Abstract
Metal artifacts notoriously pose significant challenge in computed tomography (CT), leading to inaccuracies in image formation and interpretation. Artifact reduction tools have been designed to improve cone beam computed tomography (CBCT) image quality by reducing artifacts caused by certain high-density materials. Metal artifact reduction (MAR) tools are specific algorithms that are applied during image reconstruction to minimize or eliminate artifacts degrading CBCT images. The purpose of the study is to evaluate the effect of a MAR algorithm on image quality in CBCT performed for evaluating patients before transarterial radioembolization (TARE). We retrospectively included 40 consecutive patients (aged 65 ± 13 years; 23 males) who underwent 45 CBCT examinations (Allura FD 20, XperCT Roll protocol, Philips Healthcare, Best, The Netherlands) in the setting of evaluation for TARE between January 2017 and December 2018. Artifacts caused by coils, catheters, and surgical clips were scored subjectively by four readers on a 5-point scale (1 = artifacts affecting diagnostic information to 5 = no artifacts) using a side-by-side display of uncorrected and MAR-corrected images. In addition, readers scored tumor visibility and vessel discrimination. MAR-corrected images were assigned higher scores, indicating better image quality. The differences between the measurements with and without MAR were most impressive for coils with a mean improvement of 1.6 points (95%CI [1.5 1.8]) on the 5-point likert scale, followed by catheters 1.4 points (95%CI [1.3 1.5]) and clips 0.7 points (95%CI [0.3 1.1]). Improvements for other artifact sources were consistent but relatively small (below 0.25 points on average). Interrater agreement was good to perfect (Kendall’s W coefficient = 0.68–0.95) and was higher for MAR-corrected images, indicating that MAR improves diagnostic accuracy. A metal artifact reduction algorithm can improve diagnostic and interventional accuracy of cone beam CT in patients undergoing radioembolization by reducing artifacts caused by diagnostic catheters and coils, lowering interference of metal artifacts with adjacent major structures, and improving tumor visibility.
Similar content being viewed by others
Introduction
State-of-the-art angiography units feature flat-panel (FP) detectors mounted on C-arms for rotational acquisition of projection data. Similar to multidetector computed tomography (MDCT), cone beam computed tomography (CBCT) provides a 3D dataset, which can be reformatted to generate multiplanar reconstructions in various planes, enhancing imaging versatility1. CBCT can supplement fluoroscopy and digital subtraction angiography (DSA) for more precisely planning and guiding interventional procedures as it facilitates navigation of catheters or probes to the target lesion. In addition, CBCT allows onsite assessment of treatment outcome and identification of complications2.
FP detectors provide higher spatial resolution but lower soft tissue contrast than MDCT. With its low radiation dose, high versatility, and combination of fluoroscopy and tomography, CBCT is an optimal tool for transarterial radioembolization (TARE)3,4,5,6,7. In TARE, CBCT facilitates selective embolization prior to administration of the radiotherapeutic agent (yttrium-90-loaded microspheres)8.
However, strong artifacts caused by catheters, coils, and contrast agents can severely impair image quality and compromise decision-making during TARE based on CBCT images9. Metal artifacts are generally caused by photon starvation, beam hardening, or excessive quantum noise10. These nonlinear effects are amplified when a filtered back projection reconstruction algorithm is used and lead to dark and bright streaks radiating from the artifact source (metallic coil or catheter)10,11,12. Such streaks significantly impair image quality or obscure structures close to the artifact source, such as small vessels or adjacent hepatic parenchyma and tumors. However, these structures are often part of the target volume, and hence attenuation changes need to be clearly visible in order to assess the effect of embolization.
Metal artifact reduction (MAR) techniques can be employed to reduce such artifacts and improve the diagnostic yield of images13. MAR is applied in a second step after initial reconstruction of the CBCT raw data and usually takes a few seconds, depending on computer capacity. The main benefit of highly accessible CBCT is that it yields roadmaps supposed to make procedures simpler and safer while at the same time reducing the radiation dose14. Tomographic images help in the preparation, guidance, and execution of both intra-arterial and percutaneous interventions as well as in assessing the outcome of embolization. Thus, CIRSE/SIR advisory panels already recommend CBCT guidance in their protocol guidelines for various interventional oncologic procedures, such as transarterial chemoembolization (TACE) and TARE of the liver15.
