially in patients with preoperative ATR.
Consistent with previous findings indicating a significant decrease in ocular total RMS HOAs and coma aberration, alongside a declining trend in ocular trefoil aberration following phacoemulsification for nuclear and cortical cataracts29, our aberrometry analysis revealed a reduction in postoperative ocular high-order aberration. Coma and trefoil aberrations exhibited a decreasing trend at 3 months postoperatively, although not reaching statistical significance.
The defocus curves and visual acuity results in eyes implanted with the toric IOL were comparable to outcomes seen with the well-established Eyhance IOL. Previous investigations on the Eyhance IOL indicated logMAR visual acuity values ranging from 0.20 to 0.27 at a defocus of − 1.50 D and 0.40 to 0.50 at a defocus of − 2.50 D. In our study, Eyhance Toric II IOLs exhibited logMAR visual acuity of 0.25 and 0.46 at defocus levels of − 1.50 D and − 2.50 D, respectively6,30,31,32. The mean DCIVA and DCNVA in our study aligned with previously reported values of 0.22–0.32 and 0.50–0.55, respectively, in Eyhance IOLs6,32,33.
Our study investigated toric IOL alignment relative to the intended implantation axis under full dilation 3 months postoperatively. A toric lens loses its effectiveness in correcting corneal astigmatism when rotated 30 degrees off the axis, with about one-third of the cylindrical correction’s impact diminishing for every 10-degree misalignment of the lens34. Previous studies on TECNIS toric ZCT IOL showed varying amounts of absolute axis orientation error of 2.56°–7.4235,36,37. In this study, mean IOL misalignment of this enhanced monofocal toric IOL was 0.68 ± 4.17° (range, − 9°–10°) at 3 months postoperatively and average absolute misalignment was 3.41 ± 2.44° (range, 0°–27°). In this retrospective study, assessments of toric lens alignment under full dilation were only conducted at the 3-month postoperative mark. Consequently, rotational stability between visits was indirectly evaluated through vector analysis. The angle of error demonstrated no significant difference between postoperative day 1 and postoperative 3 months.
In this retrospective study, follow-up period extended up to 3 months postoperatively. Previous investigations have reported a median IOL rotation of 0.3 degree from baseline to 1 week, 1.0 degree from 1 week to 1 month, 0.2 degree from 1 to 3 months, and 0.1 degree from 3 to 6 months38, indicating negligible rotation beyond the initial postoperative month. Therefore, we deemed the 3-month follow-up interval sufficient for assessing the rotational stability of the toric IOL. However, further research with extended follow-up durations, ideally six months or longer, would enhance our understanding of the long-term stability and efficacy of this IOL.
Although postoperative IOL rotation, as calculated through vector analysis, was minimal in this study, we identified a case with suboptimal postoperative astigmatism results. A patient with an axial length of 26.74 mm and a preoperative corneal cylinder of 1.25 D at 26 degrees in the right eye underwent cataract surgery with the DIU 150 toric IOL. On postoperative day 1, the visual and refractive outcomes were excellent, with a LogMAR UDVA of 0.00 and a refractive cylinder of 0.00 D. Although axis alignment under pupil dilation was not assessed on postoperative day 1, the angle of error was minimal at 0.38 degrees. However, by postoperative month 3, the patient presented with a LogMAR UDVA of 0.30 and a refractive cylinder of 1.00 D. Examination under full pupil dilation revealed a misalignment of 9 degrees. This postoperative rotation of the toric IOL aligns with previous literature, which indicates that toric IOL rotation is generally greater in eyes with longer axial lengths38.
Although this study exclusively presents the visual outcomes of the Eyhance toric IOLs without an active comparator, thereby precluding direct comparison of the optical performance of this IOL with traditional toric IOLs, the postoperative astigmatism results were similar to those reported for the traditional TECNIS toric ZCT or ZCU monofocal IOLs as demonstrated in a recent meta-analysis of toric monofocal IOLs39. In this meta-analysis, the percentage of eyes exhibiting residual refractive cylinder values of ≤ 0.50 D and ≤ 1.00 D ranged from 52.9 to 85.4% and 84.3 to 100% for the ZCT and ZCU lenses, respectively. These findings are consistent with the outcomes observed in this study, where a large majority of eyes demonstrated residual refractive cylinder values of ≤ 0.50 D (83.9%) and ≤ 1.00 D (95.2%). This indicates that the optical performance of the Eyhance Toric II IOLs in correcting astigmatism is comparable to that of the traditional ZCT and ZCU monofocal toric IOLs.
