Abstract
Pathophysiological processes following aneurysmal subarachnoid hemorrhage (aSAH) include upregulated underlying systemic inflammation, which is reflected by changes in different peripheral blood cells and their sub-populations. As inflammation is a crucial process that contributes to post-aSAH complications and clincal outcome, blood cell numbers and ratios in systemic circulation may predict the outcome and provide rapid and easy to quantify point of care biomarkers for these critically ill patients. To identify blood-derived cellular inflammatory parameters which allow a precise prediction of patient outcome after aSAH. In this single-center retrospective study, 19 whole blood-derived cellular inflammatory markers and clinical and demographic parameters for 101 aSAH patients were recorded within 24 h after aSAH. Clinical outcome was quantified with modified Rankin scale (mRS) on discharge. Proportional odds logistic regression (POLR) was used to model the patient outcome as the function of clinical parameters and inflammatory markers. The results were validated on a separate hold-out dataset (220 patients). The on-admission platelet count, mean platelet volume (MPV) and mean platelet volume to platelet ratio (MPR) were found to be significant and predictive of patient outcome on discharge. Mean platelet volume (MPV) and mean platelet volume to platelet ratio (MPR) predicted clinical outcome and may serve as easy to quantify point of care biomarker. The findings are potentially relevant for the management of aSAH.
Similar content being viewed by others
Introduction
Subarachnoid hemorrhage (SAH) is a debilitating and fatal disease. In about 75–85% of the cases SAH is caused by the rupture of an intracranial aneurysm1,2. The worldwide incidence estimate lies around 9 per 100,000 person-years, with high regional variability3,4. The mortality is high, reportedly around 50%5 or even higher. Many survivors suffer from permanent disability, with about 50% dependency rate6.
The long-term prognosis and recovery from SAH depend on various factors, including the extent and the localization of the hemorrhage, comorbidities and underlying conditions, and the timeliness of medical intervention. Rehabilitation and supportive care, including physical and cognitive therapy, are often necessary to regain function and manage lasting effects.
Aneurysmal SAH (aSAH) is known to induce a peripheral immune response, leading to the accumulation of immune cells in the brain parenchyma7,8. It has been shown that the resulting inflammation correlates with poor clinical status at admission9. Successful management of post-aSAH inflammation may therefore offer therapeutic benefits10,11,12.
Despite the large body of literature, the pathophysiological processes surrounding inflammation are complex, convoluted and still not completely understood. A systematic search on PubMed (https://pubmed.ncbi.nlm.nih.gov) found over 1200 articles related to (a)SAH and serum biomarkers, with varying results. To shed more light, we investigated a number of inflammation-specific markers in the serum of patients suffering from aSAH and correlated them to the clinical outcome with overarching aim to find easy to quantify point of care diagnostic biomarkers for disease progression including detection of post SAH complications.
Other studies, like13, have investigated numerous serum biomarkers and their association with the patient outcome. Large amounts of clinical and laboratory data collected for each patient make it enticing to look into possible correlations, with the danger of also discovering spurious ones. Such models may appear to be in good agreement with the data used in the modelling phase but perform poorly in practice. While this danger cannot be completely avoided, it can be mitigated. An established procedure is to use separate datasets in different modelling phases14.
Nevertheless, easy to quantify diagnostic blood biomarkers could be highly relevant to guide treatment decisions during post SAH care. For our study we have chosen markers which are routinely collected in medical practice and easy to analyse, to allow for quick clinical decisions.
Methods
Study type and population
This is a single-center retrospective study. The cohort consisted of aSAH patients admitted to our quaternary university hospital between June 2019 and September 2022 and of patients admitted between January 2014 and December 2016. The former, exploratory dataset was used to identify potential predictors and the latter to confirm the findings. Clinical parameters and full blood count were determined within 24 h after the onset of the aSAH. The patients with age ≤ 18 years, ischemic stroke, traumatic brain injury, onset of symptoms beyond 24 h, SAH due to arteriovenous malformations or vasculitis, pregnancy, signs of imminent death, or those who did not provide consent were excluded from the study. Altogether, the exploratory dataset comprised 101 patients and the confirmatory one 220. The latter was compiled only after the completion of the exploratory phase. The demographic variables, clinical parameters, and the corresponding descriptive statistics are listed in Table 1. Seventy-one patients whose data were used for exploratory analysis required extraventricular drainage (EVD), 20 vasospasms, and 47 pneumonia. Of the 17 patients who died in hospital, in 11 cases (65%), in accordance with the clearly stated patient’s will, life-sustaining treatment was withdrawn and the therapy changed to palliative. No significant differences were found between the exploratory and the confirmatory dataset (Table 2).