The purpose of this study was to retrospectively assess the effect of an onsite MAR algorithm in CBCT on qualitative image parameters in patients undergoing TARE of malignant hepatic lesions.
Materials and methods
Study population
In this retrospective study, 40 consecutive patients (aged 65 ± 13 years; 23 men, 17 women) with liver metastasis (n = 18) or primary liver tumors (n = 22) undergoing evaluation for TARE including CBCT were enrolled from January 2017 through December 2018. An interdisciplinary team of oncologists, surgeons, and radiologists decided about acceptance of patients with predominant liver cancer not amenable to resection, local ablation, or systemic treatment for TARE. Inclusion criteria were: sufficient liver function and reserve, no or minimal extrahepatic manifestation of the tumor, and life expectancy of at least 12 weeks after TARE14,15,16,17. The study was approved by the local ethics committee (EA/187/15). All patients gave written informed consent.
Image acquisition
A total of 45 CBCT examinations of the liver were performed using the same CBCT system (Allura FD 20, XperCT ND Roll protocol, Philips Healthcare, Best, The Netherlands). CBCT was performed during intra-arterial contrast agent injection into the celiac trunk using a split bolus injection protocol18. A diluted solution of Imeron® 300 [Bracco Imaging Deutschland GmbH, Konstanz, Germany] was prepared by adding the same amount of saline. The Accutron HP-D-HT® [Medtron, Saarbrücken, Germany] contrast injector was used for contrast agent administration through a 2.5F Cook Medical Cantata (Bloomington, USA) microcatheter. The first injection of 8 ml (1.5 ml/s) was performed to achieve presaturation of tumor parenchyma. 20 s following contrast agent administration, a second injection of 23 ml (1.5 ml/s) was administered. CBCT acquisition began 28 s after the start of the first injection.
CBCT was acquired at a rate of 31 images/s for 10 s using a peak tube voltage of 120 kVp. The acquired 3D volumetric CBCT images had an isotropic resolution of 0.6 mm. For extrahepatic branch embolization, coils (HILAL and TORNADO; Cook Medical, Bloomington, USA) were used. Projection data acquired were transferred to the postprocessing workstation (Philips Healthcare, Best, The Netherlands) for MAR reconstruction.
Postprocessing and metal artifact reduction algorithm
The projection data were reconstructed using a standardized field of view (FOV) of 250 × 194 mm and a matrix size of 384 × 384 × 296 pixels at a default medium voxel size and standard liver kernel (smooth) for optimal soft tissue resolution. An automated MAR algorithm19 was applied to the reconstructed data.
Qualitative assessment of CBCT with and without MAR
Qualitative evaluation of images with and without MAR was performed on regular clinical PACS workstations. Axial slices of the multiplanar reconstructions (MPRs) were presented in a side-by-side display of uncorrected and MAR-corrected images. An example of image presentation is shown in Fig. 1. Qualitative assessment was performed by four readers (interventional radiology fellows with 6 years (Rater 1), 3 years (Rater 2), and 1 year (Rater 3) of experience and 1 reader with no experience in interventional radiology (Rater 4). The readers scored artifacts caused by coils (most frequently in the gastroduodenal and right gastric artery), diagnostic catheters, surgical clips, or stents in the common hepatic duct (CHD) in uncorrected and MAR-corrected images using a five-point Likert scale (1 = artifacts affecting diagnostic information to 5 = no artifacts).
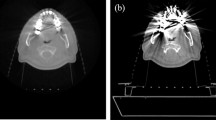
Illustration of the effect of MAR on CBCT images: shown is an image without MAR on the left (A) and an image with MAR on the right (B). The images were obtained in a patient with chronic hepatitis and hepatocellular carcinoma after coil embolization (13 × Boston Scientific and 4 × Cook, indicated by red arrows) in the gastroduodenal artery, left gastric artery, and accessory liver artery. Arrows indicate that there is marked artifact reduction after application of the MAR algorithm.