Furthermore, a recent retrospective study40 evaluated the intermediate vision performance of the Eyhance Toric II IOL in comparison with the traditional ZCT IOL. In this research, the Eyhance Toric II IOL exhibited significantly better defocus at − 1.5 D and − 2.0 D and significantly superior uncorrected intermediate visual acuity compared with the traditional ZCT Toric IOL, supporting the enhanced performance of the Eyhance Toric II IOL in intermediate vision.
Although there is no single universally accepted indication for toric IOL repositioning, criteria recommending surgical repositioning of toric IOLs typically entail misalignments greater than 10 or 15 degrees, depending on the surgeon’s decision41,42. In this study, the anterior segment OCT-measured IOL misalignment from the intended axis or the angle of error confirmed through vector analysis was within 15 degrees in all 62 eyes, and none received repositioning of toric IOL. Our results underscored the comparable rotational stability of Eyhance Toric II IOLs with toric IOLs of proven efficacy.
Previous research has indicated similar results in misalignment from intended implantation axis and visual outcomes between toric IOL implantation using a femtosecond laser and conventional phacoemulsification43,44,45. In the current study, all patients underwent femtosecond laser-assisted cataract surgery, and the refractive outcomes were comparable to those of a previous investigation on Eyhance Toric II IOL implantation with manual capsulorhexis, which reported refractive cylinder of ≤ 0.50 D in 84% and ≤ 1.00 D in 95% of patients at 3 months postoperatively8.
This study has several limitations. First, it lacked an active comparator, solely presenting the postoperative outcomes of the Eyhance Toric II IOLs. Second, all participants underwent capsulorhexis using a femtosecond laser, which potentially limited the generalizability of the results to those who underwent manual capsulorhexis. Thirdly, intermediate and near visual acuity were measured only with distance correction, and uncorrected intermediate or near visual acuity was not assessed. Fourthly, examinations under dilation are not routine clinical procedures in our medical center except for visits at 3 months postoperative, hence rotational stability between visits could not be assessed in our retrospective study. Additionally, the delta of rotation was calculated from the planned rotation, as intraoperative implantation axis was not routinely examined. Finally, all VA and defocus curves were obtained monocularly, necessitating binocular data for a more comprehensive understanding of the novel toric IOL.
To our knowledge, there has only been one previous study on the clinical outcomes of the Eyhance Toric II IOLs, showing the rotational stability of this IOL at 3 months postoperatively8. In this study, we aim to expand our comprehension of this new toric IOL by elucidating vector analysis, ocular aberration, and defocus curves subsequent to Eyhance Toric II IOL implantation. Despite these limitations, the current study provides valuable real-world data on the safety and efficacy of a new type of enhanced monofocal toric IOL.
In conclusion, the Eyhance Toric II IOLs demonstrated the ability to achieve significant improvements in UDVA and reductions in mean refractive cylinder, demonstrating good predictability, efficacy, and safety in this study. Defocus curves, along with the mean DCIVA and DCNVA, were comparable to those of previously established Eyhance™ IOLs. These findings suggest that this new enhanced monofocal toric IOL is a safe and effective treatment option for patients with cataracts and astigmatic eyes.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available because they contain information that could compromise patients’ privacy but are available from the corresponding author on reasonable request with permission from the Yonsei University College of Medicine.
References
Hoffmann, P. C. & Hutz, W. W. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J. Cataract Refract. Surg. 36, 1479–1485. https://doi.org/10.1016/j.jcrs.2010.02.025 (2010).
Yu, J. G. et al. Evaluation of biometry and corneal astigmatism in cataract surgery patients from Central China. BMC Ophthalmol. 17, 56. https://doi.org/10.1186/s12886-017-0450-2 (2017).
