Abstract
The nonspecific nature of cancer drug delivery often results in substantial toxic side effects during treatments for breast cancer. To mitigate these negative outcomes, our approach involves loading methotrexate (MTX) within carbon quantum dots (CQDs) synthesized from folic acid, which are then enveloped in exosomal membranes obtained from breast cancer cells (Ex@MTX-CQDs). Analysis utilizing nanoparticle tracking techniques has demonstrated that these Ex@MTX-CQDs maintain the physical and biochemical properties of their exosomal precursors. The release profile of MTX indicated a restricted release percentage (less than 10%) under normal physiological conditions, which is contrasted by a more consistent release rate (approximately 65%) when emulating the conditions found within tumor tissues. The toxicological assessments have confirmed that the presence of exosomes combined with leftover folic acid significantly improves the delivery efficacy of MTX directly to the cancerous cells through the binding to folate and heparan sulfate proteoglycan receptors. This process results in increased disruption of the mitochondrial membrane potential and subsequently triggers apoptosis, ultimately leading to the destruction of cancerous cells. Our research could potentially contribute to the further innovation and application of nanocarriers derived from biological sources for the targeted treatment of breast cancer.
Similar content being viewed by others
Introduction
Breast cancer (BC) stands as a considerable public health issue and is among the leading causes of mortality due to cancer in the female population globally. Despite the enhancements in traditional treatment modalities, issues such as clinical cytotoxicity and a lack of precision in targeting still significantly impede BC therapy1,2. The field of nanomedicine has introduced the use of Exosomes (Ex) as a viable therapeutic alternative. These extracellular vesicles range from 40 to 100 nm in size and are capable of being released by a majority of cells. Due to their membrane composition that closely resembles that of cellular membranes, Ex can efficaciously transport payloads into specific cells, a feature that has led to their extensive application in the realms of drug delivery and cancer treatment3,4. Ex derived from cancer cells not only have an affinity for tumor sites but also play roles in promoting tissue regeneration and orchestrating immune responses. These vesicles present several benefits when compared to other drug delivery systems, including improved bioavailability, greater stability, and diminished off-target cytotoxicity and immunogenic reactions5,6. Owing to their selective targeting faculties, these delivery vehicles limit unintentional absorption by normal cells, thereby curtailing potential toxic and adverse effects. Targeting peptides, whether in linear or looped configurations, exhibit specificity in adhering to their intended cellular targets and are distinguished by their diminutive size, minimal immune response triggering, robust penetration capabilities, and considerable stability7,8. Research by Liu et al. has described that Ex can be augmented with black phosphorus quantum dots to effectively manage bladder cancer9. The amalgamation of Ex-specific targeting peptides with other sorts of targeting sequences has been demonstrated to potentiate the directed conveyance of Ex and their therapeutic loads, with animal studies validating their efficacious treatment potential10.
Folic acid (FA) is commonly recognized as a principal targeting agent for the identification of certain cancerous cell types, such as those found in ovarian, mammary, lung, kidney, breast, brain, prostate, and throat cancers, due to the elevated levels of folate receptor (FR) expression on the surfaces of these malignant cells. These receptors, which are integral membrane proteins, are vital for the FA uptake process within cells. The high affinity of FA attachment to these receptors positions them as an appropriate candidate for FA-centered therapeutic strategies11,12. Utilization of CQDs has gained attention as a favored vector for the selective targeting and treatment of different cancers, BC included13,14. FA-tethered CQDs are distinguished by their biodegradable nature, biological compatibility, water solubility, and their attributes as antioxidants and anticancer agents15,16. These CQDs adeptly transport medicinal agents to BC cells and possess inherent antineoplastic capabilities, attributed to the FA residuals. Furthermore, the variable sizes of CQDs play a role in their cellular uptake rates, with larger CQDs encountering difficulties in cellular infiltration, whereas smaller CQDs are more likely to penetrate cells, potentially resulting in cellular harm17,18. However, despite these issues, FA-conjugated quantum dots hold promising prospects for pinpointed drug delivery systems in the context of BC treatment optimization12,19.