In addition, readers scored visualization of the renal collecting system with often persisting contrast agent (1 = unacceptable to 5 = excellent visualization), tumor visibility (1 = artifact mimicking a lesion to 5 = clearly depicted lesion), and vessel discrimination (1 = unacceptable to 5 = excellent vessel discrimination) in images without and with MAR. Finally, motion artifacts were scored using a 5-point scale (1 = unacceptable to 5 = excellent visualization).
Statistical analysis
Statistical analysis was performed using SPSS software (IBM; New York, USA), DATAtab e.U. Graz, Austria (2023.) and R software. Sample size was calculated with a two-group t-test of equivalence in means using nQuery Advisor V. 7.0 at a significance level of α = 0.05 and a desired power of 0.8. Descriptive statistics are given as mean with 95% confidence interval (CI), median with limits of the interquartile range (IQR, 25th and 75th percentile), minimum and maximum as well as absolute and relative frequencies, depending on scale.
Improvement in Likert scores with MAR algorithm compared to without were calculated with 95% confidence intervals for each artifact source and each reader. Moreover, averages over all 4 raters were calculated with 95% confidence intervals.
Kendall’s W coefficient of concordance was determined for interrater variability. Agreement between 0.81 and 1.00 was rated as perfect, between 0.61 and 0.80 as good, between 0.41 and 0.60 as moderate, between 0.21 and 0.40 as fair, and less than 0.20 as poor20,21.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. The study was approved by the local ethics committee (EA/187/15-Ethikkommission der Charité—Universitätsmedizin Berlin). Informed consent was obtained from all individual participants included in the study.
Results
Overall, MAR led to higher ratings, indicating better image quality. The differences between the measurements with and without MAR were most impressive for coils with a mean improvement of 1.6 points (95%CI [1.5–1.8]) on the 5-point likert scale, followed by catheters 1.4 points (95%CI [1.3–1.5]) and clips 0.7 points (95%CI [0.3–1.1]). Improvements for other artifact sources were consistent but relatively small (below 0,25 points on average) (Tables 1, 2, Fig. 2).
However, the differences between the measurements with and without MAR regarding vessel discrimination and tumor Visibility were not significant, with a mean improvement of 0.07 points (95%CI [− 0.04–0.18]) and 0.06 points (95%CI [0.01–0.12]), respectively. This could be explained by the overall lower diagnostic imaging quality of CBCT (Tables 1, 2, Fig. 2).
Inter-rater agreement
Apart from cases with CHD clips (in uncorrected images) and CHD stents (both MAR-corrected and uncorrected images), interrater agreement was good to perfect (Kendall’s W coefficient = 0.68–0.95) (Table 3). Use of the MAR technique led to higher Kendall’s W coefficients in most cases, indicating higher diagnostic accuracy. Specifically, differences in Kendall’s W coefficients were observed for clips (0.86 with MAR vs 0.18 without MAR), coils (0.80 with MAR vs 0.68 without MAR), and catheters, (0.86 with MAR vs 0.71 without MAR). Conversely, the differences in Kendall’s W coefficient for CHD stent were small and not significant (0.38 with MAR vs 0.19without MAR). For CHD-stents, the difference in Kendall’s W was of similar strength but agreement was generally low (0.38 with MAR vs 0.19 without MAR). Overall, the findings suggest that the use of the MAR technique can improve the overall diagnostic accuracy, especially for certain types of procedures.
Discussion
Metal artifacts notoriously pose significant challenge in CT, leading to inaccuracies in image formation and interpretation23. Artifact reduction tools have been designed to improve CBCT image quality by reducing artifacts caused by certain high-density materials. Metal artifact reduction (MAR) tools are specific algorithms that are applied during image reconstruction to minimize or eliminate artifacts degrading CBCT images24.
