Abstract
This paper documents the occurrence of the genus Umbilicaria in the Argentine Islands–Kyiv Peninsula region of the Graham Coast in the maritime Antarctic. The presence of seven Umbilicaria species (U. africana, U. antarctica, U. aprina, U. decussata, U. kappenii, U. nylanderiana and U. umbilicarioides) in the ice-free areas of the Argentine Islands–Kyiv Peninsula region were confirmed. The species of U. africana and U. aprina are documented from the studied region for the first time. This study moves the southern distribution limit of U. africana about 300 km to the south: to the Argentine Islands–Kyiv Peninsula region. The distribution maps of Umbilicaria species for the studied region and maritime Antarctica are prepared.
Similar content being viewed by others
Introduction
In the world, Umbilicaria species are widely distributed in alpine and bipolar habitats. This genus is easily distinguished by foliose thalli growing on rocks, attached to the substratum at a single point by a short strand called an umbilicus. The specimens of this genus can reach the thalli sizes from one to 30 cm in diameter or more, e.g. U. antarctica1,2. The Umbilicaria lichens grow on stable, rugged and weather-resistant acidic rocky substrates. In the Antarctic, the Umbilicaria genus is widespread and often locally abundant. It occurs less frequently on south-facing rocks and is absent on cliffs and boulders exposed to wind and salt spray1,2,3,4.
Although the Umbilicaria genus is distinct and well distinguished from other genera of lichens, the species among themselves are difficult to determine, especially in the field. The specimens of this genus are probably still overlooked during hard, polar fieldwork and limited access to the spots where Umbilicaria are abundant. This explains why the species of Umbilicaria are still discovered in Antarctica as new to science5 or as new to the region4,6. Øvstedal and Lewis Smith2 reported 10 species of Umbilicaria in the Antarctic region. In contrast, on the Argentine Islands–Kyiv Peninsula region, five species have been published from this region by Smith and Corner7 and Gremmen et al.8, and BAS Database9, including U. antarctica, U. decussata, U. kappenii, U. nylanderiana and U. umbilicarioides. Whereas on the South Shetland Islands neighbouring the Antarctic Peninsula, as many as nine species of Umbilicaria were reported1,4. In the Argentine Islands–Kyiv Peninsula region, Smith and Corner7 identified Umbilicaria species in sub-formation called fruticose lichen and moss cushion in three sociations, such as Andreaea-Dicranoweisia grimmiacea-Usnea-Umbilicaria, Andreaea-Grimmia-Usnea-Umbilicaria and Usnea-Umbilicaria decussata-Alectoria. Based on the environmental analysis, Gremmen et al.8 identified communities with Umbilicaria as the Usnea complex. These communities are developed on the rocks, debris and ledges formed by erosion, conditioned by the limited inflow of organic matter from birds and relatively low concentration of chlorides8. Water from melting snow flows into them. They are also exposed to sunlight and UV rays, and in winter they freeze without a permanent snow cover.
Lichens (symbiotic organisms, lichenized fungi) are generally very sensitive to environmental changes and respond quickly. They are sensitive because of their internal structure and physiological features. They are long-living, ectohydric organisms with a limited ability to regulate gas and water exchange. Both nutrients and toxic elements dissolved in the atmosphere are absorbed through the whole surface of the thalli10. In the 1990s rapid warming was observed in the maritime Antarctic11. It resulted in changes in the original communities of vascular plants and cryptogams12,13,14. Some changes related to this phenomenon have been shown in the Oasis Lions Ramp on King George Island, i.e. it was observed, during the long-term experiment on the dynamics of the lichen biota within the Antarctic Specially Protected Area No. 15115. According to other studies during ten years of observation, the cover of the dominant lichen Usnea antarctica Du Rietz declined by 71% in the study plots16. It seems very important, to study the actual composition of the lichen flora and communities in the maritime Antarctic to determine the direction of upcoming changes.
In this paper, the authors present data collected during their current fieldwork and historical data from previous expeditions7,8,9. Authors discuss the occurrence, status and ecological features of the Umbilicaria lichens in the area in the context of the overall distribution in the study region.
Material and methods
Study area
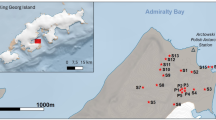
The Argentine Islands are located on a coastal shelf in the western part of the Antarctic Peninsula, 2–6 km from the coastal line of the Kyiv Peninsula (Fig. 1). It is a group of low ice-capped islands laying 6–12 km west of the Graham Coast and separated from the mainland by the Penola Strait water. They consist of more than 40 islands of different sizes. They form some groups separated by inter-island waters with depths of less than 50 m. The largest of this group are Skua Island (0.6 km2) and Galindez Island (0.4 km2), and the highest point is placed on Uruguay Island (65 m a.s.l.). The Ukrainian Academic Vernadsky Antarctic Station is located on Galindez Island, Marina Point (65° 14′ 45.2′′ S, 64° 15′ 27.7′′ W)17,18.
The Argentine Islands–Kyiv Peninsula region is composed mainly of volcanic igneous and intrusive rocks. Islands’ coasts are mostly steep and rocky, almost without abrasive terraces or beaches. The western shore of the Antarctic Peninsula is a part of the maritime Antarctic and has a mild and humid climate. The mean annual air temperature varies from − 2.4 to − 5.4 °C. The mean air temperatures of the summer months are above zero, and the highest exceed + 10 °C18. The mean temperatures of the coldest months (June–August) seldom fall beyond − 15 °C7. The absolute minimum of − 43.3 °C was detected on the Argentine Islands in August 1958, and the maximum of + 11.7 °C—in February 196018. The Argentine Islands are somewhat north of the Southern Polar Circle, so the classic polar night does not occur there. During the Antarctic winter solstice (June 22), the Sun rises above the horizon by almost 3 degrees19. The sum of the light time during the day is maximal from the second half of November until the end of January, i.e. during the polar day20. The average air humidity in the region is 86%, and the average annual precipitation is 433 mm with a coefficient of variation of 0.32. The predominant type of precipitation is snow. In some years, its thickness can reach almost 3 m, however, in rocky areas, these values are rather significant21.
Materials
The field studies were carried out in the Argentine Islands–Kyiv Peninsula region during the Ukrainian Antarctic Expeditions in the austral summer seasons of 2018/19, 2019/20, 2020/21 and 2021/22. Samples were packed into sampling bags with notes describing the sites: altitude, substrate, type of vegetation and coordinates. The geographical names of the sampling sites are given following Yevchun et al.22. In addition, some unpublished materials of Umbilicaria from the Danco Coast and Victoria Land, and specimens from King George Island curetted at the KRAM L herbarium were included to achieve a more complete view of species distribution and/or for molecular analysis.
The voucher specimens were deposited in the Herbarium of the W. Szafer Institute of Botany of the Polish Academy of Sciences in Krakow (KRAM L), (Appendix 1S). The thallus material for nuclear ITS rDNA sequence analysis was obtained from 18 specimens of Umbilicaria (GenBank Accession Numbers PP580112, PP580129). The specimens for DNA analysis were only air-dried at room temperature. The details of the analysed specimens and GenBank accession numbers are presented in Table 1. The morphology was examined using a standard stereomicroscope Nikon SMZ 645 and a compound microscope Nikon Eclipse E200. Measurements were made in water or c. 5% KOH.
DNA extraction, PCR amplification
The total DNA was extracted from fresh and herbarium materials. The small, ground samples from herbarium specimens were prepared. Next, they were extracted in acetone for 1 h to remove secondary lichen products. The acetone was discarded and the samples were dried at room temperature to let the acetone evaporate. The total genomic DNA was extracted using DNeasy Plant Mini Kit (Qiagen) following the manufacturer’s instructions. The PCR reaction was performed in 25 µl volume comprising 3 μl of DNA template, 2.5 μl of 10 × AmpliTaq 360 PCR Buffer, 2.5 μl 25 mM MgCl2, 0.5 μl dNTPs (10 mM each; Sigma Aldrich), 0.2 μl of AmpliTaq 360 DNA polymerase (Applied Biosystems), 0.2 μl of bovine serum albumin (BSA; New England Biolabs), 1 μl of forward and reverse primer (10 μM), and sterile distilled water was added to attain the final volume. The fungi primers for the nuclear ITS rDNA selected for the reaction were: nu–SSU–1752–5′ (ITS1F)28 and nu–LSU–0041–3′ (ITS4)29.
