Abstract
Incorporating selenium into high-surface-area carbon with hierarchical pores, derived from red kidney bean peels via simple carbonization/activation, yields a superior Li–Se battery cathode material. This method produces a carbon framework with 568 m2 g−1 surface area, significant pore volume, and improves the composite’s electronic conductivity and stability by mitigating volume changes and reducing lithium polyselenide dissolution. The Se@ACRKB composite, containing 45 wt% selenium, shows high discharge capacities (609.13 mAh g−1 on the 2nd cycle, maintaining 470.76 mAh g−1 after 400 cycles at 0.2 C, and 387.58 mAh g−1 over 1000 cycles at 1 C). This demonstrates exceptional long-term stability and performance, also applicable to Na–Se batteries, with 421.36 mAh g−1 capacity after 200 cycles at 0.1 C. Our study showcases the potential of using sustainable materials for advanced battery technologies, emphasizing cost-effective and scalable solutions for energy storage.
Similar content being viewed by others
Introduction
In the past few years, a considerable increase in demand for energy storage solutions with both high capacity and extended durability has been observed. This surge in demand is chiefly driven by the emergence of plug-in hybrid electric vehicles and the expansion of smart grid community networks1,2. Owing to their impressive theoretical capacity of 1675 mAh g−1 and an energy density of 2600 Wh kg−1, lithium-sulfur (Li–S) batteries are gaining recognition as a key solution, aligning with the sophisticated demands of contemporary electrochemical energy storage3,4. Nonetheless, the path to commercializing Li–S batteries encounters several obstacles, including sulfur's inherently low electronic conductivity, rapid degradation of capacity, and low coulombic efficiency (CE)5,6. Selenium (Se), a chemical counterpart of sulfur, has surfaced as an appealing option, showcasing promise as a cathode material in industrial batteries, and presenting numerous benefits. Se exhibits chemical features akin to those of sulfur, resulting in analogous electrochemical reactions during the charge and discharge cycles. Additionally, Se exhibits a significantly higher electrical conductivity of 10−5 S cm−1, surpassing that of sulfur. This characteristic enables a more effective exploitation of active materials and promotes faster electrochemical processes7. Furthermore, Se features a theoretical volumetric capacity of 3253 Ah L−1, owing to its density of 4.82 g cm−3, a figure that stands close to sulfur’s capacity of 3461 Ah L−1 at 2.07 g cm−3. This characteristic offers an expanded output voltage range and an improved volumetric energy density, bolstering its suitability for cutting-edge energy storage applications. The burgeoning interest in lithium-selenium (Li–Se) batteries stems from their potential advantages, promising a transformative path for battery technology. Nevertheless, challenges continue to impede their widespread adoption, including rapid capacity decay and the limited utilization of Se cathode materials8. To tackle these issues, researchers and engineers have focused on enhancing cathode material conductivity and effectively capturing polyselenides. This has led to the exploration of diverse host matrices, particularly porous carbons9,10,11, known for their intricate pore structures that improve conductivity by facilitating electron and ion transport. Additionally, these porous carbons effectively encapsulate Se, preventing its detachment and thus mitigating capacity decay. The integration of silica as a host matrix12 further diversifies material combinations, enhancing both performance and stability of Li–Se batteries through its unique properties that contribute to conductivity and polyselenide capture. Beyond traditional carbon and silica matrices, the incorporation of polymer matrices13,14 and metal–organic frameworks (MOFs)15,16,17 into Li–Se batteries introduces innovative strategies directly related to our core objectives. Polymer matrices address mechanical stress and improve the structural integrity of cathode materials, which is crucial for enhancing conductivity during battery cycling. MOFs, with their tunable structures and robust host–guest interactions, are effective in mitigating polyselenide dissolution. This is further complemented by unconventional host matrices like zeolites18 and layered double hydroxides (LDHs)19, which offer efficient trapping of polyselenides, thereby enhancing the overall robustness and electrochemical performance of Li–Se batteries. These examples collectively underscore the importance of designing and synthesizing porous carbon materials that can effectively address the key challenges in Li–Se batteries.
Intriguingly, the scope of host matrices extends even further to include biomass-derived porous carbon20. This emerging approach, utilizing abundant biomaterials, not only contributes to sustainable battery technology but also aligns closely with our objectives of enhancing conductivity and polyselenide capture. Biomass-derived porous carbon, with its unique porous structure and inherent superior electronic conductivity, offers an innovative avenue for tailoring pore sizes and distributions. This customization is crucial for optimal polyselenide confinement, addressing the existing issues in Li–Se batteries. The design and synthesis of such kind of biomass-derived porous carbons, specifically mesoporous activated carbon derived from red kidney bean peels (ACRKB), becomes a focal point in our research. This strategy focuses on leveraging the capabilities of this specialized carbon to boost Li–Se battery performance by enhancing conductivity and effectively trapping polyselenides.