Methotrexate, MTX, also known by the names amethopterin and 4-amino-10-methylfolic acid, is an antineoplastic agent that has been at the forefront in treating various solid neoplasms, inflammatory diseases, and blood cancers. Despite its established use, MTX falls short in targeted specificity, which results in a litany of deleterious effects, including alopecia, nausea, somatic pain, liver toxicity, and bone marrow depression20,21,22. Additionally, the suboptimal pharmacokinetic profile and limited therapeutic window of MTX curtail the efficacy of traditional drug delivery methods. Even though these methods manifest extended circulation times and heightened targeted action in preliminary in vitro studies and in vivo within animal frameworks, exhaustive ancillary investigations are necessary to ascertain their safety and effectiveness for clinical application in humans23.
During this investigation, we designed an approach for the targeted delivery of MTX, a chemotherapeutic compound, to BC cells by utilizing FA-CQDs. We enhanced the targeting ability of MTX by incorporating it into FA-CQDs, which were subsequently integrated within Ex sourced from BC cells (Ex@MTX-CQDs) aiming to target BC therapeutically. Ex and FA can selectively target cancer cells through HSPG and folate receptors, and safeguard the integrity of MTX24,25. By merging with the lipid bilayer of the cancer cell membrane, Ex and FA-CQDs accelerate the delivery and uptake of MTX into the cancer cells, prompting the drug’s activation precisely at the intended site12. The premise of the research suggests that utilizing a nanocarrier that engages with multiple receptors to deliver MTX might significantly enhance treatment outcomes for BC, while concurrently reducing the possible side effects of MTX. The synthetic journey began with the creation of CQDs made from FA through a hydrothermal process (Fig. 1)26. Afterwards, these freshly synthesized FA-CQDs were integrated with MTX, undergoing a series of optimizations to refine the pharmaceutical qualities, ensuring elevated drug loading, followed by thorough characterization. This integration of MTX with CQDs enshrouded in Ex derived from BC cells is postulated to represent a novel and efficacious method for the sharp and specific transport of medical agents, namely MTX, to BC cells. The resulting Ex@MTX-CQDs hold the prospect of becoming an advanced treatment pathway for combating BC.
Results and discussion
Characterization of nanocarrier
FA-based CQDs were synthesized according to the reported method26. The CQDs underwent a successful drug-loading phase with MTX, giving rise to MTX-CQDs nanohybrids27. These MTX-CQDs were then incorporated into the Ex that were isolated and refined via established methods, leading to the creation of Ex@MTX-CQDs28,29. FT-IR analysis confirmed the formation of Ex@MTX-CQDs (see Fig. 2A). Characteristics such as the asymmetric stretch at 1250 cm−1 and the bend at 1315 cm−1 were linked to the C–N and C=N bonds present in the pterin moiety. The peak at 3421 cm−1 indicated O–H stretch vibrations. The peak at 3387 cm−1 was indicative of N–H stretch vibrations, and those at 1670 cm−1 and 1402 cm−1 denoted C=O stretch and C–O bend vibrations, respectively. Notably, in the FTIR spectrum of MTX-CQDs, the stretching frequency associated with C=C/C–N near 1550 cm−1 intensified post-MTX integration with CQDs30. Concerning the Ex spectrum, the stretching vibrations at 988 cm−1 demonstrated the presence of carbohydrate elements like glycans found in glycoproteins. The frequency range between 800 and 1300 cm−1, which pertained to the PO2− stretch, related to phosphate entities in the backbone of DNA or as constituents in modified lipids like phospholipids. Additionally, the amide stretches related to proteins were evidenced by peaks at 1314 cm−1 (amide III), 1544 cm−1 (amide II), and 1656 cm−1 (amide I). Protein second structures, like α-helices and β-sheets, yielded peaks around 1651–1655 cm−1, 1620–1640 cm−1, and 1671–1695 cm−1, respectively. CH2 and CH3 vibrations, typical of lipids and proteins, presented peaks around 1394 cm−1. The presence of C–O–C ester functionalities in lipids like phospholipids and glycerides was evidenced by peaks spanning 950–1210 cm−1. The spectral region of 1800–2600 cm−1 was discarded due to unspecific disturbances, while peaks in the high-frequency area from 2700 to 3010 cm−1 corresponded to CH2 and CH3 stretching common in lipid structures. By comparing the spectra of Ex and Ex@MTX-CQDs, a new shoulder peak was noted at 1520 cm−1, attributed to the aromatic skeletal vibrations and stretches of C–N and C=N linked to CQDs were apparent in the Ex@MTX-CQDs range from 1100 to 1300 cm−1, signaling the presence of MTX-CQDs within the Ex.