The reactions run with the following parameters for the nuclear ITS rDNA: initial denaturation at 95 °C for 5 min, then 5 cycles of denaturation at 95 °C for 30 s, annealing at 54 °C for 30 s, and extension at 72 °C for 1 min, followed by 33 cycles of denaturation at 95 °C for 30 s, annealing at 48 °C for 30 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 10 min and a 4 °C hold. PCR products were visualized by running 3 μl of the PCR product on 1% agarose gels. The PCR products were subsequently purified using the mixture of exonuclease (10 U/μl, 5000 U, EurX) and shrimp alkaline phosphatase (1 U/μl, 1000 U, Applied Biosystems). Purified PCR products were sequenced by Macrogen (Amsterdam, Netherlands) using the same amplification primers. The ITS1–5.8S–ITS2 rDNA sequences were generated bidirectionally and read by GATC Biotech AG (Germany). Consensus sequences were obtained manually with Geneious Prime 2022.1.1. (https://www.geneioues.com). The ITS1–5.8S–ITS2 rDNA sequences generated in this study have been deposited in the NCBI nucleotide sequence database (https://www.ncbi.nlm.nih.gov) under accession numbers (Table 1).
Phylogenetic analyses
The newly obtained sequences were compared in GenBank using BLASTn search30. Highly similar sequences were chosen for phylogenetic analyses (Table 1). The sequences were aligned with the MAFFT v.7.49031,32 plugin implemented in Geneious Prime 2022.1.1 (https://www.geneioues.com) and adjusted manually. The maximum-likelihood (ML) analysis was conducted in IQ-TREE version 1.6.1233. IQ-TREE with the -m TEST options was run so that ModelFinder34 would find the best-fitting substitution model. In total, 5000 ultrafast bootstrap pseudoreplicates were performed to calculate support for the analysed tree35,36. The calculated tree was rooted with Xylopsora friesii (Ach.) Bendiksby and Timdal KY947740. The results were visualised using FigTree 1.4.4. On the diagram, the posterior probabilities and ML bootstrap values are placed on well-supported branches [SH-aLRT support (%) / ultrafast bootstrap support (%)].
Results and discussion
Species diversity of the genus Umbilicaria
In the surveyed area seven Umbilicaria species were determined in the materials originating from the ice-free areas. The list of recorded species comprises: U. africana (Jatta) Krog and Swinscow, U. antarctica Frey and I.M. Lamb, U. aprina Nyl., U. decussata (Vill.) Zahlbr., U. kappenii Sancho, Schroeter and Valladares, U. nylanderiana (Zahlbr.) H. Magn. and U. umbilicarioides (Stein) Krog and Swinscow. Information is given below concerning their morphological characteristics and results of phylogenetic analysis.
Morphological characteristics
The investigated species of Umbilicaria, based on their morphological features, can be divided into two distinct groups: one with rhizines on the lower surface of their thalli (i.e.: U. africana, U. antarctica, U. aprina, U. kappenii, and U. umbilicarioides) and the other without rhizines (i.e.: U. decussata and U. nylanderiana).
Umbilicaria decussata, one of the species without rhizines, is very distinct and relatively easily distinguished from other species of the genus, particularly due to the upper surface with white wrinkled structures organised in a reticular pattern. The upper side is generally dark grey to grey-brown and scabrous, with a white necral layer in the central part, and sharp ridges radiating from the centre, fading into a reticulate pattern of weak ridges towards the periphery. However, this is quite a variable species, the most remarkable extremes being specimens with a completely smooth upper surface, and specimens with a laciniate outline to the margin2. On the other hand, the lower surface is completely sooty black (except for the margin, which is grey to brown), covered with unicellular, rarely bicellular thalloconidia a characteristic feature of the species. U. decussata most frequently reproduces vegetatively by thalloconidia35,36 and has rarely been recorded with fertile thalli in Antarctica2,4. Similarly, in our study, among all recorded sites fertile specimens were found only on Black Island (KRAM-L 72735).
Although U. decussata is easily distinguished from other species of the genus, it is sometimes confused with U. krasheninnikovii, which has a very similar upper side of its thallus. Meanwhile, U. krasheninnikovii differs clearly in the appearance of its lower surface which is pale brown, without black patches, and lacks thalloconidia. However, in the field, both species can be confused due to similarities in the appearance of the upper side of their thalli1. The lack of records of U. krasheninnikovii in the investigated area may be due to the species being overlooked during field surveys because the first field identification is based only on the upper surface.
Umbilicaria nylanderiana, similarly to U. decussata has no rhizines. It is also characterized by unicellular thalloconidia created in sooty black patches on the lower surface. However, it can be distinguished from U. decussata by the presence of brown vermiform ridges on the upper surface of the thallus. The thallus of U. nylanderiana is usually monophyllous, though in our study a few polypyllous thalli were also found, particularly in wind-exposed habitats. The Antarctic specimens grow only up to ca. 2 cm in diameter2. Thus, they are considerably smaller compared to specimens from other regions where this species usually reaches 3–5 cm in favourable conditions and up even to 15 cm across37.
In the past U. nylanderiana was often mistakenly reported from Antarctica as U. hyperborea (Ach.) Hoffm.,4. In fact, in our study, juvenile specimens of U. nylanderiana (up to 0.5 cm in diameter), with a brown lower side of the thallus, without black patches and thalloconidia (similar as in the mature specimens of U. hyperborea) were often observed in the Wilhelm Archipelago area. The pale brown lower side of their thalli is typical for the juvenile specimens of U. nylanderiana that have not yet started producing black thalloconidia. While U. nylanderiana and U. hyperborea are generally easily distinguished by their morphology, in the case of the latter which has rather more delicate and fragile thallus, with brown, vermiform folded upper surface, apothecia that tend to be embedded among the folds in smaller specimens, these differences might be inconspicuous. It is likely, therefore, that records of U. hyperborea from Green Island9 and other records of this species from Antarctica38 or elsewhere in the Southern Hemisphere may relate to juvenile forms of U. nylanderiana, whereas the distribution of U. hyperborea seems to be restricted to boreal zone with less harsh climatic conditions39.
Among the species characterised by the presence of the rhizines, only U. umbilicarioides have dark brown to black rhizines richly branched at the top part (tree-like). Similarly, U. cristata also possesses multi-divided rhizines from one point and barred multicellular thalloconidia, but rhizines of that species are pale and grouped on the lower surface. Umbilicaria umbilicarioides has multi-cellular thalloconidia produced on the apices of richly branched, black rhizines placed mainly in marginal parts of the lower surface. The other rhizinate Umbilicaria species are recognized by only simple, dichotomous or rarely irregularly multi-branched rhizines.
Specimens of U. umbilicarioides investigated during current work are very variable. They had mono- to polyphyllous thalli, from one to 3–4 cm in diameter, only exceptionally larger up to 6 cm. These large specimens were characterized by ashy grey to blackish upper surface, often pale grey pruinose with well visible black rhizines in the marginal part. However, the upper side of some of the investigated specimens was pale brown. Probably these thalli were not light exposed. The lower sides of the thalli were smooth and pale beige-brown, sometimes nearly pinkish with only a dark and scabrous part around the umbilicus to scabrous and black throughout. The rhizines were abundant, mostly black terete, richly branched, shrubby to coralloid, and thallyles frequent, especially in the materials from Skua Island. These rhizines' tops are covered with black multi-cellular thalloconidia, appearing as a black rim around the thallus when it is seen from above. The Antarctic specimens are generally sterile2. However, single specimens with immature apothecia were reported from Galindez Island40 and King George Island1.