Sodium (Na), a member of the same family of alkali metals as lithium (Li), undergoes electrochemical reactions with Se that mirror those observed with Li. The comparative prevalence of sodium over lithium in the planet's outer layer suggests a more cost-effective prospect for future battery technologies. Hence, delving into the ideal carbon host for sodium-selenium (Na–Se) batteries is of paramount importance21, facilitating the progress of economical and effective energy storage technologies.
Our research details a straightforward, cost-effective method for synthesizing hierarchical porous activated carbon from red kidney bean peels (ACRKB), proposing an environmentally friendly and economical alternative for the cathode matrix in Li–Se batteries. Our hierarchical porous carbon stands out among other carbonaceous materials due to its abundant pores, facilitating efficient Se loading, enhancing electron and ion transport, and effectively trapping polyselenides. As a cathode material, the Se@ACRKB composite exhibited remarkable capacity, enhanced rate capability, and superior cycling stability over 1000 cycles. Moreover, our investigation extends to Na–Se batteries, where the developed composite exhibited promising outcomes, maintaining a cycling stability of 421.36 mAh g−1 at 0.1 C after 200 cycles, coupled with impressive rate performance. This enhanced functionality underscores the adaptability and promise of the hierarchical porous carbon framework for various energy storage applications.
Experimental section
Synthesis of ACRKB
Red kidney beans were obtained from a local market and employed as a bio-precursor for synthesizing porous carbon. After a thorough washing process with distilled water and ethanol, the beans were soaked in water for an hour, their peels were removed, and then they were desiccated in an oven at 80 °C for 24 h. Following this, carbonization was carried out at 900 °C at 10 °C min−1 for one hour. The resulting carbonized materials underwent a KOH treatment using a modified version of a previously established procedure22. Specifically, 4.5 g of carbonized red kidney bean peels and 9 g of KOH (1:2) were evenly mixed in 75 ml of water and agitated for 8 h using a magnetic stirrer. The resulting uniform black slurry was then shifted to an oven and heated at 60 °C for a duration of 12 overnight. Afterward, the sample was moved to a quartz tube furnace crucible with alumina and heated under an Ar gas environment with a ramping rate of 10 °C min−1 to 700 °C for 2 h. The resulting black powder was rinsed with a 10% HCl to eliminate excess KOH, subjected to multiple washes with both water and ethanol. This sample was labeled as ACRKB.
Loading of Se into ACRKB
The melt-diffusion method was utilized for the incorporation of Se into the active carbons. For Se@ACRKB, 0.5762 g of Se powder was thoroughly mixed with 0.28810 g of ACRKB using an agate mortar for 30 min. The mixture thus obtained was positioned in a ceramic boat and subsequently moved to a tube furnace under an Ar gas environment. To facilitate the diffusion of Se into the pores of ACRKB, the temperature was gradually raised to 300 °C at a ramping of 3 °C min-1 and kept for 4 h. Afterward, the temperature was gradually elevated to 400 °C, progressing at a speed of 2 °C per minute, to assist in evaporating the remaining Se. The furnace was allowed to undergo natural cooling until it reached the ambient room temperature. The resulting composite, weighting approximately 0.5 g, was designated as Se@ACRKB.
Materials characterization
We conducted an examination of the crystal structures of activated carbon and its composites employing a PANalytical X'Pert Powder diffractometer, outfitted with a Cu-Kα radiation source (1.54 Å wavelength), covering a range of 10–70 °C. To determine the surface chemical states of the materials, we utilized X-ray photoelectron spectroscopy (XPS) with the Thermo Scientific K-Alpha XPS system. Selenium loading within the Se@ACRKB composites was confirmed by thermogravimetric analysis (TGA) (STA 449 F5), spanning a temperature range from 30 to 800 °C at an incremental rate of 10 °C per minute. The specific surface area and pore distribution were characterized employing the Brunauer–Emmett–Teller (BET) method (ASAP 2460), through nitrogen adsorption and desorption isotherms. To ensure accurate measurements, the samples underwent evacuation and outgassing at 200 °C for eight hours before the adsorption experiments using a Quantachrome autosorb-IQ gas adsorption analyzer. Analyses of the surface structure and elemental composition of the synthesized materials were conducted utilizing a field emission scanning electron microscope (FESEM; JEOL, JSM-IT800; Tokyo, Japan) and a transmission electron microscope (TEM) from JEOL (JEM-F200).