The photoluminescence attributes of the synthesized Ex@MTX-CQDs were determined via PL spectroscopy. The CQDs showed a peak emission at 450 nm (as depicted in Fig. 2B). Owing to the inherent robust fluorescence properties of FA-derived CQDs when combined with Ex, notably discernible fluorescence was activated in the Ex@MTX-CQDs formation under a UV lamp at 365 nm (Fig. S1). Nevertheless, the fluorescent intensity from the CQDs was reduced after coating, which in turn formed Ex@MTX-CQDs. There are several possible explanations for this observation, which have been attributed to factors such as self-absorption, filtering effects, inner fluorescence reabsorption, internal and external transformation phenomena, limited quenching, an extinction effect, and intersystem crossing31. Furthermore, the fluorescence quantum yield was measured, with the CQDs displaying a yield of 0.54 in contrast to 0.21 for the Ex@MTX-CQDs, highlighting their substantial fluorescence efficacy.
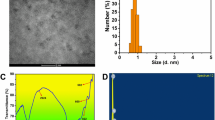
MTX-CQDs were characterized by a nanometric size distribution spanning from 2 to 8 nm (with an average particle size (APS) reported as 2.69 ± 0.51 nm and the polydispersity index, PDI, at 0.65 ± 0.05) as seen in Fig. 3A, fitting well for integration into extracellular vesicles28,29. In contrast, the blank Ex and Ex@MTX-CQDs demonstrated an APS of 96.63 ± 30.38 nm and 106.64 ± 31.13 nm (with a PDI of 1.00 ± 0.05 and 0.36 ± 0.05) as depicted in Figs. 3B and S4, respectively. The dimensions of the Ex@MTX-CQDs were increased after encapsulating MTX-CQDs, which appear appropriate for utilization as a delivery platform32. Surface charge readings of the MTX-CQDs and Ex@MTX-CQDs presented values at − 14.72 ± 5.10 mV and − 6.30 ± 4.81 mV, respectively, which are indicative of the systems’ dispersion stability and the colloidal steadiness of the nanoformulation33,34.
It is imperative that the Ex maintain their inherent structure and functional attributes post-extraction from the origin cells to fulfill their role in targeting tumor cells. Western blotting revealed that the Ex-based formulation (Ex@MTX-CQDs) preserved its essential markers, notably CD81 and CD63, as illustrated in Fig. 3C 35. Full-length gels are shown in Fig. S2 for CD81 and CD63. High expression levels of these proteins in the Ex@MTX-CQDs were noted. Additionally, the presence of these proteins in Ex-DC@CQDs ensures the selective activation of MTX in the tumor’s favorable conditions, signifying the potential of the Ex to act as a delivery system preserving both structural and functional fidelity. Moreover, the absence of Calnexin protein verified that there was no cellular contamination in the isolated exosomes36.
The surface structure and overall integrity of the developed nanoformulations were scrutinized through SEM and TEM imaging modalities. These methods substantiated that MTX-CQDs maintained a spherical morphology with a homogenous size below 15 nm [SEM: 10–15 nm (Fig. 4A), TEM: 5–9 nm (Fig. 4C)]37. Conversely, the Ex@MTX-CQDs manifested in a larger dimension [SEM: 61–84 nm, TEM: 50–150 nm (Fig. 4B,D)]. These morphological characteristics were further corroborated by DLS measurements. Incorporating the MTX-CQDs into the Ex led to a predictable escalation in size. Such nanocarrier dimensions ranging from 50 to 300 nm are advantageous since they contribute to enhanced accumulation at tumor sites because of the enhanced permeability and retention (EPR) effect32.