Variable definition and data collection
Patients’ demographic and clinical data were recorded at admission. For the exploratory analysis, eighteen CBC-derived inflammation blood markers (Table 3) were selected from the blood analysis performed at the central hospital laboratory. The markers which were found to be significant were used in subsequent confirmatory analysis. SAH was determined by computerized tomography (CT) scan and evidence of an aneurysm was subsequently confirmed by CT angiography or digital subtraction angiography. Patient outcome at discharge was quantified using the Glasgow outcome scale (GOS) and modified Rankin scale (mRS) at discharge (Fig. 1).
Data analysis
The outcome was quantified by modified Rankin scale (mRS) at discharge and summarized in three ordered categories: favorable (0 ≤ mRS ≤ 2), poor (3 ≤ mRS ≤ 5), and death (mRS = 6). The said boundary between favorable and poor outcome is the most common in the literature15. Age, sex, the presence of extraventricular drainage (EVD), DCI, pneumonia, or vasospasms, the modified Fisher score, the WFNS score, and all inflammation markers listed in Table 3 were treated as potential predictor variables in proportional odds logistic models.
The WFNS score, which is easily and routinely determined, is known to be highly correlated with or even the most predictive parameter for the patient outcome16,17,18,19. In order to enforce our models to outperform it, we decided to use it as a fixed predictor in every model. Next, in the exploratory phase, a series of models with one additional predictor was fitted. Significant predictors (p < 0.05)—i.e. those which improved the simple, WFNS-only model—were recorded. Colinearity between predictors was tested using linear regression. The findings from the exploratory phase were validated on the confirmatory dataset. The dependence between a single predictor variable and the trichotomized outcome was analyzed using one-way ANOVA. If ANOVA indicated significant differences between outcome levels, post-hoc t-test was used to identify them. For multiple predictor variables, the dependency was modeled by proportional odds logistic regression (POLR). Multiple regression was performed using all significant predictors found by univariate analysis.
All computations were performed in Python (www.python.org), using NumPy20, SciPy21, and Statsmodels22 libraries.
Ethical approval
The study was performed according to the guidelines of the Helsinki declaration and was approved by the local ethics committee of the medical faculty of the Heinrich-Heine-University Düsseldorf, Germany (reference number: 2019-787-bio). The informed consent was obtained by the treating neurosurgeon. The manuscript was prepared according to the STROBE guidelines.
Results
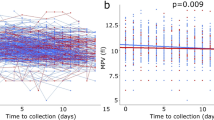
In the exploratory phase, WFNS and age were shown to be highly significant predictors for the outcome (p < 0.0001 for both). We also identified platelets, MPV, and MPR as significant predictors (p < 0.05), even when taken as a second independent variable next to the WFNS score. The three markers were strongly linearly related (p < 0.001), which is unsurprising, given that all three are related to platelets. In particular, the inverse of the MPR was nearly perfectly colinear with platelets (R2 = 0.941). In the confirmatory phase, using a separate dataset, all three markers proved to be significant predictors (POLR, p = 0.009, p = 0.013 and p = 0.011 for platelets, MPV and MPR, respectively). No significant difference in the distribution of the markers was found between the two sets (Table 4). All three markers were also colinear with the WFNS score (p = 0.02, p = 0.04, and p = 0.005) and, in addition, platelets count and MPR were colinear with age (p < 0.0001 and p = 0.0001, respectively). No collinearity between MPV and age was observed (p = 0.4).
However, ANOVA and the post-hoc t-tests showed that these three markers didn’t significantly differ over all outcome levels. Platelet levels differed significantly only between patients with favorable outcome and the deceased (p = 0.007), but not between the patients with poor outcome and the other two groups. A similar result was observed for MPV (p = 0.018 for differences between favorable outcome and death), but there was also a trend (p < 0.1) between the groups with favorable and poor outcome. MPR showed the best differentiation capability, with significant differences between favorable on one side and both poor outcome and death on the other (p = 0.042 and p = 0.017, respectively). There was also a statistical trend (p < 0.1) between MPR values for patients with poor outcome and the deceased (Fig. 2a–c).