Electrochemical characterization
For the electrochemical analyses, the electrode composition was a mixture consisting of 70% by weight of the active substance, 20% carbon black, and 10% polyvinylidene fluoride (PVDF). This mixture was homogenized with 550 µL of N-methylpyrrolidone (NMP) over 10 h. Subsequently, the uniform mixture was spread onto a copper foil and desiccated at 70 °C overnight. The resultant coated foil was then sectioned into 12 mm diameter discs. For electrochemical testing, CR2032 coin-type half-cells were assembled within an argon-saturated glove box, maintaining oxygen and water vapor levels below 0.1 ppm. In the Li–Se coin cells, a 16 mm diameter lithium foil functioned as the counter electrode, while in Na–Se batteries, a sodium foil was used for the same purpose. The cells were separated using Celgard 2400 polymeric films. The electrolyte for the LiPF6 was a 1:1 volumetric mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC); for NaClO4, it was a mix of EC and diethyl carbonate (DEC). Half-cells underwent cyclic voltammetry (CV) analysis at room temperature, cycling between 0.5 and 3.0 V at a scan rate of 0.1 mV s−1, utilizing an IVIumSTAT electrochemical workstation. Cycle stability and rate performance were evaluated using a Neware battery analyzer (BTS 4000). Specific capacities were determined according to the weight of the active Se material. Electrochemical impedance spectroscopy (EIS) was performed with a Solartron SI1287 analytical electrochemical interface, spanning a frequency range from 0.01 to 1 × 106 Hz at an AC amplitude of 10 mV.
Results and discussions
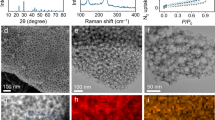
Figure 1 illustrates the overview of the fabrication process employed to create porous activated carbon and its composite with Se (Se@ACRKB), utilizing red kidney bean peels as the carbon precursor. Initially, the red kidney beans underwent washing with water and ethanol, followed by immersion in water to extract the peels. Subsequent carbonization of the dried peels occurred at 900 °C under an argon atmosphere. The resulting carbon was activated with potassium hydroxide (KOH) at 700 °C for 2 h, yielding the highly porous ACRKB23. SEM study was conducted to explore the structural changes and elemental composition of the carbon obtained from red kidney bean peels. Initially, the carbonized red kidney beans peel exhibits a pore-free structure (Fig. 2a). However, after undergoing the KOH activation process, a significant transformation took place, leading to the proliferation of pores throughout the ACRKB structure (Fig. 2b). This advancement is probably a consequence of the interplay between the carbon matrix and the activation agent, leading to the creation of a porous structure, subsequently enhancing the material's functionality. Furthermore, as evident in Fig. 2c, the HRTEM image clearly demonstrates the presence of micropores on the ACRKB, confirming the material's intricate microstructure conducive to improved functionality in battery systems. Moreover, the XPS analysis revealed the presence of nitrogen in the carbon matrix, with trace amounts identified as pyridinic nitrogen (N-PD) at 398.3 eV, graphitic nitrogen (N-G) at 400.6 eV, and pyridine N-oxide nitrogen (N-PDO) at 402.4 eV (Fig. S1). These nitrogen atoms, through the strong polar interactions, can effectively enhance its interaction with polar discharge products, thereby suppressing the shuttle effect24.
FESEM images of (a) carbonized red kidney bean peels, (b) activated carbon derived from red kidney bean peels (ACRKB), (c) HRTEM of ACRKB, and (d) EDS mapping of the Se@ACRKB composite, corroborating the dispersion of (e) carbon, (f) selenium, and (g) N2 adsorption–desorption isotherms of ACRKB and Se@ACRKB composite, along with (h) BJH pore size distribution of ACRKB.
We employed a straightforward melt-diffusion process to fabricate the Se@ACRKB composite. The SEM image of Se@ACRKB revealed a relatively smooth surface, suggesting the successful penetration of elemental Se into the smaller pores inherent to ACRKB, resulting in effective dispersion (as shown in Fig. 2d). Although the SEM image primarily shows the surface of the Se@ACRKB composite, the EDS elemental mapping images (Fig. 2e,f) further verified the spread of Se within the porous carbon matrix. These combined results confirm the successful infiltration and effective dispersion of Se, ensuring optimal electrochemical performance by enhancing efficient charge transfer and ion diffusion25,26.