Multicellular thalloconidia are also present in U. africana, but they are produced on the lower surface of the thallus in sooty black patches. The specimens of U. africana found in Antarctica are generally small and reach only up to ca. 4 cm in diameter, the specimens from other regions are bigger, for instance in South America they reach up to 6 cm in diameter. The biggest specimens, up to 14 cm in diameter, were found in Africa.
The Antarctic specimens of U. africana can be confused with the juvenile specimens of U. antarctica and U. aprina. However, U. africana can easily be distinguished by pale to pale grey, simple to dichotomously branched or occasionally richly branched rhizines, circular in section, grey to brownish grey on the upper surface with dark marginal rhizines giving a ciliate appearance and by multi-cellular thalloconidia (4–10-celled, 20–40 µm diam.) bared in sooty black patches on the lower surface not on rhizines1. Umbilicaria antarctica and U. aprina also bared thalloconidia in black patches on the lower surface and have rhizines on the lower surface but differ in the presence of unicellular, rarely 1-septate thalloconidia.
Moreover, in the field, U. antarctica is often easily distinguished by its conspicuous size, with individual thalli reaching even 30 cm or more across1,2. However, in our study, such large specimens were recorded only on Darboux Island on the high steep rock walls. Umbilicaria antarctica is very variable and only the largest specimens can easily be distinguished during fieldwork. The other species of Umbilicaria generally grow only up to 7 cm in diameter, and only the thalli of U. kappenii and less frequently of U. aprina can reach up to 15 cm in diameter. Small specimens of U. antarctica can easily be confused with these two species. The most important characteristics distinguishing U. kappenii are the presence of dark brown soredia on the upper side and the lack of thalloconidia on the lower side of the thallus. In contrast, U. antarctica lacks soredia but its lower side is covered with unicellular thalloconidia which makes the lower side of the thallus distinctly black. While U. aprina also possesses black unicellular thalloconidia, it can be distinguished from U. antarctica by the lack of strap-like rhizines and its upper side of the thallus is more or less reticulate scabrous over the umbilicus.
Umbilicaria kappenii has dark brown soredia on the upper side of the margin and lacks thalloconidia on the lower side of the thallus whereas U. antarctica has unicellular thalloconidia and lacks soredia. The Lower side of U. kappenii is light brown, purple-brown or ash-grey, sometimes only brown-black around the umbilicus whereas U. antarctica is black on the lower side.
Phylogenetic analysis
The phylogenetic analyses involved 57 nucleotide sequences. The new 19 fungal ITS nrDNA sequences were aligned with 38 sequences available in the Genbank to produce a matrix (Table 1). The 19 newly obtained sequences were between 565 and 859 bp long. There was a total of 598 positions in the final dataset. The Bayesian inference and maximum-likelihood analyses yielded consistent tree topologies. The results are shown (Fig. 2) on a consensus tree obtained in ML analyses. The posterior probabilities and the ML bootstrap values are placed above the well-supported branches (SH-aLRT support 82%, ultrafast bootstrap support 96%).
Maximum likelihood phylogenetic tree of the ITS1-5 8S-ITS2 rDNA sequences of Umbilicaria species. Newly generated sequences are given in bold. The tree is rooted with sequences of Xylospora friesii. The posterior probabilities and ML bootstrap values are placed on well-supported branches [SH-aLRT support (%) / ultrafast bootstrap support (%)].
The highest support (100% ultrafast bootstrap support) was observed in two clades, one with the sequences of U. nylanderiana and the other with U. umbilicarioides. Two newly received sequences of U. nylanderiana from Galindez and Winter Islands were clade together with the sequences of U. nylanderiana from the GenBank from Russia and Bolivia. These sequences are separated very well on the cladogram, with 97.8% SH-aLRT support and 100% ultrafast bootstrap support. The clade with U. umbilicarioides has also high support (97.3% and 100%, respectively) and encloses sequences obtained from Antarctic and Chilean material from the GenBank. The population of U. umbilicarioides from Antarctica seems to be genetically homogenous. The newly received sequences are very similar although the material was collected from different scattered locations (such as Galindez, Skua, Uruguay, Winter and Western Corner Islands). It is important to indicate that some untypical small brownish thalli, initially classified as belonging to U. cristata based on their morphological characteristics, during the genetic analyses of ITS nuDNA region (sequences PP580124 and PP580125, Fig. 2) claded together with U. umbilicarioides; thus, these specimens most likely represent juvenile forms of U. umbilicarioides.
Umbilicaria africana and U. aprina were also separated into well-supported clades. The newly received sequences of U. aprina from Antarctica clade together with sequences from the GenBank from the Himalayas and Antarctica. The samples of U. aprina were separated very well on the cladogram, with 95.6% SH-aLRT support and 96% ultrafast bootstrap support. However, the population of U. africana seems to be diverse having two well-supported branches. One with the sequences from Galindez Island (PP580112), Chile (GenBank KY 947844) and King George Island (PP580116) (with support 90/97) and the other with the sequences from Bolivia (PP580112) and Peru (GenBank HM161482) (85.4/96) (Fig. 2). It is important to note that the specimen from King George Island (PP580116) was previously reported by Krzewicka and Smykla1 as U. cristata as it had all morphological characteristics typical of that species. However, our current genetic analyses demonstrated that this material represented juvenile specimens of U. africana. It seems likely that all previous reports of U. cristata from Antarctica may refer to juvenile specimens of U. africana or U. umbilicarioides—the species also distinguished by the ciliate appearance. Unfortunately, there are no sequences of U. cristata available in GenBank. Therefore, re-examination and molecular analyses of other specimens determined previously as U. cristata are necessary to verify their taxonomic position.
The two newly received sequences of U. decussata from Antarctica clade with sequences of U. decussata from the GenBank. However, it is worth indicating an unusual position of the sequence of U. krasheninnikovii within this clade (Fig. 2). The sequences received from the specimens of U. krasheninnikovii seem to be generally very problematic in molecular analyses. For instance, three different ITS sequences of U. krasheninnikovii from the GenBank (i.e.: AY603134, JQ739994, KY947857) surprisingly clade in three separate clades (Fig. 2). However, the sequence AY603134 clade with U. decussata likely belongs to the latter species. During this study, we also prepared the ITS sequences (not included in the final analysis) from U. krasheninnikovii specimens collected previously on King George Island (KRAM-L 471331) possessing all typical key features of the species (confirmed by Umbilicaria specialist—B. Krzewicka). Surprisingly, ITS sequence of this specimen of U. krasheninnikovii clades together with U. antarctica. Maybe it is a result of the morphological structure of U. krasheninnikovii thallus which probably is easily settled by other diasporas of Umbilicaria that are sequenced instead of the main material.
The clade with sequences of U. antarctica and U. kappenii is well-supported (99.2/100). The support for individual clades is considerably lower, for instance for U. antarctica up to 82.2% SH-aLRT support and 97% ultrafast bootstrap support. While both these species can easily be distinguished by their morphological and anatomical features, they remain genetically closely related, forming one clad. For this reason, based on molecular data alone, Otto et al.41 treated both taxa as one species U. antarctica. According to recent trends, modern taxonomy should be based on genetics and classical taxonomy41,42. The differences between U. antarctica and U. kappenii have also been discussed in detail in previous literature1,2,5. Therefore, contrary to the suggestion of Otto et al.27 in this study we consider both taxa as valid separate species.
Antarctic species of Umbilicaria not recorded in the study area
Of the 10 Umbilicaria species recorded in Antarctica1,2 only four were not found within the study area, including U. cristata Dodge and Baker, U. krascheninnikovii (Savicz) Zahlbr., U. saviczii Llano and U. aff. thamnodes Hue.
Umbilicaria cristata is an Antarctic endemic reported from South Georgia I. and South Orkneys Is., the Antarctic Peninsula (rare) and the continental Antarctic (scarce)2. In our study, all specimens with ciliate appearance that resembled U. cristata turned out to be small, juvenile specimens of U. africana or U. umbilicarioides which are also characterized by ciliate appearance.