Yang, L. H. & Tang, X. The research progress in treating astigmatism at the time of cataract surgery. Zhonghua Yan Ke Za Zhi 47, 573–576 (2011).
Poll, J. T., Wang, L., Koch, D. D. & Weikert, M. P. Correction of astigmatism during cataract surgery: Toric intraocular lens compared to peripheral corneal relaxing incisions. J. Refract. Surg. 27, 165–171. https://doi.org/10.3928/1081597X-20100526-01 (2011).
Alpins, N. A. Vector analysis of astigmatism changes by flattening, steepening, and torque. J. Cataract Refract. Surg. 23, 1503–1514. https://doi.org/10.1016/s0886-3350(97)80021-1 (1997).
Unsal, U. & Sabur, H. Comparison of new monofocal innovative and standard monofocal intraocular lens after phacoemulsification. Int. Ophthalmol. 41, 273–282. https://doi.org/10.1007/s10792-020-01579-y (2021).
Vision, J. Johnson & Johnson Vision: Markteinführung der TECNIS Eyhance® Toric II Intraokularlinse in Europa.
Zeilinger, J., Hienert, J., Ruiss, M., Pilwachs, C. & Findl, O. Rotational stability of a new toric intraocular lens with an advanced optical profile. J. Cataract Refract. Surg. 49, 584–588. https://doi.org/10.1097/j.jcrs.0000000000001158 (2023).
Grunstein, L. L. & Miller, K. M. Astigmatism management at the time of cataract surgery. Expert Rev. Ophthalmol. 6, 297–305. https://doi.org/10.1586/eop.11.25 (2011).
Ninomiya, Y. et al. Toric intraocular lenses in eyes with with-the-rule, against-the-rule, and oblique astigmatism: One-year results. J. Cataract Refract. Surg. 42, 1431–1440. https://doi.org/10.1016/j.jcrs.2016.07.034 (2016).
Faria-Correia, F., Lopes, B., Monteiro, T., Franqueira, N. & Ambrosio, R. Jr. Scheimpflug lens densitometry and ocular wavefront aberrations in patients with mild nuclear cataract. J. Cataract Refract. Surg. 42, 405–411. https://doi.org/10.1016/j.jcrs.2015.10.069 (2016).
Hyon, J. Y. & Yeo, H. E. Rotational stability of a single-piece hydrophobic acrylic intraocular lens during removal of ophthalmic viscosurgical devices. Am. J. Ophthalmol. 149, 253-257.e251. https://doi.org/10.1016/j.ajo.2009.08.014 (2010).
Kaindlstorfer, C., Kneifl, M., Reinelt, P. & Schönherr, U. Rotation of a toric intraocular lens from neodymium:YAG laser posterior capsulotomy. J. Cataract Refract. Surg. 44, 510–511. https://doi.org/10.1016/j.jcrs.2018.02.018 (2018).
Dick, H. B. & Schultz, T. Laser-assisted marking for toric intraocular lens alignment. J. Cataract Refract. Surg. 42, 7–10 (2016).
Lucisano, A., Ferrise, M., Balestrieri, M., Busin, M. & Scorcia, V. Evaluation of postoperative toric intraocular lens alignment with anterior segment optical coherence tomography. J. Cataract Refract. Surg. 43, 1007–1009. https://doi.org/10.1016/j.jcrs.2017.05.025 (2017).
Alpins, N. Surgically induced astigmatism. Aust. N. Z. J. Ophthalmol. 22, 217. https://doi.org/10.1111/j.1442-9071.1994.tb01722.x (1994).
Mingo-Botin, D. et al. Comparison of toric intraocular lenses and peripheral corneal relaxing incisions to treat astigmatism during cataract surgery. J. Cataract Refract. Surg. 36, 1700–1708. https://doi.org/10.1016/j.jcrs.2010.04.043 (2010).
Elkadim, M. & El-Shehawy, A. Novel Microsoft Excel spreadsheet calculator for vector analysis of mass astigmatism data and comparison between Alpins and Thibos methods. Delta J. Ophthalmol. 23, 292–297 (2022).