Figure 2g presents the nitrogen adsorption–desorption isotherms for assessing the porous structure of ACRKB. These isotherms display characteristics of both Type I and IV, suggestive of the presence of both micro and mesopores within ACRKB. Employing the Brunauer–Emmett–Teller technique, the specific surface area of ACRKB was determined to be 568 m2 g−1. This significant surface area, coupled with a noteworthy micropore area and volume, highlights the impact of KOH activation on pore formation. Pore size distribution analysis (Fig. 2h) confirmed a hierarchical porous configuration characterized by micro and mesopores, with sizes predominantly in the lower nanometer range. These pores are instrumental in two ways: they provide storage space for Se and also create routes for electrolyte to penetrate, thereby enhancing the overall effectiveness of the material. Upon Se encapsulation, the specific surface area of Se@ACRKB decreased markedly, as detailed in Tables S1 and S2, which provide comprehensive BET surface area and corresponding DFT pore size data for ACRKB and Se@ACRKB. This reduction in surface area is indicative of Se’s successful integration into ACRKB's pores. The encapsulation of selenium within these pores serves a dual purpose: it diminishes the depletion of active selenium, thus boosting its effective use, and additionally acts to obstruct the development of extended chain polyselenides, consequently lessening the shuttle effect27. Notably, previous research by Lee et al. suggested that liquid electrolyte permeation is limited in densely compressed electrodes28. Consequently, the porous matrix of ACRKB and its intricate architecture likely enhance wettability in the Se@ACRKB cathode, facilitating superior electrolyte infiltration into the cathode's core. Such structural advantages are critical for improving the overall performance and efficiency of battery systems.
Furthermore, the X-ray diffraction (XRD) profiles of ACRKB exhibit clear peaks around 23.3° and 43.39°, which belong to the (002) and (101) planes of graphite, respectively. These spectral features indicate the typical structure of disordered activated carbon (shown in Fig. 3a). Following the Se loading via melt-diffusion, the XRD profiles of the Se@ACRKB composite exhibit broadening and the lack of Se peaks, signifying the non-crystalline structure of ACRKB. This suggests the effective distribution of Se throughout ACRKB’s pores, with no Se particles located outside the mesoporous structure29. To quantify the Se loading in the Se@ACRKB composites, TGA was performed in an argon environment, as illustrated in Fig. 3b. The observed decrease in weight from 300 to 600 °C can be attributed to the evaporation of Se. The Se percentage within Se@ACRKB composite was calculated to be approximately 45%. Notably, the TGA curve of Se@ACRKB demonstrates a significantly delayed Se evaporation rate and a higher evaporation temperature. This effect can be ascribed to the porous carbon matrix’s ability to hinder Se evaporation, showcasing the strong affinity of Se for the diverse pores within ACRKB.
Electrochemical performance of Se@ACRKB for Li–Se batteries
To comprehensively examine the electrochemical reaction mechanism of Li–Se batteries employing carbonate electrolytes, we carried out cyclic voltammetry (CV) tests on coin cells equipped with Se@ACRKB as the cathode and a Li plate as the anode. These tests were performed at a scan rate of 0.1 mV/s for the initial nine cycles, as depicted in Fig. 4a. During the first cycle, oxidation and reduction peaks emerged at 1.973 V and approximately 1.71 V, respectively, signifying a single-step Se-to-Li2Se conversion during cycling30,31. In subsequent cycles, overlapping charging and discharging peaks observed around 1.98 V and 1.70 V, showcasing the remarkable reversibility and stability of the electrochemical properties within Li–Se batteries employing Se@ACRKB as the cathode32,33. These distinct CV tests unveiled that the ACRKB matrix facilitated the direct solid reaction of Se within the pores, effectively mitigating the shuttle effect associated with electrolyte-soluble intermediate phases such as Li2Sen (3 ≤ n ≤ 8)34. Figure S2 presents the CV curves for bare ACRKB over the first five cycles. The Galvanostatic charging and discharging (GCD) patterns for the relevant coin cell, conducted at a 0.2 C current rate, are shown in Fig. 4b. The voltage stability noticeable in the GCD traces align with the peaks apparent in the CV analysis (Fig. 4a), underscoring the straightforward reversible process of Se ↔ Li2Se. Impressively, in the second cycle, the battery reached a noteworthy discharge capacity of 609.13 mAh g−1. Additionally, the battery consistently held a significant reversible capacity of 470.76 mAh g−1 even beyond 400 cycles.
Electrochemical characterizations of Li–Se cells equipped with Se@ACRKB cathode. (a) CV plots of the cell during the initial nine cycles, obtained at a rate of 0.1 mV s−1. (b) Charging and discharging profiles of cell at 0.2 C, illustrating the shapes of the 2nd, 3rd, 100th, 200th and 400th cycles, respectively. (c) Rate capabilities of cell at various rates. (d) Cycle stability at 0.2 C for 400 cycles and (e) Long term cycle stability of battery at 1 C for 1000 cycles.