Umbilicaria krascheninnikovii was reported from the South Orkney Islands and the South Shetland Islands (Livingstone and King George Islands), and south of the Antarctic Peninsula to ca. 70ºS2,43,44. In the North Hemisphere, it was previously reported from Svalbard at 78° N45. Currently, this species is known also from many other localities in the N Hemisphere, including the Arctic and the Himalayas46,47.
Umbilicaria saviczii occurs in the continental Antarctic1,2. This species is likely to be found in the examined area.
Umbilicaria aff. thamnodes was reported only from Charcot Island and Alexander Island2. Probably this Himalayan species should be found in other localities in Antarctica.
Species distribution
The species of U. africana and U. aprina are documented from the studied region for the first time. The occurrence of U. antarctica U. decussata, U. kappenii, U. nylanderiana and U. umbilicarioides in the Argentine Islands–Kyiv Peninsula region is confirmed. The occurrence of Umbilicaria species in the study area was briefly discussed above. The complete list of records with notes on local distribution for all Umbilicaria species recorded in the Argentine Islands–Kyiv Peninsula region and some new registrations outside this region is given in Appendix 1S. The maps of species distribution are provided in Figs. 3, 4, 5, 6, 7, 8 and 9.
Umbilicaria africana
It is reported for the first time from the area investigated in the present study. The species turned out to be rare and was found in only two localities, including Galindez and Skua Islands (Fig. 3, Appendix 1S). The discovery of the sites of Umbilicaria africana in the studied region makes it possible to shift the southern limit of its distribution in the maritme Antarctic about 300 km southward to the Argentine Islands region. On both islands, the occurrence of U. africana was restricted to the sheltered north-facing volcanic rock slopes where it was found outside the splash zone at an altitude of ca. 20–40 m a.s.l. The species was generally growing together with U. kappenii, U. umbilicarioides, and other fruticose and crustose lichens.
Umbilicaria africana is known from the high mountains of East Africa, where it occurs at altitudes between 3000 and 5000 m a.s.l.48, from South America where it is often the most common high alpine Umbilicaria species found along the entire Andean Mountain Range from Venezuela to Chile at elevation between 4300 and 5000 m a.s.l.35,36,49,50,51,52, and from Antarctica1,2,4. Moreover, U. africana has also been reported from Mt. Adjuno in Java46. However, as noted by Wei and Jiang46, these specimens exhibit morphological inconsistencies with the species’ original description. Therefore, their identification is only provisional and requires more detailed taxonomic evaluation. This type of disjunction distribution pattern, excluding Australia and New Zealand, is exceptional for lichens of the Southern Hemisphere and suggests that this species may represent an ancient phytogeographical element developed in the western regions of Gondwana4.
In Antarctica, U. africana has been found in both the maritime Antarctic region, where it was reported from Livingston Island4 and King George Island1,44, and the continental Antarctic in the northern Victoria Land and Wilkes Land4. It has also been found in the Subantarctic in South Georgia2,9. The species seems to be rare, though locally abundant1, and usually found inland at elevations 25–200 m a.s.l.4,44 on vertical, north-facing rocks, growing among other species of the genus1.
Umbilicaria antarctica
From the Argentine Islands–Kyiv Peninsula region, the species was reported as abundant and frequent7,8 and recorded at many sites, including Black Island, Galindez Island, Petermann Island, Uruguay Island, Rasmussen Island, the Berthelot Islands (with Green Island), Booth Island, Cape Tuxen and two continental points near Lemaire Channel9. Our current study confirmed most of the previous reports from the Argentine Islands–Kyiv Peninsula region and added some new distribution records, including from other areas such as Beneden Head and Cape Sterneck on the Danco Coast or Hook Island on the Graham Coast (Appendix 1S). With a total of 68 recorded sites (including 36 new records), U. antarctica is the most common and widespread Umbilicaria species in the region (Fig. 4).
The species is particularly abundant in coastal sites on sheltered, north-facing moist boulders or cliff faces, where it often provides almost complete cover. It is found on a variety of volcanic and/or intrusive rocks at altitudes 6–500 m a.s.l. However, it has an irregular range (Fig. 4, Appendix 1S). It does not occur in the splash zone and on the low outer islands, such as Cruls, Roca and Anagram, which are exposed to strong winds and extensive sea spray during storms. It also does not occur in areas exposed to ornithogenic influence, such as Great Yalour Island, which indicates its nitrophobic preferences. Typically grows with Usnea antarctica, alone or with other species of Umbilicaria.
This is an Antarctic endemic known previously as U. rufidula (Hue) Filson, the synonym name. The species is widely distributed and locally abundant, particularly across the northern maritime Antarctic, including the South Sandwich Islands, the South Orkney Islands, the South Shetland Islands and the Antarctic Peninsula1,2, but only rarely found in the continental Antarctic2,53.
Umbilicaria aprina
Our current work provides the first documented records of U. aprina in the Argentine Islands–Kyiv Peninsula region. Though it has been reported nearby, from a few sites along the west coast of the Antarctic Peninsula40, unfortunately exact locations of these collections are unknown. In the investigated area U. aprina appears to be rare, and was found only on two of the largest Argentine Islands, i.e.: Galindez and Skua Islands (Fig. 5). On both islands the occurrence of U. aprina was restricted to the coastal sites where it was found outside the splash zone at an altitude of ca. 16–20 m a.s.l., on rather dry sheltered north-facing volcanic rock outcrops. However, none were found in habitats flushed by meltwater or at margins of temporary melt streams, indicated previously2 as its typical habitat. The species was found growing together with other species of the genus, notably: U. antarctica, U. kappenii, U. nylanderiana, U. umbilicarioides, and other fruticose and crustose lichens.
Umbilicaria aprina is a species with disjunct bipolar-alpine distribution, it occurs in both Polar Regions and is also known from the high mountains across all continents except Australia2,48,54. However, for a long time, U. aprina was known only from Dedschen Mts. in Ethiopia, it was recorded at 4700 m a.s.l.55 and from Ruwenzori Mts. in Uganda56. It was, therefore, considered as an African endemic. Later it was found in Scandinavia57 and in the American Arctic region58,59. Subsequently, it was also recorded across the Arctic region of Eurasia59,60,61,62,63, in the Himalayans64 and in high mountains of Europe, including the Alps65, Carpathians66 and Pyrenees67 in South America along the Andes35,36,51,68 and again in Africa on Kilimanjaro48.
In Antarctica, U. aprina is known mostly from the continent, where it is widespread and locally abundant2,4,40, with records spanning elevation between 10 and 1700 m a.s.l.2. On the other hand, in the maritime Antarctic, it seems to be rare and some of its previous records from King George and Livingston Islands, the maritime Antarctic69, are doubtful2,4; however, the occurrence of U. aprina on King George Island was confirmed in our previous treatment1.
Umbilicaria decussata
From the Argentine Islands–Kyiv Peninsula region U. decussata has been reported as frequent, associated with gently sloping to vertical and dry wind-exposed rocks7,8 and was found at several collection sites, including Black Island, Galindez Island, Petermann Island, Rasmussen Island, Green Island (the Berthelot Islands), Uruguay Island, Cal between Mt. Mill and Mt Balch and the Mount Mill summit9. During the present study, U. decussata was confirmed to be relatively frequent and locally abundant, and was recorded at 21 surveyed localities (12 new) of the studied region and 1 previously unknown site outside this region—Lagoon Island at Fallières Coast (Fig. 6, Appendix 1S).
In line with previous reports1,2,4, U. decussata was found growing on wind-exposed dry rocks and cliff faces, predominantly on volcanic but also intrusive substratum (e.g. Boudet Island), and within broad altitudes ranging from low-elevation coastal sites (e.g. Boudet Island, 5 m a.s.l.) to inland high elevation sites (i.e. Gaies Cliff, 545 m a.s.l.). However, it does not occur on the low outer islands, such as Cruls, Roca and Anagram Islands, which are exposed to strong winds and extensive sea spray during storms. It was also not found on Great Yalour Island and the southern part of Petermann Island, hosting large penguin colonies and exposed to intensive ornithogenic fertilization. Observations from the Arctic38 and New Zealand37 suggest, however, that moderate ornithogenic fertilization provided by perching flying birds may provide favourable conditions for the species' growth. The species grows alone or with other species of the genus and other fruticose and crustose lichens.