Drug loading and release studies
The encapsulation efficiency and drug loading capabilities of the designed nanocarrier (Ex@MTX-CQDs) were quantified at 79.43 ± 4.7% and 11.09 ± 1.2%, respectively. The release of MTX from the Ex@MTX-CQDs was considered at both pH 5 and 7.4 (Fig. 5A). The findings showed that around 65% of MTX was released within 48 h under tumor tissue conditions at pH 5, while only about 10% MTX released under physiological conditions at pH 7.4. This release profile denotes a markedly increased discharge rate of MTX from Ex@MTX-CQDs in acidic media, which mimics the tumor microenvironment, compared to the significantly restrained release rate at the normal physiological pH level of 7.4. Such acidic conditions could potentially alter the nanocarrier’s composition, which consists of carbohydrate and phospholipid moieties, thus facilitating a more rapid and extensive release of MTX from the nanocarrier as opposed to what is observed in basic or neutral environments38,39. Compared to a neutral pH, the burst release of MTX from Ex@MTX-CQDs was 6.5 times greater at pH 5. The negligible MTX release from the nanocarrier implies a reduced likelihood of adverse effects on normal tissues, which is an advantageous property for the carrier’s precision in navigating to the intracellular compartments like lysosomes and endosomes (pH 5), subsequently boosting the anticancer efficacy40,41.
Cell viability
The MTT assay was performed to assess cell viability in MCF-7 cell lines including free MTX, CQDs, Ex, MTX-CQDs, and Ex@MTX-CQDs. Over a 48-h observation window, a dose-correlated diminishing in cellular viability was observed (Fig. 5B). Noteworthy was the fact that Ex@MTX-CQDs demonstrated a lower half-maximal inhibitory concentration (IC50) (200 μg/mL) relative to MTX-CQDs (250 μg/mL) in MCF-7 cells at equal concentration of MTX, indicating an enhanced efficacy of the former. Marked was the pronounced cytotoxic effect of Ex@MTX-CQDs against cancerous cells when compared with free MTX and other treatment samples. However, CQDs and Ex showed a higher cell viability of about > 85%, confirming their cytocompatibility nature. The elevated antagonistic action against cancer cells may be attributed to multiple factors including amplified cellular uptake, Ex and FA-mediated targeting to cancer cells through HSPG and folate receptors, as well as efficient disruption of endosomes alongside escalated drug liberation under the cell’s internal pH conditions. By comparison, free MTX was less toxic, likely due to swift expulsion by the p-glycoprotein efflux mechanism42,43,44.
DAPI staining
DAPI staining, an efficacious approach to ascertain cell apoptosis and necrosis due to its nucleus-binding aptitude, was paired with fluorescence imaging for apoptotic feature inspection. Within the MCF-7 cell line, cells in exposure to a range of treatments including CQDs, Ex, MTX, MTX-CQDs, and Ex@MTX-CQDs (as shown in Fig. 6) were submitted to morphological evaluations. Cells subjected to Ex@MTX-CQDs disclosed the most conspicuous reduction in viability, identified by intensified blue fluorescence indicative of nuclei fragmentation and chromatin condensation. Conversely, cells receiving CQDs and Ex treatment displayed uniformly tinted nuclei devoid of condensation. The Ex and FA present in Ex@MTX-CQDs design contributed to apoptosis induction and cancer cell targeting via HSPG and folate receptors while safeguarding MTX’s structure24,25.