The examination of rate capability in the coin cell unveiled impressive performance across various current densities, with gradual capacity reductions ranging from 563 mAh g−1 at 1 C to 366.12 mAh g−1 at 3 C. Remarkably, the battery sustained an impressive capacity of 493.8 mAh g−1, even when operated at an elevated rate of 3.5 C. Upon returning to the original current density, specific capacities rebounded with a slight decrease, showcasing the Se@ACRKB cathode's excellent rate capability (the performance of bare ACRKB is shown in Fig. S3). Furthermore, the cell demonstrated remarkable cyclic performance at a rate of 0.2 C, as shown in Fig. 4d, indicating exceptional retention of specific capacity by maintaining 470.76 mAh g−1 after 400 cycles. When subjected to a long-term stability assessment at a high rate of 1 C (Fig. 4e), the material exhibited exceptional resilience, preserving a capacity of 387.58 mAh g−1 even after 1000 cycles. Through comprehensive analysis involving cyclic voltammetry and discharge–charge studies, we elucidated the electrochemical behaviors of the Se@ACRKB cathode, highlighting its potential as an appealing choice for stable and high-capacity cathode materials in Li–Se batteries. These findings emphasize the outstanding cycle and rate performances of porous activated carbon derived from red kidney bean peels, reaffirming its exceptional suitability for energy storage applications.
To emphasize the significance of the Se@ACRKB composite in the context of Li–Se batteries, we conducted a comparative analysis with previously studied materials for Se in Li–Se batteries, as detailed in Table S3. This comparison underscores the exceptional cycling stability of the Se@ACRKB composite, achieving the highest specific capacity of 387.58 mAh g−1 after 1000 cycles at 1 C. The impressive electrochemical efficacy of the Se@ACRKB composite can be credited to its substantial electrical conductivity and porosity, effectively enabling the incorporation and confinement of Se within the pores35,36.
EIS was utilized to evaluate the resistance characteristics of the Se@ACRKB cathode, both before and after cycle testing, with the results depicted in Fig. 5. The Nyquist graphs for the Se@ACRKB cathode revealed a marked semicircle in the high-frequency zone, which seamlessly transitioned into a linear trend in the low-frequency region. This semicircle is predominantly indicative of the charge transfer impedance (Rct), with its diameter providing an estimate of the resistance encountered by lithium ions and electrons as they traverse the electrode/electrolyte interface during electrochemical reactions37. Concurrently, the linear portion relates to Warburg factor (W), which signifies the diffusion-related resistance of lithium ions within the active material38. The Rct values demonstrated a consistent decrease, signifying enhanced charge transfer rates throughout the cycling period and suggesting progressively reduced resistance to charge transfer within the composite material39. This pattern aligns with the capacity performance of the electrodes, implying that the Se@ACRKB composite cathode has undergone kinetic improvements. Enhancements in surface area and pore volume have been associated with these physical and electrochemical improvements, resulting in enhanced charge transfer at the electrode/electrolyte interface40,41,42. Notably, the Rct values for the Se@ACRKB composite before and after cycling were measured at 170.8 Ω and 31.21 Ω, respectively. The downward trend in Rct values throughout the Se@ACRKB electrode's cycling process indicates improved electrical conductivity, reflecting an enhancement in the overall performance of the composite material43.
Additionally, the lithium-ion diffusion coefficient (DLi+) was obtained employing the following equation:
In the given equation, ‘A’ signifies the surface area of the electrode, and ‘n’ corresponds to the number of moles of electrons exchanged in the redox reaction, which, in this case, is 2. ‘F’ denotes the Faraday constant (96,500 C mol−1), ‘C’ indicates the lithium-ion concentration (set at 1 mol L−1), ‘R’ is the universal gas constant (8.314 J K−1 mol−1), and ‘T’ is the standard room temperature, measured at 298 K. The parameter ‘b’ reflects the slope obtained from plotting Z′ against the square root of frequency within the low-frequency scope, as depicted in Fig. 5b. The DLi+ for the Se@ACRKB cathode, calculated before and after 1000 cycles, is approximately 3.92 × 10−13 m2 s−1 and 1.20 × 10−13 m2 s−1, respectively. This indicates an enhancement in the overall electrochemical kinetics of the cathode material. Further evaluation reveals that the Se@ACRKB electrode exhibits efficient Li⁺ diffusion and charge transfer processes. These characteristics are instrumental in its superior capacity reversibility and remarkable rate capability, as exemplified in Fig. 4c, d,e44.