Umbilicaria decussata is a cosmopolitan, distinctly high alpine species. It is widespread across, both the Northern and Southern Hemisphere, with distribution spanning all the continents and both Polar Regions, it is generally known only from scattered localities. For instance, in South America it occurs at altitudes 4500–5300 m a.s.l. along the entire Andean chain from Patagonia to Venezuela35,36,37,38,49,50,51 but nowhere is common or abundant. In Australia, it was found only in the south-western territories along the Australian Alps and in Tasmania, where it is rare with the local population represented by only a few small thalli growing on exposed rock ridges that serve as bird perching sites37. In Europe, this species is known from all high mountain ranges but also nowhere is common or abundant39, except for Svalbard where it is a common species45,62
On the other hand, in Antarctica this is a generally common and widely distributed species, growing on dry and exposed rocks from coastal sea level sites to high altitude far-inland sites at over 2000 m a.s.l.1,2,70,71. It is considered rare in the northern maritime Antarctic (i.e. South Orkneys and South Shetlands), and it becomes increasingly more common and abundant with increasing latitude, especially in the southern Antarctic Peninsula and around the Continent2. However, our previous survey on King George Island, South Shetlands1, documented U. decussata as one of the most frequent species of the genus, and locally very abundant. Moreover, also in the area of Arthur Harbour (Antarctic Peninsula), it seems to be relatively common7,8. Thus, the view on latitudinal abundance pattern for U. decussata may not reflect the real distribution, but this could be mainly due to lack of floristic exploration and paucity of data.
Umbilicaria kappenii
Accordingly, in the Argentine Islands–Kyiv Peninsula region this species was previously reported only from Black and Petermann Islands5,9. Thus, a total of 45 localities of the Argentine Islands–Kyiv Peninsula region (41 previously unknown) and two outside this region (including a new record from Cape Sterneck) documented during our current study (Fig. 7, Appendix 1S) change considerably perception of the species occurrence in the area; U. kappenii appears to be one of the most common and abundant species of the genus. It is remarkable that only U. antarctica, generally recognized as the most common and abundant species of Umbilicaria across the entire maritime Antarctic1,2, was recorded more frequently and at more sites during our survey. This finding is in line with previous studies1,5 suggesting that U. kappenii is probably much more frequent and abundant across the maritime Antarctic, but is often overlooked in the field due to its high resemblance to U. antarctica.
While U. kappenii is widespread and often very abundant in the area, and growing both on volcanic and intrusive rocks (i.e. Rasmussen Island), similarly to other species of the genus, its occurrence is generally limited to sheltered north-exposed rock walls. Moreover, its distribution in the area appears to be restricted to coastal sites within altitudes of 3–80 m a.s.l. and, in contrast to reports from other areas5,44, it was not found at higher elevations and sites distant from the coastline. The species was also not found on the low outer islands, such as Cruls, Roca and Anagram Islands, that are exposed to strong winds and extensive sea spray during storms. The lack of the species on Great Yalour Island and other areas exposed to strong ornithogenic influence indicates its nitrophobic preferences. Surprisingly, this taxon was reported previously from the southern part of Petermann Island9 which hosts large penguin colonies. However, this record was not confirmed during our study.
Umbilicaria kappenii typically grows alone or mixed with other species of the genus, notably with U. antarctica and/or U. umbilicarioides (rarely also with U. nylanderiana or U. africana), and other fruticose and crustose lichens7,8. The co-occurrence of U. kappenii with U. antarctica is particularly noticeable, both species were often found together growing in intermixed stands, suggesting very similar ecological preferences for these two Antarctic endemics. However, with occurrence spanning the entire Antarctic and altitudes up to 750 m a.s.l.2, U. antarctica demonstrates a considerably broader range of ecological and geographical distribution.
Umbilicaria kappenii is an Antarctic endemic with occurrence restricted to the maritime Antarctic. It appears to have a continuous distribution range throughout the entire region, extending from the South Orkneys in the north, along the west coast of the Antarctic Peninsula and its offshore islands, to as far south as Adelaide and Lagoon Islands (68°S)1,2,5. It also seems to have a relatively broad altitudinal range growing between 2 and 300 m a.s.l., though most commonly found on dry north-facing rocks in coastal areas2,5. However, despite its seemingly wide altitudinal and latitudinal distribution range across the entire maritime Antarctic the species seems to be rare. For instance, during our previous survey on King George Island1 it was found only in two sites. Similarly, Olech44 despite long long-lasting and detailed survey of the same area reported only two additional sites.
Umbilicaria nylanderiana
In the Argentine Islands–Kyiv Peninsula region U. nylanderiana was previously recorded from the Argentine Islands–Galindez Island?8, Rasmussen Island and probably Green Island9. Consistently, during our survey, the species was found to be rare and never abundant, and was recorded only at seven surveyed sites (probably five previously unknown) in the Argentine Islands–Kyiv Peninsula region, including Black, Galindez, Grotto, Skua, Storozh and Winter Islands, as well as at one previously unknown site, i.e. to the south of the studied area on Blaiklock Island on the Fallières Coast (Fig. 8). It was found only in coastal sites at altitudes of 8–20 m, growing on north-facing walls of different heights, on both volcanic and intrusive rocks. Its occurrence was typically associated with other species of the genus, notably with U. umbilicarioides and U. kappenii, and only rarely with U. decussata, U. antarctica or U. aprina, and fruticose and crustose lichens. However, as U. nylanderiana can be easily overlooked in the field due to its strong resemblance to U. decussata and U. krascheninnikovii1,4, it is likely to be more frequent and future surveys may provide additional records.
This is a bipolar and high alpine species known from the mountain, polar and sub-polar habitats across all the continents, except Africa. While its distribution was primarily associated with the Northern Hemisphere, where it is generally common and widespread4,39, recent surveys demonstrated its widespread distribution also across the Southern Hemisphere, including South America where it was found along the entire Andean chain at altitudes ranging between 4100 and 5300 m a.s.l.35,36,49,50,51,72, in the Australian Alps and Tasmania above 1000 m a.s.l.37 and the Southern Alps in New Zealand73.
In Antarctica distribution of U. nylanderiana is restricted to the maritime Antarctic, with very few records scattered from the South Orkneys in the north2,9, across South Shetlands1,4,44 and the west coast of the Antarctic Peninsula and its offshore islands, to as far south as 68°S in Marguerite Bay on Léonie Island and Rothera Point on Adélaide Island2,9. It was found only in coastal sites at elevations between 5 and 200 m a.s.l., growing on ridges and vertical or sloping north-facing volcanic rocks, often together with U. decussata1,4,44. Although, generally rare and known only from very few sites it is locally abundant with populations in some environments more numerous than other Umbilicaria species2,4.
Umbilicaria umbilicarioides
In the Argentine Islands–Kyiv Peninsula region U. umbilicarioides was previously reported as a frequent and locally abundant component of vegetation on Galindez Island7,8. Additionally, it was recorded only in a relatively few sites, including Galindez, Uruguay, Rasmussen, Skua and Green Islands, and Cape Tuxen9.
During the current study, U. umbilicarioides was widespread and was recorded also at several other sites across the entire Argentine Islands–Kyiv Peninsula region (Fig. 9, Appendix 1S). Thus, currently with a total of 45 sites (19 previously unknown) recorded in the studied region, U. umbilicarioides, along with U. antarctica and U. kappenii, appear to be one of the most widespread and frequently recorded species of the genus. However, despite the broad distribution of U. umbilicarioides, it was not found on the low outer islands, such as Cruls, Roca and Anagram Islands, that are exposed to strong winds and extensive sea spray during storms. It was also not found on Great Yalour Island and the southern part of Petermann Island hosting large penguin colonies and exposed to intensive ornithogenic fertilization. Thus, the local distribution of U. umbilicarioides appears to be confined to more sheltered coastal sites and altitudes between 3 and 42 m a.s.l., where it was found growing on sheltered north-facing walls of different heights, on both volcanic and intrusive substratum. It grows alone or with other Umbilicaria species, notably with U. antarctica and less frequently with other species of the genus. We also found a new stand of U. umbilicarioides, on Hook Island south of the studied region.