Association between platelet-related markers and the patient outcome (trichotomized mRS score): (a) platelet count; (b) MPV; (c) MPR. Only MPR showed a significant difference between patients with a favorable outcome and both other groups. The difference between poor outcome and death was not significant, but a trend (p < 0.1).
As age is an important factor for overall health, whose impact rises strongly as it advances, we analyzed the association between the three predictors and the outcome separately for two groups: patients under 60 (133 patients) and patients aged 60 and over (86 patients) (Fig. 3a–f). Although the trends remain the same—decreasing platelet counts and increasing MPV and MPR for worse outcomes—the differences are significant only for MPV for the elderly patients. In part, this can be explained by the reduction in set sizes, as small effects are statistically harder to detect on smaller sets. MPV, however, turns to be a much better predictor in the elderly group than for all patients taken together, with highly significant differences (p < 0.01) between patients with favorable outcome and the other two groups, and a trend (p = 0.076) between unfavorable outcome and the deceased.
Subgroup analysis by age for the platelet-related markers: (a) platelet count; (b) MPV; (c) MPR, all three for patients under 60. Panels (d–f) show the same for patients aged 60 and more. A significant difference can only be observed for MPV in the latter group, but the directions of change for values between outcome groups remain the same.
In the multiple regression model, using the WFNS score, the age, and all three markers as the predictors, platelets and MPR turned out not to be significant, and a trend (p = 0.07) was observed for MPV. This loss of significance was expected, due to the above collinearities. Figure 4a–d show the ROC curves and the decision curves for predicting favorable outcome and death using the multiple regression model.
Discussion
We investigated several known blood markers involved in inflammation processes, together with clinical parameters, for their predictivity of patient outcome after aSAH. As the number of markers was substantial in relation to the data size, special attention was paid to ensure that the results are reliable and not resulting from random noise in the data. We have therefore reason to believe that our findings are generalizable beyond data used in this study.
Patient’s initial status, reflected in the WFNS score, is—unsurprisingly—higly predictive of the outcome, confirming the ample evidence reported in the literature. Patients with a low initial WFNS score tend to recover better, while patients with a higher WFNS score or poor clinical status at admission are likely to have a worse outcome. At the same time, the WFNS score is plagued by subjectivity and inaccuracies in early assessment and can be supplemented by laboratory results. The role of platelet count and related indicators, MPV and MPR, needs to be explained within the body of the available knowledge. The current literature on platelets and prediction of post SAH complications is controversial.
While some studies indicate that MPR might serve as a predictor of DCI or overall outcome after aSAH23,24, others haven’t found such an association25. A recent study found no association between platelet count and DCI after aSAH26. We applied different ratios of blood cells including mean platelet volume (MPV) and mean platelet volume to platelet ratio (MPR) to evaluate the predictive value of systemic platelet numbers and consequently platelet mediated inflammation. The association between MPR and patient outcome, found in our study, is significant to differentiate and classify different outcome groups (good vs poor outcome) based on modified Rankin scale. Our analysed ratios, however, were not sufficient to differentiate between poor outcome group and the group of patient with in-hospital death. This might be due to high spread of MPR values in the group with poor outcome, causing a significant overlap with the values in the smaller group of deceased patients (Fig. 2c). By its definition, MPR is bound to vary more when the platelet counts are low, and this tends to be the case for patients with poor outcome or death (Fig. 2a), although only the difference between favorable outcome and death was significant. Other studies27,28 found no significant association between the platelet count and the outcome, but the raw numbers point in the same direction. The latter study found a significant negative correlation between platelets and the SAH volume, suggesting a possible link.
Altogether, our findings suggests that MPR might play a secondary role as the outcome predictor. Nevertheless, combining MPV and MPR can be a valuable tool to rapidly recognize the patients with poor outcome after SAH and hence, may help in treatment decision making during intensive care.
Limitations
The study was retrospective by design, based on a single-center cohort. The primary outcome (mRS) was determined retrospectively based on the medical records. We only investigated mRS at discharge, which is indicative of, but not the same as the long-term outcome, e.g. six months after discharge. Although we used two different datasets for exploration and confirmation, further studies on external and ideally larger cohorts are needed to corroborate the findings. Investigating the distribution of predictor values for different outcome categories showed that log-odds for “death” violate the linearity assumption, thus weakening the predictions. Nevertheless, the overall direction points in the same way as under the linearity assumption. Also, the study was phenomenological and further basic research into the exact mechanisms of inflammation and its consequences is warranted.