Electrochemical performance of Se@ACRKB for Na–Se batteries
Following the exceptional performance exhibited by the Se@ACRKB cathode in Li–Se batteries, we extended our investigation to explore its application in Na–Se batteries, operating within a voltage range of 0.5–3.0 V (vs. Na/Na+). The CV curves presented in Fig. 6a demonstrate a single pair of redox peaks, reminiscent of the phenomena observed in Li–Se batteries (Fig. 4a). This similarity indicates a direct conversion of Se to Na2Se and vice versa, without the formation of sodium polyselenides (NaPSe)29. The unique composite structure of the Se@ACRKB carbon matrix facilitates this direct conversion process, effectively incorporating Se into the porous carbon framework. Consequently, the final discharge product (Na2Se) forms without any intermediate phases.
Electrochemical performance measurements of Na–Se cells equipped with Se@ACRKB cathode. (a) CV curves of the cell during the first nine cycles, obtained at 0.1 mV s−1. (b) Rate capabilities of cell at various rates. (c) Charging and discharging curves of cell at 1C, illustrating the shapes of the 2nd, 3rd, 4th, and 40th cycles, respectively. (d) Cycle stability of Na–Se battery at 1 C and 0.1 C for 200 cycles. (e) Nyquist plots of cell before cycling and after 100 cycles; and (f) Z′ versus W−1/2 in the low-frequency range.
In sodium-selenium battery configurations, the Se@ACRKB cathode is distinguished by its superior rate performance, as depicted in Fig. 6b. It impressively maintains a reversible capacity of 490.69 mAh g−1 at a low current density of 0.1 C. This capacity remains robust, showcasing a specific capacity of 355.31 mAh g−1 even as the current density reaches to 1.2 C. The GCD curves for these Na–Se batteries, aligning with the voltage trends observed in the cyclic voltammetry studies, are presented in Fig. 6c. The cathode’s durability was assessed over time through cycle stability tests, initially conducted at a density of 1 C for 48 cycles and then at 0.1 C for an additional 200 cycles. As Fig. 6d shows, after the 200 cycles, the cathode consistently conveyed a specific capacity of 421.36 mAh g−1, with a CE exceeding 98%. Such efficiency distinguishes it from most Se@carbon materials discussed in contemporary scientific literature, as detailed in Table S4.
The results highlight the Se@ACRKB composite cathode's significant capacity, supported by the ACRKB structure's porous nature that effectively facilitates the sodiation/desodiation processes of Se45. EIS studies, performed before and after 100 cycles, showed a reduction in impedance, as depicted in the Nyquist plots of Fig. 6e, indicating enhanced charge transfer dynamics46. Concurrently, Fig. 6f presents the fitting plots from which the diffusion coefficients were derived. Specifically, the diffusion coefficients of sodium ions (DNa+) were calculated to be DNa+ = 4.834 × 10−13 m2 s−1 before cycling and 5.193 × 10−13 m2 s−1 after cycling, respectively. These enhancements, along with the measured diffusion coefficients, validate the Se@ACRKB cathode’s superior functionality in both Li–Se and Na–Se battery configurations.
Conclusion
To conclude, our research underscores the importance of hierarchical porous carbon derived from carbonized red kidney bean peels (ACRKB) as a durable and highly efficient cathode matrix for both Li–Se and Na–Se batteries. Encapsulating 45 wt% of elemental Se into ACRKB’s pores, the resulting Se@ACRKB composite demonstrated superior cathode functionality in Li–Se batteries, achieving a reversible capacity of 609.13 mAh g−1 in its second cycle and retaining 470.76 mAh g−1 after 400 cycles at a 0.2 C rate. Furthermore, the Se@ACRKB composite showed significant potential in Na–Se batteries, exhibiting a stable cycling capacity of 421.36 mAh g−1 following 200 cycles at 0.1 C, in addition to its exceptional rate capability. These results, driven by the porous architecture facilitating efficient Se loading, enhanced electron/ion transport, and the composite’s ability to sustain the solid–solid reaction of Se, thereby avoiding the possible loss of active material, collectively highlight its consistent delivery of exceptional performance and cost-effectiveness in sustainable energy storage applications.
Data availability
Data will be available on request by contacting the corresponding author, Dr. Wang at wangyong@ujs.edu.cn OR via Dr. Ali, via ahmed.refaie@science.menofia.edu.eg.
References
Thackeray, M. M., Wolverton, C. & Isaacs, E. D. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 5(7), 7854–7863 (2012).
Kong, P.-Y. & Karagiannidis, G. K. Charging schemes for plug-in hybrid electric vehicles in smart grid: A survey. IEEE Access 4, 6846–6875 (2016).
Zhao, H. et al. A review on anode for lithium-sulfur batteries: Progress and prospects. Chem. Eng. J. 347, 343–365 (2018).