Specimens of this species were previously reported from Antarctica as U. propagulifera (Vain.) Llano which is currently treated as a synonym name of U. umbilicarioides. Its distribution seems to be confined to the Southern Hemisphere, where it has been reported from alpine and subalpine environments in South America38, Africa48, Australia37, New Zealand72 and Antarctica1,2. In some previous treatments U. umbilicarioides was considered as having bipolar-alpine disjunct distribution, but the records from Northern Europe were mistakenly reported as U. propagulifera74,75; according to Hestmark76, they actually represent the species U. dendrophora (Poelt) Hestmark. However, for some Umbilicaria specimens from the Carpathian Mountains in Romania and Poland, initially determined based on their morphology as U. cylindrica group (without thalloconidia), their sequences in molecular analyses (ITS and LSU) were unexpectedly claded together with sequences of U. umbilicarioides from Antarctica77. Thus, the occurrence of U. umbilicarioides in the mountains of the Northern Hemisphere should still be considered.
In Antarctica, the distribution of U. umbilicarioides seems to be restricted to the maritime Antarctic and the Subantarctic. Although it has also been reported from Enderby Land in the continental Antarctic, this record is dubious2. Thus, the known distribution of U. umbilicarioides spans numerous records scattered from South Georgia9, South Orkneys2,9, South Shetlands1,43,78 and many sites along the west coast of the Antarctic Peninsula and its offshore islands, to as far south as 69.5° S on Engel Picks at Wilkins Coast9. Although locally frequent and abundant, especially on dry rocks and pebbles on raised beaches in the mid-southern Antarctic Peninsula, e.g. in northern Marguerite Bay, is generally considered rare2.
Possible local dispersal mechanisms
Umbilicaria species are not evenly distributed in the study area (Figs. 3, 4, 5, 6, 7, 8 and 9). It is not clear what the reason for this distribution is, but there are two main factors in the spread of diasporas—wind and birds79,80. The species of this genus reproduce mainly by producing vegetative propagules such as soredia, isidia, thalloconidia, conidia or thalli fragmentation and thallyles, whereas production of generative spores is very rare on this area38,67,81. Propagules are likely carried here mainly by the wind. In winter they are transported between islands through frozen channels, while in summer they move mainly within neighbouring islands. This is evidenced by the local distribution of these species, which is concentrated in the coastal zone.
The birds are the second factor in the dispersal of diasporas in this area. The fragments of Umbilicaria specimens were discovered in nests of Stercorarius maccormicki on Winter, Grotto and Skua Islands, and in nests of Larus dominicanus on Galindez Island, on the north part of Petermann Island and Booth Island. The fragments of the thallus of Umbilicaria antarctica (KRAM-L 70414) and Umbilicaria kappenii (KRAM-L 72656) were discovered in nests of Larus dominicanus located on the northern coast of the eastern arm of Booth Island. The nearest site of both species was several dozen meters higher on the slope. In addition, birds could collect fragments of the Umbilicaria kappenii thallus on the top of the ridge of the eastern arm of Booth Island, where this species grew on rocks (KRAM-L 72403). Whereas the thalli of Umbilicaria antarctica could be collected by birds 4 km to the northeast on the northern head of Booth Island in a green oasis where birds also collected rare moss Warnstorfia sarmentosa (Wahlenb.) Hedenäs for nest building. This moss species occurs only in this one place in this part of the region. In the case of birds, species with more noticeable thalli may have an advantage in transport. This would explain the wide local range of the species with the largest sizes, such as U. antarctica and U. kappenii (Figs. 4 and 7). Birds also use lichen thalli for mating behaviour. At the beginning of the summer season, fragments of lichen thallus abandoned by gulls during the mating season are regularly recorded near the Vernadsky station on Galindez Island. Specimens of lichens abandoned by birds can be next transported by the wind. In the region, probably both dispersal mechanisms of foliose lichens are important.
Conclusions
Our study demonstrates that the Argentine Islands–Kyiv Peninsula region is characterized by a relatively high diversity of the Umbilicaria species (7 taxa).
Umbilicaria africana and U. aprina were recorded for the first time in the region. The discovery of the species U. africana here shifts the southern limit of its distribution by approximately 300 km south.
Umbilicaria antarctica is the most frequent species in the region. This taxon is a dominating species in the maritime Antarctic. Umbilicaria umbilicaroides which was until now treated as rare in Antarctica is widely distributed in the study area. It is one of the most numerous species in this area, along with U. antarctica and U. kappenii.
Umbilicaria decussata is a widespread species in the maritime Antarctic, however, it is less frequent in the study area. It was also discovered on an inland nunatak on the Kyiv Peninsula—the next registration of this species inland in this Peninsula region.
Umbilicaria nylanderiana is a rare species in this region. During the current study, it was found exclusively on the Argentine Islands.
Data availability
The voucher specimens were deposited in the Herbarium of the W. Szafer Institute of Botany Polish Academy of Sciences in Krakow (KRAM L), Lubicz 46, 31-512 Kraków, Poland. The thallus material for nuclear ITS rDNA sequence analysis was obtained from 18 specimens of Umbilicaria (GenBank accession numbers PP580112, PP580129).
References
Krzewicka, B. & Smykla, J. The lichen genus Umbilicaria from the neighbourhood of Admiralty Bay (King George Island, maritime Antarctic), with a proposed new key to all Antarctic taxa. Polar Biol. 28, 15–25. https://doi.org/10.1007/s00300-004-0638-9 (2004).
Øvstedal, D. O. & Lewis Smith, R. I. Lichens of Antarctica and South Georgia: A Guide to their Identification (Cambridge University Press, 2001).
Lindsay, D. C. New records for Antarctic Umbilicariaceae. Br. Antarctic Surv. Bull. 21, 61–69 (1969).
Sancho, L. G., Kappen, L. & Schroeter, B. The lichen genus Umbilicaria on Livingston Island, south Shetland Islands, Antarctica. Antarctic Sci. 4(2), 189–196. https://doi.org/10.1017/S0954102092000294 (1992).
Sancho, L. G., Schroeter, B. & Valladares, F. Umbilicaria kappenii (Umbilicariaceae) a new lichen species from Antarctica with multiple mechanisms for the simultaneous dispersal of both symbionts. Nova Hedwigia 67, 279–288. https://doi.org/10.1127/nova.hedwigia/67/1998/279 (1998).
Convey, P., Lewis Smith, R. I., Peat, H. J. & Pugh, P. J. A. The terrestrial biota of Charcot Island, eastern Bellingshausen Sea, Antarctica: An example of extreme isolation. Antarctic Scie. 12, 406–413 (2000).
Smith, R. I. L. & Corner, R. W. M. Vegetation of the Arthur Harbour - Argentine Islands region of the Antarctic Peninsula. Br. Antarctic Surv. Bull. 33–34, 89–122 (1973).
Gremmen, N. J. M., Huiskes, A. H. L. & Francke, J. W. Epilithic macrolichen vegetation of the Argentine Islands, Antarctic Peninsula. Antarctic Sci. 6(4), 463–471. https://doi.org/10.1017/S0954102094000702 (1994).
BAS Database BAS Antarctic Plant Database. https://apex.nerc-bas.ac.uk/f?p=148:1:24192077027123. Accessed 20 May 2023 (2023).
Nash, T. H. (ed.) Lichen Biology 2 (Cambridge University, 2008).
Turner, J. et al. Antarctic climate change during the last 50 years. Int. J. Climatol. 25, 279–294. https://doi.org/10.1002/joc.1130 (2005).
Fowbert, J. A. & Lewis Smith, R. I. Rapid population increases in native vascular plants in the Argentine Islands Antarctic Peninsula. Arctic Alpine Res. 26(3), 290–296 (1994).