Cywinski, A. & Bloch, D. Usefulness of the “Toric Alignment Check” module of the iTrace analyser in assessing the alignment of an intraocular Toric lens in relation to the axis of corrected corneal astigmatism postoperatively. J. Clin. Rev. Case Rep. 8, 84–90 (2023).
Potvin, R. et al. Prospective multicenter study of toric IOL outcomes when dual zone automated keratometry is used for astigmatism planning. J. Refract. Surg. 29, 804–809. https://doi.org/10.3928/1081597x-20131115-03 (2013).
Zhang, Z., Li, H., Zhou, J., Zhang, Y. & Zhang, S. Clinical evaluation of toric intraocular lens implantation based on iTrace wavefront keratometric astigmatism. BMC Ophthalmol. 20, 450. https://doi.org/10.1186/s12886-020-01726-0 (2020).
Sheppard, A. L. et al. Clinical outcomes after implantation of a new hydrophobic acrylic toric IOL during routine cataract surgery. J. Cataract Refract. Surg. 39, 41–47. https://doi.org/10.1016/j.jcrs.2012.08.055 (2013).
Waltz, K. L., Featherstone, K., Tsai, L. & Trentacost, D. Clinical outcomes of TECNIS toric intraocular lens implantation after cataract removal in patients with corneal astigmatism. Ophthalmology 122, 39–47. https://doi.org/10.1016/j.ophtha.2014.06.027 (2015).
Kaufmann, C. et al. Astigmatic neutrality in biaxial microincision cataract surgery. J. Cataract Refract. Surg. 35, 1555–1562. https://doi.org/10.1016/j.jcrs.2009.03.048 (2009).
Goggin, M., Moore, S. & Esterman, A. Toric intraocular lens outcome using the manufacturer’s prediction of corneal plane equivalent intraocular lens cylinder power. Arch. Ophthalmol. 129, 1004–1008. https://doi.org/10.1001/archophthalmol.2011.178 (2011).
Krall, E. M. et al. Vector analysis of astigmatism correction after toric intraocular lens implantation. J. Cataract Refract. Surg. 41, 790–799. https://doi.org/10.1016/j.jcrs.2014.07.038 (2015).
Park, H. J. et al. Comparison of the astigmatic power of Toric intraocular lenses using three Toric calculators. Yonsei Med. J. 56, 1097–1105. https://doi.org/10.3349/ymj.2015.56.4.1097 (2015).
Ernest, P. & Potvin, R. Effects of preoperative corneal astigmatism orientation on results with a low-cylinder-power toric intraocular lens. J. Cataract Refract. Surg. 37, 727–732. https://doi.org/10.1016/j.jcrs.2010.11.026 (2011).
Zhu, X. et al. Objective functional visual outcomes of cataract surgery in patients with good preoperative visual acuity. Eye 31, 452–459. https://doi.org/10.1038/eye.2016.239 (2017).
Yangzes, S., Kamble, N., Grewal, S. & Grewal, S. P. S. Comparison of an aspheric monofocal intraocular lens with the new generation monofocal lens using defocus curve. Indian J. Ophthalmol. 68, 3025–3029. https://doi.org/10.4103/ijo.IJO_985_20 (2020).
Salgado-Borges, J., Borges, A., Ferreira, I., Gonzalez-Meijome, J. M. & Faria-Ribeiro, M. Optical characterization and through-focus performance of two advanced monofocal intraocular lenses. Graefes Arch. Clin. Exp. Ophthalmol. https://doi.org/10.1007/s00417-023-06322-8 (2023).
Danzinger, V. et al. Intraindividual comparison of an enhanced monofocal and an aspheric monofocal intraocular lens of the same platform. Am. J. Ophthalmol. https://doi.org/10.1016/j.ajo.2023.11.006 (2023).
Goslings, O., Veraart, H., van de Laar-Muskens, J. & Pinero, D. P. Clinical outcomes with an aspheric monofocal and a new enhanced monofocal intraocular lens with modified optical profile. Graefes Arch. Clin. Exp. Ophthalmol. 261, 2315–2326. https://doi.org/10.1007/s00417-023-06128-8 (2023).