Yan, Y. et al. Activated porous carbon materials with ultrahigh specific surface area derived from banana peels for high-performance lithium–sulfur batteries. J. Mater. Sci.: Mater. Electron. 29(13), 11325–11335 (2018).
Zhao, M. et al. Lithium–sulfur batteries under lean electrolyte conditions: Challenges and opportunities. Angew. Chem. Int. Edit. 59(31), 12636–12652 (2020).
Lv, D. et al. High energy density lithium–sulfur batteries: Challenges of thick sulfur cathodes. Adv. Energy Mater. 5(16), 1402290 (2015).
Sun, J. et al. State-of-the-art and future challenges in high energy lithium-selenium batteries. Adv. Mater. 33(10), 2003845 (2021).
Lu, B., Wang, Z.-R. & Sun, Q. Electrochemical behaviors and electrochemical performances of lithium-selenium battery using selenium/carbon as cathode in different electrolytes. J. Electroanal. Chem. 921, 116654 (2022).
Khan, M. et al. Recent advancements in selenium-based cathode materials for lithium batteries: a mini-review. Electrochem 3(2), 285–308 (2022).
Jin, J. et al. Advances and challenges of nanostructured electrodes for Li–Se batteries. J. Mater. Chem. 5(21), 10110–10126 (2017).
Jiang, S. et al. Selenium encapsulated into 3D interconnected hierarchical porous carbon aerogels for lithium–selenium batteries with high rate performance and cycling stability. J. Power Sources 267, 394–404 (2014).
Lin, S. et al. Three-dimensional ordered porous nanostructures for lithium-selenium battery cathodes that confer superior energy-storage performance. ACS Appl. Mater. Interfaces 13(8), 9955–9964 (2021).
Xiang, H. et al. A review on electronically conducting polymers for lithium-sulfur battery and lithium-selenium battery: Progress and prospects. J. Energy Chem. 58, 523–556 (2021).
Li, J. et al. Facile synthesis of hollow carbonized polyaniline spheres to encapsulate selenium for advanced rechargeable lithium–selenium batteries. J. Alloys Compd. 619, 794–799 (2015).
Ye, W. et al. ZIF-67@ Se@ MnO2: a novel Co-MOF-based composite cathode for lithium–selenium batteries. J. Phys. Chem. C 123(4), 2048–2055 (2018).
Lulu, L., Lu, Y. & Li, D. Application of lithium–selenium batteries using covalent organic framework composite cathodes. Acta Phys.-Chim. Sin 35(7), 734–739 (2019).
Deng, N. et al. Rational design and preparation of covalent organic frameworks and their functional mechanism analysis for lithium-ion and lithium sulfur/selenium cells. Energy Storage Mater. 46, 29–67 (2022).
Dong, Y. et al. Advanced design of cathodes and interlayers for high-performance lithium–selenium batteries. SusMat 1(3), 393–412 (2021).
Wang, X. et al. The rational design of nickel-cobalt selenides@ selenium nanostructures by adjusting the synthesis environment for high-performance sodium-ion batteries. Inorg. Chem. Front. 9(3), 547–558 (2022).
Du, Y. et al. Biomass carbon materials contribute better alkali-metal–selenium batteries: a mini-review. Batteries 8(9), 123 (2022).
Huang, X. L. et al. An emerging energy storage system: Advanced Na–Se batteries. ACS Nano 15(4), 5876–5903 (2021).
Li, B. et al. Eggplant-derived microporous carbon sheets: Towards mass production of efficient bifunctional oxygen electrocatalysts at low cost for rechargeable Zn–air batteries. Chem. Commun. 51(42), 8841–8844 (2015).
Kim, M. et al. Sorghum biomass-derived porous carbon electrodes for capacitive deionization and energy storage. Microporous Mesoporous Mater. 312, 110757 (2021).
Eng, A. Y. S. et al. Tunable nitrogen-doping of sulfur host nanostructures for stable and shuttle-free room-temperature sodium-sulfur batteries. Nano Lett. 21(12), 5401–5408 (2021).
Kim, J. K. & Kang, Y. C. Encapsulation of Se into hierarchically porous carbon microspheres with optimized pore structure for advanced Na–Se and K-Se batteries. ACS Nano 14(10), 13203–13216 (2020).
Wu, X. et al. Encapsulation of Se in dual-wall hollow carbon spheres: Physical confinement and chemisorption for superior Na–Se and K–Se batteries. Carbon 187, 354–364 (2022).
Babu, D. B. & Ramesha, K. Constraining polyselenide formation in ether based electrolytes through confinement of Se in microporous carbon matrix for Li–Se batteries. Electrochim. Acta 219, 295–304 (2016).