Conclusion
Three easy-to-determine inflammation markers, platelet count, MPV, and MPR, are related to patient outcome after suffering aSAH. Together with the WFNS score, they can be used to indicate whether a patient is at a higher-than-expected risk of poor outcome and adjust the treatment as needed.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AISI:
-
Aggregate index of systemic inflammation
- aSAH:
-
Aneurysmal subarachnoid hemorrhage
- AUC:
-
Area under the curve
- CBC:
-
Complete blood count
- CT:
-
Computerized tomography
- DCI:
-
Delayed cerebral ischemia
- dNLR:
-
Derived neutrophil-to-lymphocyte ratio
- EVD:
-
Extra-ventricular drainage
- GOS:
-
Glasgow outcome scale
- MLR:
-
Monocyte-to-lymphocyte ratio
- MPV:
-
Mean platelet volume
- MPR:
-
Mean platelet volume to platelet ratio
- mRS:
-
Modified Rankin scale
- NLR:
-
Neutrophil-to-lymphocyte ratio
- NLPR:
-
Neutrophil-to-lymphocyte times platelet ratio
- PLR:
-
Platelet-to-lymphocyte ratio
- ROC:
-
Receiver operating characteristics
- SII:
-
Systemic inflammation index
- SIRI:
-
Systemic inflammation response index
- WBC:
-
White blood cell count
- WFNS:
-
World federation of neurosurgeons
References
Priebe, H. Aneurysmal subarachnoid haemorrhage and the anaesthetist. Br. J. Anaesth. 99(1), 102–118 (2007).
van Gijn, J. & Rinkel, G. Subarachnoid haemorrhage: Diagnosis, causes and management. Brain 124(Pt 2), 249–278 (2001).
Linn, F., Rinkel, G., Algra, A. & van Gijn, J. Incidence of subarachnoid hemorrhage: Role of region, year, and rate of computed tomography: A meta-analysis. Stroke 27(4), 625–629 (1996).
de Rooij, N., Linn, F., van der Plas, J., Algra, A. & Rinkel, G. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 78(12), 1365–1372 (2007).
Long, B., Koyfman, A. & Runyon, M. Subarachnoid hemorrhage: Updates in diagnosis and management. Emerg. Med. Clin. N. Am. 35(4), 803–824 (2017).
le Roux, A. & Wallace, M. Outcome and cost of aneurysmal subarachnoid hemorrhage. Neurosurg. Clin. N. Am. 21(2), 235–246 (2010).
Moraes, L. et al. Immune cells subpopulations in cerebrospinal fluid and peripheral blood of patients with Aneurysmal Subarachnoid Hemorrhage. Springerplus 4, 195 (2015).
Chaudhry, S. R. et al. Differential polarization and activation dynamics of systemic T helper cell subsets after aneurysmal subarachnoid hemorrhage (SAH) and during post-SAH complications. Sci. Rep. 11, 14226 (2021).
Savarraj, J. P. J. et al. Systematic model of peripheral inflammation after subarachnoid hemorrhage. Neurology 88(16), 1535–1545 (2017).
Ahn, S.-H. et al. Inflammation in delayed ischemia and functional outcomes after subarachnoid hemorrhage. J. Neuroinflamm. 16, 213 (2019).
Muhammad, S. & Hänggi, D. Inflammation and anti-inflammatory targets after aneurysmal subarachnoid hemorrhage. Int. J. Mol. Sci 22(14), 7355 (2021).
Muhammad, S. et al. Targeting high mobility group box 1 in subarachnoid hemorrhage: A systematic review. Int. J. Mol. Sci. 21(8), 2709 (2020).
Nie, Z., Lin, F., Li, R., Chen, X. & Zhao, Y. A pooled analysis of preoperative inflammatory biomarkers to predict 90-day outcomes in patients with an aneurysmal subarachnoid hemorrhage: A single-center retrospective study. Brain Sci. 13(2), 257 (2023).
Johnson, R. A. & Wichern, D. W. Applied Multivariate Statistical Analysis 595 (Pearson Prentice Hall, 2007).