Flow-cytometry
Cell apoptosis was assessed using flow cytometry on MCF-7 cells stained with FITC-annexin V/propidium iodide (PI) following treatment with MTX, CQDs, Ex, MTX@CQDs, and Ex@MTX-CQDs for 48 h. Analysis revealed that cells treated with CQDs and Ex showed negativity for both FITC-annexin V and PI, suggesting the safety of the nanocarrier for drug delivery applications. Additionally, the apoptotic effects of MTX@CQDs, Ex@MTX-CQDs, and free MTX were compared, with higher apoptosis rates observed compared to nanocarriers without drugs. Therefore, MTX can be expertly transported into the tumor cells using our developed nanocarriers that can facilitate circumventing MDR efflux known as “stealth” endocytosis45,46. Summary of results can be found in Fig. 6.
Cellular uptake study
The use of nano-carriers for anti-cancer drug conveyance stands as a promising countermeasure against multi-drug resistance (MDR) phenomena43,44. Receptor-mediated nanocarriers present innovative means to overcome MDR by bolstering unidirectional endocytosis into malignant cells. As depicted in cellular uptake research (Fig. 7), internalization by MCF-7 cells was significantly superior with Ex@MTX-CQDs when compared to CQDs and MTX-CQDs. This suggests that MTX is adeptly transferred into tumor cells via the devised multi-receptor-facilitated nano-vehicles, potentially eluding MDR-related efflux, known colloquially as “stealth” endocytosis. Ex-paired with FA-CQDs efficiently bolstered MTX infiltration into cancer cells by merging with cellular membranes and favoring MTX’s activation within malignant cells47. This demonstrates that conventional MTX cellular entry was augmented by the active internalization of CQDs/Ex, which might also address MDR arising from therapeutic efflux in tumor cells. Additionally, as time elapsed to 3 h, the intensity of blue fluorescence uplifted, thus bolstering the intracellular drug concentration and raising cytotoxicity. These findings parallel those of the MTT and DAPI staining outcomes, corroborating successful Ex@MTX-CQDs cell internalization. Taken together, the results indicate that Ex@MTX-CQDs harbor potential as a focused drug nanocarrier for improved breast cancer (BC) treatment.
Materials and methods
Materials
Anti-CD63, CD81, and Calnexin antibodies were supplied from Santa Cruz Biotechnology, Inc. (Santa Cruz, California, USA). Folic acid (FA), Methotrexate (MTX), MTT dye [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide], and all other chemicals and biological reagents were purchased from Sigma Aldrich Co. (Germany). Penicillin–Streptomycin (Pen-Strep, 100×) was obtained from Serana Europe GmbH, Germany. Trypsin–EDTA 0.25% (1×), Fetal bovine serum (FBS), and Dulbecco’s Modified Eagle’s medium (DMEM), were provided from Gibco, Life Technologies Limited, USA. The human breast cancer cell line (MCF-7) was purchased from Pasteur Institute.
Methods
Preparation of folic acid-based quantum dots (FA-CQDs)
N-doped CQDs were fabricated employing FA as the organic precursor via hydrothermal synthesis37. An FA solution was made homogeneous by dissolving 10 mg of FA in 15 mL of ultrapure water, followed by ultrasonication for 5 min. This solution was then subjected to a heat treatment process at 200 °C for 8 h to induce carbonization. The end product of this procedure was a translucent solution with a light yellow to brown tint. The final step involved centrifuging the solution at a speed of 11,000 rpm for a quarter of an hour to eliminate any solid residue. The clear supernatant collected was subsequently stored at a freezing point away from light to be preserved for subsequent usage.
MTX loading
For the incorporation of MTX, 1.0 mL of an MTX solution (1.0 mg/mL) was mixed into 5.0 mL of a CQDs PBS buffer solution (0.45 mg/mL), and the mixture was continuously agitated for 6 h in conditions of low temperature and absence of light48. The mixture was then subject to multiple rounds of dialysis against PBS buffer within a dialysis membrane (MWCO ∼1000 Da) to eliminate any MTX that had not been integrated. The buffer outside the dialysis bag was replaced with fresh PBS every 3 h, persisting until the UV–Vis Spectroscopy indicated no MTX absorption at 372 nm in the resultant PBS solution. The concentration of MTX not encapsulated was established by recording the absorbance at 326 nm from the collected solutions.