Wiley, W. F. & Bafna, S. Intra-operative aberrometry guided cataract surgery. Int. Ophthalmol. Clin. 51, 119–129. https://doi.org/10.1097/IIO.0b013e31820f226d (2011).
Brar, S., Rathod, D. P., Nikhil, R. & Ganesh, S. Clinical outcomes, predictability and rotational stability following implantation of Eyecryl toric versus TECNIS toric intraocular lenses—A comparative study. Int. Ophthalmol. 41, 3769–3780 (2021).
Jung, N. Y., Lim, D. H., Hwang, S. S., Hyun, J. & Chung, T. Y. Comparison of clinical outcomes of toric intraocular lens, Precizon vs Tecnis: A single center randomized controlled trial. BMC Ophthalmol. 18, 292. https://doi.org/10.1186/s12886-018-0955-3 (2018).
Brar, S., Rathod, D. P., Nikhil, R. P. & Ganesh, S. Clinical outcomes, predictability and rotational stability following implantation of Eyecryl toric versus TECNIS toric intraocular lenses—A comparative study. Int. Ophthalmol. 41, 3769–3780. https://doi.org/10.1007/s10792-021-01944-5 (2021).
Shah, G. D. et al. Rotational stability of a toric intraocular lens: Influence of axial length and alignment in the capsular bag. J. Cataract Refract. Surg. 38, 54–59. https://doi.org/10.1016/j.jcrs.2011.08.028 (2012).
Al-Mohtaseb, Z. et al. Toric monofocal intraocular lenses for the correction of astigmatism during cataract surgery: A report by the American Academy of Ophthalmology. Ophthalmology 131, 383–392. https://doi.org/10.1016/j.ophtha.2023.10.010 (2024).
Hwang, H. S., An, D., Kim, H. S. & Kim, E. C. Comparison of visual efficacy and patient’s satisfaction between two Toric IOLs, enhanced for intermediate vision and monofocal. BMC Ophthalmol. https://doi.org/10.21203/rs.3.rs-3963398/v1 (2024).
Haripriya, A., Gk, S., Mani, I. & Chang, D. F. Comparison of surgical repositioning rates and outcomes for hydrophilic vs hydrophobic single-piece acrylic toric IOLs. J. Cataract Refract. Surg. 47, 178–183. https://doi.org/10.1097/j.jcrs.0000000000000415 (2021).
Visser, N., Bauer, N. J. & Nuijts, R. M. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J. Cataract Refract. Surg. 39, 624–637. https://doi.org/10.1016/j.jcrs.2013.02.020 (2013).
Lai, K. R., Zhang, X. B., Yu, Y. H. & Yao, K. Comparative clinical outcomes of Tecnis toric IOL implantation in femtosecond laser-assisted cataract surgery and conventional phacoemulsification surgery. Int. J. Ophthalmol. 13, 49–53. https://doi.org/10.18240/ijo.2020.01.07 (2020).
Espaillat, A., Perez, O. & Potvin, R. Clinical outcomes using standard phacoemulsification and femtosecond laser-assisted surgery with toric intraocular lenses. Clin. Ophthalmol. 10, 555–563. https://doi.org/10.2147/OPTH.S102083 (2016).
Clark, K. D. Toric intraocular lens outcomes with a new protocol for IOL selection and implantation. J. Fr. Ophtalmol. 41, 145–151. https://doi.org/10.1016/j.jfo.2017.08.007 (2018).
Funding
Financial support: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2023-00302193). The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
H.J. established the database, performed statistical analyses, and drafted the article; T.K. conceived the idea of the project; and I.J., H.K.L., and K.Y.S. contributed to revising the intellectual content. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jung, H., Jun, I., Lee, H.K. et al. Real-world outcomes of cataract surgeries using a new type of enhanced monofocal toric intraocular lens. Sci Rep 14, 27758 (2024). https://doi.org/10.1038/s41598-024-65746-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-65746-2