Gerighausen, U., Bräutigam, K., Mustafa, O. & Peter, H. U. Expansion of vascular plants on an Antarctic Islands a consequence of climate change? In Antarctic Biology in a Global context (eds Huiskes, A. H. L. et al.) 79–83 (Backhuys Publihers, 2003).
Parnikoza, I. et al. Current status of the Antarctic herb tundra formation in the Central Argentine Island. Glob. Change Biol. 15, 1685–1693. https://doi.org/10.1111/j.1365-2486.2009.01906.x (2009).
Olech, M. & Słaby, A. Changes in the lichen biota of the Lions Rump area, King George Island, Antarctica, over the last 20 years. Polar Biol. https://doi.org/10.1007/s00300-015-1863-0 (2016).
Bokhorst, S., Convey, P., Huiskes, A. & Aerts, R. Usnea antarctica, an important Antarctic lichen, is vulnerable to aspects of regional environmental change. Polar Biol. 39(3), 511–521. https://doi.org/10.1007/s00300-015-1803-z (2016).
Gozhyk, P. F. et al. Karta reliefa dna melkowodnoi zony arkhypelaga Argentinskich ostrovov v raione Ukrainskoj antarktycheskoi stancii «Akademik Vernadsky» [The map of bottom relief of shallow deep zone of Argentine Islands archipelago in the region of Ukrainian Antarctic station «Academican Vernadsky»]. Geologycheskyi Zhurnal 1, 128–131 (2002).
Parnikoza, I., Berezkina, A., Moiseyenko, Y., Malanchuk, V. & Kunakh, V. Complex survey of the Argentine Islands and Galindez Island (maritime Antarctic) as a research area for studying the dynamics of terrestrial vegetation. Ukr. Antarctic J. 1(17), 73101. https://doi.org/10.33275/1727-7485.1(17).2018.34 (2018).
Govorukha, L. S. Short geographical and glaciological characteristic of Argentine Islands archipelago. Ukr. Antarctic Center Bull. 1, 17–19 (1997).
Tymofeev, V. E. Organization of meteorological studies and comparison investigation of atmospheric processes in 1996 on Antarctic Station Vernadsky. First Ukrainian Antarctic expedition 1996–1997. Bull. Ukr. Antarctic Centre 1C, 49–52 (1997).
Martazinova, V. F., Tymofeyev, V. E. & Ivanova, Y. K. Current regional climate of the Antarctic Peninsula and Akademik Vernadsky station. Ukr. Antarctic J. 9, 231–248. https://doi.org/10.33275/1727-7485.9.2010.411 (2010).
Yevchun, H. et al. The toponymy of the Argentine Islands area, the Kyiv Peninsula (West Antarctica). Ukr. Antarctic J. 2, 127–157. https://doi.org/10.33275/1727-7485.2.2021.683 (2021).
Davydov, E. A., Peršoh, D. & Rambol, G. Umbilicariaceae (lichenized Ascomycota) – Trait evolution and a new generic concept. Taxon 66(6), 1282–1303 (2017).
Hestmark, G. et al. Single origin and subsequent diversification of central Andean endemic Umbilicaria species. Mycologia 103(1), 45–56. https://doi.org/10.3852/10-012 (2011).
Ivanova, N. V., DePriest, P. T., Bobrova, V. K. & Troitsky, A. V. Phylogenetic analysis of the lichen family Umbilicariaceae based on nuclear ITS1 and ITS2 rDNA sequences. Lichenologist 31, 477–489 (1999).
Pérez-Ortega, S., Ortiz-Álvarez, R., Allan Green, T. G. & de los Ríos, A. Lichen myco- and photobiont diversity and their relationships at the edge of life (McMurdo Dry Valleys, Antarctica). FEMS Microbiol. Ecol. 82(2), 429–448 (2012).
Ott, S., Brinkmann, M., Wirtz, N. & Lumbsch, H. T. Mitochondrial and nuclear ribosomal DNA data do not support the separation of the Antarctic lichens Umbilicaria kappenii and Umbilicaria antarctica as distinct species. Lichenologist 36, 227–234. https://doi.org/10.1017/S0024282904014306 (2004).
Gardes, M. & Bruns, T. D. ITS primers with enhanced specificity for basidiomycetes – Application to the identification of mycorrhizae and rusts. Mol. Ecol. 2, 113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x (1993).
White, T. J., Bruns, T., Lee, S. & Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications (eds Innis, M. A. et al.) 315–322 (Academic Press Inc, 1990).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. https://doi.org/10.1093/nar/25.17.3389 (1997).
Katoh, K., Misawa, K., Kuma, K. & Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30(14), 3059–3066. https://doi.org/10.1093/nar/gkf436 (2002).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30(4), 772–780. https://doi.org/10.1093/molbev/mst010 (2013).
Nguyen, L.-T., Schmidt, H. A., von Haesele, A. & Minh, B. Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 32, 268–274. https://doi.org/10.1093/molbev/msu300 (2015).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 14(6), 587–589. https://doi.org/10.1038/nmeth.4285 (2017).
Hestmark, G. New observations and records for Umbilicaria (Umbilicariaceae) in Bolivia. Bryologist 112, 833–838. https://doi.org/10.1639/0007-2745-112.4.833 (2009).
Minh, B. Q., Nguyen, M. A. T. & von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 30, 1188–1195. https://doi.org/10.1093/molbev/mst024 (2013).
Kantvilas, G. & Louwhoff, S. The lichen genus Umbilicaria Hoffm in Tasmania. Muellera 25, 3–20. https://doi.org/10.5962/p.292233 (2007).
Llano, G. A Monograph of the Lichen Family Umbilicariaceae in the Western Hemisphere (Office of Naval Research, 1950).
Krzewicka, B. The lichen genera Lasallia and Umbilicaria in the Polish Tatra Mts. Pol. Bot. Stud. 17, 1–88 (2004).
Filson, R. B. Studies in Antarctic lichens 6: Further notes on Umbilicaria. Muelleria 6, 335–347 (1987).
Lücking, R., Leavitt, S. D. & Hawksworth, D. L. Species in lichen-forming fungi: Balancing between conceptual and practical considerations, and between phenotype and phylogenomics. Fungal Divers. 109, 99–154. https://doi.org/10.1007/s13225-021-00477-7 (2021).
Lücking, R., Moncada, B. & Forno, M. PhyloKey: A novel method to rapidly and reliably identify species in complex, species-rich genera, and an opportunity for ‘non-molecular museomics’. Lichenologist 55(5), 181–192. https://doi.org/10.1017/S0024282923000415 (2023).
Olech, M. Annotated Checklist of Antarctic Lichens and Lichenicolous Fungi (The Institute of Botany of the Jagiellonian University, 2001).
Olech, M. Lichens of King George Island (Antarctica. The Institute of Botany of the Jagiellonian University, 2004).
Øvstedal, D. O., Tønsberg, T. & Elvebakk, A. The lichen flora of Svalbard. Sommerfeltia 33, 1–39. https://doi.org/10.2478/v10208-011-0013-5 (2009).
Wei, J. & Jiang, Y. The Asian Umbilicariaceae (Ascomycota). Mycosystema Monographicum Series No 1 (International Academic Publishers, 1993).
Davydov, E. A., Himelbrant, D. E. & Stepanchikova, I. S. Contribution to the study of Umbilicariaceae (lichenized Ascomycota) in Russia. II. Kamchatka Peninsula. Herzogia 24, 251–263 (2011).
Krog, H. & Swinscow, T. D. V. The lichen genera Lasallia and Umbilicaria in East Africa. Nord. J. Bot. 6, 75–85. https://doi.org/10.1111/j.1756-1051.1986.tb00861.x (1986).
Galloway, D. J. & Quilhot, W. Checklist of chilean lichen-forming and lichenicolous fungi Gayana. Botanica 55(2), 111–185 (1998).
Hestmark, G. The lichen genus Umbilicaria in Ecuador. Nordic J. Bot. 34, 257–268. https://doi.org/10.1111/njb00952 (2016).