The severity of raw data corruption depends on the volume and geometry of a metal object. For instance, one or more metallic embolization coils deposited in small vessels cause heavier corruption of raw projection data than a straight aligned catheter. In addition, metal constituents of coils, usually platinum with a high density and therefore high attenuation, may cause more severe artifacts than catheters. Factors contributing to metal artifacts include beam hardening, scatter, noise, photon starvation, and edge effects. These factors can corrupt the data and lead to inaccurate representations of tissues near the metal during reconstruction. As a result, analysis of tissue close to metal within the body might be unreliable or even impossible, depending on the amount and composition of the metal present25.
Cone beam CT is used in a wide range of clinical settings, including image-guided radiation therapy, image-guided interventions, and diagnostic applications requiring dedicated scanners such as breast, dental, and extremity imaging26.
Recent advances in MAR technology have been shown to contribute to the reduction of metal artifacts in conventional MDCT by using monoenergetic extrapolation from dual-energy or iterative reconstruction from single-energy datasets27. MAR algorithms in CBCT have already been investigated in the field of interventional neuroradiology and have been proven to increase visibility of hemorrhage, brain parenchyma, and vessels after stent or coil placement9,28. The segmentation algorithm automatically detects the metal trace in the raw data, which simplifies handling of the algorithm without any presets29,30.
In patients undergoing TARE, detection of aberrant vessels, residual vascularization of gastrointestinal organs, or extrahepatic reflux is necessary to prevent adverse effects such as duodenal or gastric ulcers. Therefore, high-resolution and accurate imaging of hepatic vasculature, including aberrant vessels, is essential. However, image quality of CBCT can be significantly compromised by metal-induced artifacts from intraprocedural catheters, previously placed coils for embolization of vessels, or high levels of intravascular contrast agent. An efficient and straightforward onsite implementation of this technique would greatly enhance the diagnostic and therapeutic value of CBCT.
The current retrospective study of a prototype metal artifact reduction (MAR) algorithm in patients who underwent CBCT for radioembolization showed that use of MAR significantly reduced coil- and catheter-induced artifacts and, to a lesser degree, also artifacts induced by other materials such as stents in the CHD.
The high interrater agreements we found in the present analysis—especially the higher agreement seen for MAR-corrected images—indicate that qualitative scores and image characteristics are reproducible. The MAR algorithm works for different kinds of artifact sources, in the present study we found the most pronounced effect for artifacts caused by angiographic catheters and metallic coils. Qualitative assessment showed significant improvement in terms of artifact reduction and visibility of blood vessels and liver parenchyma.
The current study has several limitations. The retrospective study design which might introduce inherent limitations, particularly related to patient selection bias and the subjectivity in image analysis. While our findings demonstrate the efficacy of the MAR algorithm in CBCT during TARE, our analysis was retrospective, focusing on qualitative parameters, while we did not prospectively investigate its influence on everyday clinical decisions.
Furthermore, the qualitative assessment of image quality by different readers is subjective and might introduce inter-reader variability, which we attempted to mitigate through interrater agreement analysis. In the present study, we reported a good to perfect interrater agreement, yet subjective scoring by readers still poses a possible risk of variability. To address this, future studies should incorporate quantitative metrics for image quality assessment. Quantitative measures can provide a more objective and reproducible evaluation of image quality improvements introduced by the MAR algorithm which might be specifically relevant to further validate the clinical impact of the MAR algorithm on decision-making as well as improving procedural accuracy, patient safety and patient outcomes.
In addition, the present study was conducted using a specific CBCT system and MAR algorithm, which may limit the generalizability of the findings. Different CBCT systems and MAR algorithms might yield varying results. Therefore, comparative prospective studies across multiple platforms are necessary to determine the efficacy of different MAR techniques universally. This would ensure that our findings are applicable to a wider range of clinical practices and imaging technologies.
Nevertheless, it is plausible to suggest that MAR correction contributes to clearer image interpretation, potentially saving time and minimizing radiation exposure as well as enhancing diagnostic confidence29,30.
Another limitation is the relatively small and heterogenous study population, comprising patients with various types of metallic coils and diagnostic catheters, each contributing differently to image artifacts, which may limit the generalizability of the findings. Further prospective studies with larger patient cohorts are necessary to validate these results and ensure they are applicable across diverse clinical settings in order to provide a more comprehensive understanding of MAR techniques and their diagnostic and therapeutic impact.