The percentage of MTX successfully contained within Ex@MTX-CQDs was assessed by employing HPLC, which utilized a reverse phase C18 column, Endcapped (5 μm), in conjunction with a PDA detector adjusted to 293 nm. The mobile phase for the analysis consisted of a blend of MeOH (90 parts) and water (10 parts) with an addition of 0.1% formic acid. During the analysis phase, the temperature of the autosampler compartment was strictly maintained at 4 °C, and each sample injection volume was maintained at 20 μL49. The metrics for determining the quantitative drug loading (% LE) and the MTX entrapment rate (% EE) in Ex@MTX-CQDs were calculated according to designated formulae Eqs. (1), (2).
Exosome isolation and encapsulation of MTX-CQDs
Gradient ultracentrifugation was applied for the exosome isolation, employing a technique outlined in previous studies50,51. Isolate exosomes. Initially, centrifugation at 500 g for 30 min was performed, followed by clearance of micro-vesicles through centrifugation at 20,000 g for 2 h and filtration using 0.22 μm syringe filters. Subsequently, ultra-centrifugation at 100,000 g for 2 h was conducted to pellet the exosomes. The resulting pellet was then resuspended in 1 mL phosphate buffer saline (PBS), subjected to another round of centrifugation at 100,000g to eliminate protein contamination, and stored at − 80 °C after removal of the supernatant. All centrifugation procedures were carried out at 4 °C. The protein content in the resultant solution was quantified utilizing a bicinchoninic acid assay.
The preserved Ex proceeded through a coating process with MTX-CQDs, applying an extrusion/sonication technique. Concisely, Ex were amalgamated with MTX-CQDs dissolved in PBS and subjected to periodic sonication (20 kHz for 5 min, repeated thrice) to ensure a uniform blend. Next, the mixture was extruded using a membrane with pores measuring 0.22 μm, purging any conglomerates or sizable entities. This extruded blend was rinsed in PBS to discard unaffixed Ex. The environment was consistently controlled at 4 °C throughout these steps. The coated Ex with MTX-CQDs (Ex@MTX-CQDs) were subsequently stored at 4 °C and kept in an opaque and aseptic environment to impede photolytic degradation and avoid contamination52. These Ex were characterized through methodologies like electron microscopy, nanoparticle tracking analysis, and Western blot to verify the occurrence of Ex-specific markers including CD63, and CD81, confirming the successful isolation and integrity of the Ex.
Characterization of prepared nanocarrier
FT-IR spectroscopy (Bruker Instruments, model Aquinox 55, Germany) was employed to discern potential chemical bonds and interactions between MTX and other components. Spectral data from the prepared samples were acquired utilizing the customary KBr pellet method across a spectrum of 400–4000 cm−1. To obtain the UV–Vis absorption spectra of the samples, a Shimadzu spectrophotometer (model 2450) was utilized. The photoluminescent (PL) spectra were obtained with a spectrofluorometric (LS45, PerkinElmer). Particle size distributions and zeta potential values were ascertained using Dynamic Light Scattering (DLS, Malvern model MAL1032660) and measurements were performed after a 100-fold dilution in triply distilled water. Repeated thrice, these measures were conducted to guarantee precision. Transmission Electron Microscopy (TEM, EM 900, Carl Zeiss, Germany) and Scanning Electron Microscopy (SEM, LEO 1430VP scanning electron microscope, samples were coated with gold, SEM magnification: 205 and 90 kx for MTX-CQDs and Ex@MTX-CQDs, respectively) were implemented for examining the structural form of the refined formulations.