Lee, S. G. & Jeon, D. H. Effect of electrode compression on the wettability of lithium-ion batteries. J. Power Sources 265, 363–369 (2014).
Rashad, M. & Asif, M. Recycling biowaste to synthesize nitrogen-doped highly porous activated carbon scaffolds for selenium stuffing with superior electrochemical properties. ACS Appl. Energy Mater. 4(3), 2786–2796 (2021).
Zhang, H. et al. Encapsulating selenium into macro-/micro-porous biochar-based framework for high-performance lithium-selenium batteries. Carbon 95, 354–363 (2015).
Jia, D. et al. High performance of selenium cathode by encapsulating selenium into the micropores of chitosan-derived porous carbon framework. J. Alloys Compd. 746, 27–35 (2018).
Liu, S., Lu, Q. & Zhao, C. Hierarchical porous carbon/selenium composites derived from abandoned paper cup as Li–Se battery cathodes. Solid State Sci. 84, 15–22 (2018).
Sun, K. et al. Selenium/pomelo peel-derived carbon nanocomposite as advanced cathode for lithium-selenium batteries. Ionics 21(9), 2477–2484 (2015).
Ma, C. et al. Porous bamboo-derived carbon as selenium host for advanced lithium/sodium–selenium batteries. Energy Technol. 8(9), 1901445 (2020).
Zhang, Q. et al. Selenium-infused ordered mesoporous carbon for room-temperature all-solid-state lithium–selenium batteries with ultrastable cyclability. ACS Appl. Mater. Interfaces 12(14), 16541–16547 (2020).
Xue, P. et al. Selenium@ Hollow mesoporous carbon composites for high-rate and long-cycling lithium/sodium-ion batteries. Chem. Eng. J. 392, 123676 (2020).
Hoseini, A. H. A. et al. Synthesis of soybean-derived porous carbon as selenium host for high-performance lithium-selenium batteries. Electrochim. Acta 429, 140954 (2022).
Barai, A. et al. A study on the impact of lithium-ion cell relaxation on electrochemical impedance spectroscopy. J. Power Sources 280, 74–80 (2015).
Shu, H. et al. Li fast ion conductive La0.56Li0.33TiO3 inlaid LiFePO4/C microspheres with enhanced high-rate performance as cathode materials. Electrochim. Acta 152, 368–377 (2015).
Li, Q. et al. Electrochemistry of selenium with sodium and lithium: Kinetics and reaction mechanism. ACS Nano 10(9), 8788–8795 (2016).
Xie, L. et al. Effect of pore structure and doping species on charge storage mechanisms in porous carbon-based supercapacitors. Mater. Chem. Front. 4(9), 2610–2634 (2020).
Saji, V. S. & Lee, C.-W. Selenium electrochemistry. RSC Adv. 3(26), 10058–10077 (2013).
Huang, H., Rao, P. & Choi, W. M. Carbon-coated silicon/crumpled graphene composite as anode material for lithium-ion batteries. Curr. Appl. Phys. 19(12), 1349–1354 (2019).
Ren, Y. et al. Ultrathin Si nanosheets dispersed in graphene matrix enable stable interface and high rate capability of anode for lithium–ion batteries. Adv. Funct. Mater. 32(16), 2110046 (2022).
Sun, W. et al. Confined selenium in N-doped mesoporous carbon nanospheres for sodium-ion batteries. ACS Appl. Mater. Interfaces 13(14), 16558–16566 (2021).
Luo, C. et al. Selenium@ mesoporous carbon composite with superior lithium and sodium storage capacity. ACS Nano 7(9), 8003–8010 (2013).
Acknowledgements
This study was financially supported by the Jiangsu Distinguished Professors Project (No. 1711510024), the funding for Scientific Research Startup of Jiangsu University (No. 4111510015, 19JDG044), the Jiangsu Provincial Program for High-Level Innovative and Entrepreneurial Talents Introduction, the National Natural Science Foundation of China (No. 22008091), Natural Science Foundation of Guangdong Province (2023A1515010894), and the Open Project of Luzhou Key Laboratory of Fine Chemical Application Technology (HYJH-2302-A).
Author information
Authors and Affiliations
Contributions
All authors listed have significantly contributed to the development and the writing of this article and all authors are participated equally in this research project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Khan, M., Mahmood, F., Ali, M. et al. Synthesis of hierarchical porous carbon scaffold derived from red kidney bean peels for advanced Li–Se and Na–Se batteries. Sci Rep 14, 17749 (2024). https://doi.org/10.1038/s41598-024-67254-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-67254-9