Krzewicka, B. & Flakus, A. New records of the genus Umbilicaria (Umbilicariaceae, lichenized Ascomycota) from Bolivia. Cryptogamie Mycologie 31(4), 441–451 (2010).
Sipman, H. J. M. & Topham, P. The genus Umbilicaria (lichenized ascomycetes) in Colombia. Nova Hedwigia 54(1–2), 63–75 (1992).
Castello, M. & Nimis, P. L. The lichen vegetation of Terra Nova Bay (Victoria Land, Continental Antarctica). Bibliotheca Lichenologica 58, 43–55 (1995).
McCarthy, P. M. Checklist of the Lichens of Australia and its Island Territories. Australian Biological Resources Study, Canberra. http://www.anbg.gov.au/abrs/lichenlist/introduction.html. Accessed 7 March 2023 (2023).
Nylander, W. Synopsis Lichenum 2 (1863).
Frey, E. & Motyka, J. Les lichens des hautes altitudes au Ruwenzori Résultates botaniques de l‘expédition scientiique Belge au Ruwenzori, 1932 VI. l’Inst R. Colon. Belge Sect. Sci. Nat. Mèdicales 2, 1–31 (1936).
Hasselrot, T. E. Lavar från Hälsingland och Härjedalen, samlade av M Östman [Lichens from Hälsingland and Härjedalen, collected by M Östman]. Arkiv Bot. 30A(13), 1–80 (1943).
Hale, M. E. Lichens from Baffin Island. Am. Midl. Nat. 51(1), 232–264. https://doi.org/10.2307/2422220 (1954).
Ryvarden, L. Umbilicaria aprina Nyl., a rare lichen. Bryologist 71, 366–368. https://doi.org/10.2307/3241125 (1968).
Hestmark, G. Thalloconidia in the genus Umbilicaria. Nordic J. Bot. 9, 547–574 (1990).
Davydov, E. A. & Zhurbenko, M. Contribution to Umbilicariaceae (lichenized Ascomycota) studies in Russia I mainly Arctic species. Herzogia 21, 157–166 (2008).
Krzewicka, B. & Maciejowski, W. Lichen species from the northeastern shore of Sørkapp Land (Svalbard). Pol. Biol. 31, 1319–1324. https://doi.org/10.1007/s00300-008-0469-1 (2008).
Davydov, E. A. On the status of Umbilicaria aprina var halei and U. canescens (Umbilicariaceae, lichenized Ascomycota). Phytotaxa 533(1), 091–097. https://doi.org/10.11646/phytotaxa.533.1.6 (2022).
Poelt, J. Die Gattung Umbilicaria (Umbilicariaceae) (Flechten des Himalaya 14). Khumbu Himal Ergeb. Forshungsunternehmens Nepal Himalaya 6, 397–435 (1977).
Nimis, P. L. & Martellos, S. A Second Checklist of the Lichens of Italy, with a Thesaurus of Synonyms (Monografie Monografie 4. del Museo Regionale di Scienze Naturali Aosta, 2003).
Krzewicka, B. & Osyczka, P. Umbilicaria aprina Nyl – A new lichen species from central Europe. Acta Soc. Bot. Pol. 71, 171–174. https://doi.org/10.5586/asbp.2002.020 (2002).
Hestmark, G. Umbilicaria aprina new to the Pyrenees. Graphis Scr. 27(1–2), 42–45 (2015).
Crespo, A. & Sancho, L. G. Umbilicaria aprina Nyl en el Hemisferico Sur (Andes peruanos). Lazaroa 4, 357–360 (1982).
Olech, M. Lichens of the Admiralty Bay region, King George Island (South Shetland Islands, Antarctica). Acta Soc. Bot. Pol. 58(3), 493–512. https://doi.org/10.5586/asbp.1989.038 (1989).
Castello, M. Lichens of the Terra Nova Bay area, northern Victoria Land (continental Antarctica). Studia Geobot. 22, 3–54 (2003).
Smykla, J., Krzewicka, B., Wilk, K., Emslie, S. D. & Śliwa, L. Additions to the lichen flora of Victoria Land, Antarctica. Pol. Pol. Res. 32(2), 123–138. https://doi.org/10.2478/v10183-011-0009-5 (2011).
Sipman, H. J. M., Hekking, W. & Aguirre, C. J. Checklist of Lichenized and Lichenicolous Fungi from Colombia (Instituto de Ciencias Naturales, Facultad de Ciencias, Universidad Nacional de Colombia, 2008).
Galloway, D. J. Flora of New Zealand Lichens. PD Hasselberg, New Zealand Government Printer. Wellington (1985).
Topham, P. B., Seaward, M. R. D. & Bylińska, E. A. Umbilicaria propagulifera new to the Northern Hemisphere. Lichenologist 14(1), 47–52. https://doi.org/10.1017/S0024282982000061 (1982).
Seaward, M. R. D., Bylińska, E. A. & Topham, P. B. The distribution and ecology of Umbilicaria propagulifera (Vainio) Llano. Nova Hedwigia 38, 703–716 (1983).
Hestmark, G. Umbilicaria dendrophora. Mycotaxon 46, 211–215 (1993).
Krzewicka, B., García, M. A., Johansen, S. D., Sancho, L. G. & Martín, M. P. Morphological and nuclear ribosomal DNA data support distinguishing two new species of Umbilicaria (Umbilicariaceae, Ascomycota) from Europe. Lichenologist 41(6), 631–648. https://doi.org/10.1017/S0024282909990120 (2009).
Andreev, M. P. De lichenoflora Insulae King-George (Antarctic) notula. Novitates Sistematiki Nizshikh Rastenii 25, 118–124 (1988).
Ivanets, V. et al. Skua and plant dispersal: Lessons from the Argentine Islands–Kyiv Peninsula region in the maritime Antarctic. Nordic J. Bot. https://doi.org/10.1111/njb.03326 (2022).
Parnikoza, I. et al. Spread of Antarctic vegetation by the kelp gull: Comparison of two maritime Antarctic regions. Pol. Biol. 41, 1143–1155. https://doi.org/10.1007/s00300-018-2274-9 (2018).
Cao, S. et al. Distribution patterns of haplotypes for symbionts from Umbilicaria esculenta and U. muehlenbergii reflect the importance of reproductive strategy in shaping population genetic structure. BMC Microbiol. 15(1), 212. https://doi.org/10.1186/s12866-015-0527-0 (2015).
Acknowledgements
The Ukrainian authors are grateful to the Ukrainian army for defending the country from a brutal Russian invasion, which enables them to conduct scientific research. We thank Capitan of “Selma” Yacht Capitan Piotr Kuzniar.
Funding
This research was funded by the Ukrainian State Special-Purpose Research Programme in Antarctica for 2011–2023, by the Institute of Nature Conservation of the Polish Academy of Sciences within the projects “Biodiversity of the Antarctic terrestrial ecosystems as an indicator of environmental changes” and “Antarctic call: biodiversity, monitoring and protection of the vulnerable environment”. The study was supported by statutory funds of the W. Szafer Institute of Botany Polish Academy of Sciences. Ivan Parnikoza was supported by the project of the Polish Academy of Science supporting the stay of Ukrainian scientists in Poland.
Author information
Authors and Affiliations
Contributions
Conceptualization—B.K., I.P., J.S.; Methodology—B.K., I.P.; Validation—B.K.; Formal Analysis—B.K., I.P.; Investigation—I.P., V.I.; Data Curation—B.K.; Writing–Original Draft Preparation—B.K., I.P.; Writing–Review and Editing, B.K., I.P., J.S., V.I., H.Y.; Visualization B.K., H.Y.; Supervision—B.K.; Project Administration—I.P.; Funding Acquisition I.P.,B.K., J.S.; ALL authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Krzewicka, B., Parnikoza, I., Ivanets, V. et al. Diversity and distribution of the lichen genus Umbilicaria in the Argentine Islands–Kyiv Peninsula region, the maritime Antarctic. Sci Rep 14, 17310 (2024). https://doi.org/10.1038/s41598-024-65806-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-65806-7