Additionally, while improved visualization of contrast agent in gastrointestinal organs due to MAR might prevent residual vascularization or extrahepatic reflux, this aspect was not addressed in the present study. Furthermore, all patients were examined using a standard CBCT protocol for abdominal interventions. Protocols with higher radiation doses might further diminish metal artifacts, though MAR techniques aim to reduce these artifacts without increasing radiation exposure.
Conclusion
In conclusion, use of a metal artifact reduction (MAR) algorithm enhances diagnostic and interventional precision in cone beam CT when used in patients undergoing radioembolization. Such an algorithm effectively reduces artifacts caused by diagnostic catheters and coils, minimizes the interference of metal artifacts with adjacent critical structures, and improve tumor visibility.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CT:
-
Computed tomography
- CBCT:
-
Cone beam computed tomography
- MAR:
-
Metal artifact reduction
- TARE:
-
Transarterial radioembolization
- FP:
-
Flat-panel
- MDCT:
-
Multidetector computed tomography
- DSA:
-
Digital subtraction angiography
- TACE:
-
Transarterial chemoembolization
References
Kalender, W. A. The use of flat-panel detectors for CT imaging. Radiologe. 43(5), 379–387 (2003).
Kakeda, S. et al. Usefulness of cone-beam volume CT with flat panel detectors in conjunction with catheter angiography for transcatheter arterial embolization. J. Vasc. Interv. Radiol. 18(12), 1508–1516 (2007).
Gupta, R. et al. Flat-panel volume CT: Fundamental principles, technology, and applications. Radiographics. 28(7), 2009–2022 (2008).
Kwok, Y. M. et al. Effective dose estimates for cone beam computed tomography in interventional radiology. Eur. Radiol. 23(11), 3197–3204 (2013).
Schegerer, A. A. et al. Dose and image quality of cone-beam computed tomography as compared with conventional multislice computed tomography in abdominal imaging. Invest. Radiol. 49(10), 675–684 (2014).
Lüdemann, W. M. et al. C-arm cone beam CT for intraprocedural image fusion and 3D guidance in portal vein embolization (PVE). Cardiovasc. Intervent. Radiol. 41(3), 424–432 (2018).
Böning, G. et al. Clinical experience with real-time 3-D guidance based on C-arm-acquired cone-beam CT (CBCT) in transjugular intrahepatic portosystemic stent shunt (TIPSS) placement. Cardiovasc. Intervent. Radiol. 41(7), 1035–1042 (2018).
Adamus, R. et al. Image guiding techniques and navigation for TACE, SIRT and TIPS. Radiologe. 53(11), 1009–1016 (2013).
Psychogios, M. N. et al. Impact of a new metal artefact reduction algorithm in the noninvasive follow-up of intracranial clips, coils, and stents with flat-panel angiographic CTA: Initial results. Neuroradiology. 55(7), 813–818 (2013).
Prell, D. et al. Metal artifact reduction for clipping and coiling in interventional C-arm CT. AJNR Am. J. Neuroradiol. 31(4), 634–639 (2010).
Prell, D., Kalender, W. A. & Kyriakou, Y. Development, implementation and evaluation of a dedicated metal artefact reduction method for interventional flat-detector CT. Br. J. Radiol. 83(996), 1052–1062 (2010).
Prell, D. et al. Reducing metal artifacts in computed tomography caused by hip endoprostheses using a physics-based approach. Invest. Radiol. 45(11), 747–754 (2010).
Shellikeri, S. et al. Metal artefact reduction algorithm for correction of bone biopsy needle artefact in paediatric C-arm CT images: A qualitative and quantitative assessment. Clin. Radiol. 71(9), 925–931 (2016).
Bajpai, S. et al. Image-guided treatment in the hepatobiliary system: Role of imaging in treatment planning and posttreatment evaluation. Radiographics. 35(5), 1393–1418 (2015).
Bapst, B. et al. Cone beam computed tomography (CBCT) in the field of interventional oncology of the liver. Cardiovasc. Intervent. Radiol. 39(1), 8–20 (2016).