Andersen, C. R., Fitzgerald, E., Delaney, A. & Finfer, S. A systematic review of outcome measures employed in aneurysmal subarachnoid hemorrhage (aSAH) clinical research. Neurocrit. Care 30, 534–541 (2018).
Zhao, L. et al. Postoperative red blood cell distribution width predicts functional outcome in aneurysmal subarachnoid hemorrhage after surgical clipping: A single-center retrospective study. Front. Neurol. 13, 1036433 (2022).
Hammer, A. et al. Dynamics of outcome after aneurysmal subarachnoid hemorrhage. Aging 12(8), 7207–7217 (2020).
Nguyen, T. et al. Predictive validity of the prognosis on admission aneurysmal subarachnoid haemorrhage scale for the outcome of patients with aneurysmal subarachnoid haemorrhage. Sci. Rep. 13(1), 6721 (2023).
Hofmann, B. B. et al. Clinical outcome prediction of early brain injury in aneurysmal subarachnoid hemorrhage: The SHELTER-Score. Neurocrit. Care 40(2), 438–447 (2024).
Harris, C. et al. Array programming with NumPy. Nature 585, 357–362 (2020).
Virtanen, P. et al. SciPy 1.0: Fundamental Algorithms for scientific computing in python. Nat. Methods 17(3), 261–272 (2020).
Seabold, S. & J. Perktold, J. Econometric and statistical modeling with Python. In 9th Python in Science Conference, 2010.
Ray, B. et al. Trends of platelet volume index predicts delayed cerebral ischemia after subarachnoid hemorrhage. World Neurosurg. 111, e624–e631 (2018).
Wang, Z., Pei, W., Chen, L., Ning, Y. & Luo, Y. Mean platelet volume/platelet count ratio is associated with poor clinical outcome after aneurysmal subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 29(11), 105208 (2020).
Ray, B. et al. Systemic response of coated-platelet and peripheral blood inflammatory cell indices after aneurysmal subarachnoid hemorrhage and long-term clinical outcome. J. Crit. Care 52, 1–9 (2019).
Raatikainen, E. et al. Platelet count is not associated with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as defined by the 2010 consensus definition. J. Neurol. Sci. 436, 120227 (2022).
Yun, S., Yi, H. J., Lee, D. H. & Sung, J. H. Clinical significance of platelet to neutrophil ratio and platelet to lymphocyte ratio in patients with aneurysmal subarachnoid hemorrhage. J. Clin. Neurosci. 92, 49–54 (2021).
Rzepliński, R., Kostyra, K., Skadorwa, T., Sługocki, M. & Kostkiewicz, B. Acute platelet response to aneurysmal subarachnoid hemorrhage depends on severity and distribution of bleeding: An observational cohort study. Neurosurg. Rev. 44, 2647–2658 (2021).
Funding
Open Access funding enabled and organized by Projekt DEAL. We are thankful to Stiftung Neurochirurgische Forschung (DGNC), EANS Research Funds, Forschungskommission HHU Düsseldorf and BMBF, to S. Muhammad.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.M.; Methodology: I.F., S.M.; Data collection and curation: R.L., D.D., I.F., S.S., B.H.; Formal analysis and investigation: I.F., R.L.; Writing—original draft preparation: I.F.; Writing—review and editing: S.M., R.L., D.D., S.S., B.H.; Funding acquisition: S.M., J.F.C.; Resources: S.M., J.F.C.; Supervision: S.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fischer, I., Lala, R., Donaldson, D.M. et al. Prognostic value of platelet levels in patients with aneurysmal Subarachnoid Hemorrhage. Sci Rep 14, 16743 (2024). https://doi.org/10.1038/s41598-024-67322-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-67322-0
Keywords
This article is cited by
-
Deep immunophenotyping in aneurysmal subarachnoid hemorrhage: a prospective and controlled clinical study
Journal of Neuroinflammation (2025)
-
Hematocrit-to-Hemoglobin Ratio as a Novel Independent Predictor for In-Hospital Mortality and Delayed Cerebral Ischemia in Critically Ill Patients with Aneurysmal Subarachnoid Hemorrhage Requiring Neurosurgical or Endovascular Treatment: A Retrospective Analysis
Neurocritical Care (2025)
-
Trajectory of mean platelet volume changes after aneurysmal subarachnoid hemorrhage in patients with or without delayed cerebral ischemia
Scientific Reports (2024)