Bester, L. et al. Transarterial chemoembolisation and radioembolisation for the treatment of primary liver cancer and secondary liver cancer: A review of the literature. J. Med. Imaging Radiat. Oncol. 58(3), 341–352 (2014).
Clark, H. P. et al. Staging and current treatment of hepatocellular carcinoma. Radiographics. 25(Suppl 1), S3–S23 (2005).
Jonczyk, M. et al. Visibility of hypovascularized liver tumors during intra-arterial therapy using split-bolus single-phase cone beam CT. Cardiovasc. Intervent. Radiol. 42(2), 260–267 (2019).
Meyer, M., Kalender, W. A. & Kyriakou, Y. A fast and pragmatic approach for scatter correction in flat-detector CT using elliptic modeling and iterative optimisation. Phys. Med. Biol. 55(1), 99–120 (2010).
McHugh, M. L. Interrater reliability: The kappa statistic. Biochem. Med. (Zagreb). 22(3), 276–282 (2012).
Brennan, P. F. & Hays, B. J. The kappa statistic for establishing interrater reliability in the secondary analysis of qualitative clinical data. Res. Nurs. Health. 15(2), 153–158 (1992).
Sofue, K. et al. Improved image quality in abdominal CT in patients who underwent treatment for hepatocellular carcinoma with small metal implants using a raw data-based metal artifact reduction algorithm. Eur. Radiol. 27(7), 2978–2988 (2017).
Gjesteby, L. et al. A dual-stream deep convolutional network for reducing metal streak artifacts in CT images. Phys. Med. Biol. 64(23), 235003 (2019).
Oliveira, M. R. et al. Influence of CBCT metal artifact reduction on vertical radicular fracture detection. Imaging Sci. Dent. 51(1), 55–62 (2021).
Wellenberg, R. H. H. et al. Metal artifact reduction techniques in musculoskeletal CT-imaging. Eur. J. Radiol. 107, 60–69 (2018).
Shi, L. et al. Single-pass metal artifact reduction using a dual-layer flat panel detector. Med. Phys. 48(10), 6482–6496 (2021).
Mennecke, A. et al. Evaluation of a metal artifact reduction algorithm applied to post-interventional flat detector CT in comparison to pre-treatment CT in patients with acute subarachnoid haemorrhage. Eur. Radiol. 27(1), 88–96 (2017).
Pjontek, R. et al. Metal artifact reduction for flat panel detector intravenous CT angiography in patients with intracranial metallic implants after endovascular and surgical treatment. J. Neurointerv. Surg. 8(8), 824–829 (2016).
Jonczyk, M. et al. Radiation exposure during TACE procedures using additional cone-beam CT (CBCT) for guidance: Safety and precautions. Acta Radiol. 59(11), 1277–1284 (2018).
Hamie, Q. M. et al. Prototype metal artefact reduction algorithm in flat panel computed tomography—evaluation in patients undergoing transarterial hepatic radioembolisation. Eur. Radiol. 28(1), 265–273 (2018).
Acknowledgements
We would like to thank Bettina Herwig for her language editing assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, M.J., A.E. and E.C.; methodology, M.J. and E.C.; interventions, D.G., G.W.,B.G. and M.J.; software, G.B., E.C., J.K., M.J., C.H., W.L., S.K.P.,T.N. and A.E.; validation, M.J., G.B., and E.C.; formal analysis, E.C., A.E.; investigation, E.C., M.J., G.B.; resources, B.G.; data curation, S.P., W.L., M.J.; writing—original draft preparation, E.C.; writing—review and editing, M.J., A.E., G.B.; visualization, E.C., A.E., S.K.P. and T.N.; supervision, M.J.; project administration, M.J. and A.E.; A.E. and M.J. contributed equally. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Can, E., Böning, G., Lüdemann, W.M. et al. Evaluation of a prototype metal artifact reduction algorithm for cone beam CT in patients undergoing radioembolization. Sci Rep 14, 16399 (2024). https://doi.org/10.1038/s41598-024-66978-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-66978-y