In vitro release assay
The dialysis bag method facilitated the study of Ex@MTX-CQDs’ controlled release45. The Ex@MTX-CQDs (equivalent to 1 mg MTX) were enclosed within a dialysis bag (MWCO∼12 kDa) and immersed into a 200 mL buffer of pH 7.4 or pH 5 with 0.1% (w/v) Tween 80. This setup was agitated magnetically at 500 rpm, with the condition remaining at 37 °C. Samples of 1 mL were systematically withdrawn at a specific time and replaced with fresh buffer. UV–Vis spectroscopy assessed these samples at 326 nm using a related fresh buffer as a control sample, and this process was replicated three times. The concentration of the released drug was determined with Eq. 3.
where n, Cn, Cn.s, Cs, Vs, and Vt are the number of withdrawal steps of the releasing medium, the concentration of the released MTX (ppm), the concentration obtained from the calibration curve (ppm), the concentration of the previous sample obtained from calibration curve (ppm), the volume of sample (1 mL) and the total volume of release solution (200 mL), respectively. Finally, the percentage of cumulative MTX release was determined using Eq. (4).
Cell culture
The MCF-7 cell line was adopted for cellular studies. Culturing was performed in DMEM medium augmented with 10% v/v FBS, 100 I.U. penicillin, and 100 µg/mL streptomycin, under conditions of 37 °C temperature, 90% humidity, and 5% CO2.
Western blotting
Proteins were extracted from cultured cells by incubation in RIPA buffer placed within an ice container, extending between 20 and 30 min. The lysates were then separated at 4 °C and 12,000 rpm for 20 min through centrifugation. The protein levels were quantitated employing the BCA protein assay protocol and segregating through 10–15% SDS-PAGE. Post-separation, these proteins were translocated to a PVDF sheet and blocked with 5% BSA solution at ambient temperature for an hour. Following triple washes with TBST, the detection of proteins was performed by incubating the membrane with primary antibodies specific to the targeted proteins overnight at 4 °C. A secondary antibody was introduced and incubated for another 2 h, then washed before visualization with ECL reagents using a Chemi Doc system. GAPDH served as an internal standard.
Cytotoxicity study
The cytotoxicity of free MTX, CQDs, Ex, MTX-CQDs, and Ex@MTX-CQDs against the MCF-7 cells was gauged through the MTT assay53. Cells were initially seeded in 96-well plates at designated densities and allowed to attach overnight. Various concentrations (5–80 μM) of the MTX, MTX-CQDs, and Ex@MTX-CQDs samples at equal concentrations of MTX were applied to the cells in RPMI media supplemented with 10% FBS, with a subsequent 48 h incubation. Besides, CQDs and Ex were considered to investigate their cytocompatibility45. Post incubation, the MTT reagent was added post medium removal, which after a 3.5-h dark incubation at 37 °C, facilitated formazan formation. The solubilization of formazan ensued with a DMSO blend’s addition. Absorbance was recorded at 570 nm via a microplate reader with cellular viability standardized to that of the control cell.
DAPI staining
DAPI staining was utilized for the detection of nuclear condensation and fragmentation in apoptotic cells, induced by the samples (negative control, CQDs, Ex, MTX, MTX-CQDs, and Ex@MTX-CQDs). MCF-7 cells (5 × 105 cells/well) were cultured on glass coverslips and handled with samples at IC50 for 48 h. After the treatment, cells were washed, fixed with paraformaldehyde, penetrated with Triton X-100, stained with DAPI, and then apoptosis was examined using fluorescence microscopy. All experiments were executed in triplicate.
Cellular uptake
To assess the uptake of CQDs, MTX-CQDs, and Ex@MTX-CQDs by endometrial cells, they were labeled with a lipophilic fluorescent dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate (DIL), through incubation with 2 μmol of DIL dye for 20 min with regular agitation. The human endometrial epithelial cell line (Ishikawa, 5 × 104 cells per mL) was cultured in DMEM medium supplemented at 37 °C with 10% FBS in a humidified atmosphere with 5% CO2. Upon reaching the appropriate confluency, Ishikawa cells were exposed to DIL-labeled samples at concentrations of 15 and 30 μg/mL, as well as an equivalent amount of free DIL, for 2 and 4 h. Subsequently, the cells were harvested, washed thrice with PBS, and subjected to analysis using a fluorescence microscope (Olympus microscope Bh2-RFCA, Tokyo, Japan). The fluorescence intensity of cells treated with unlabeled exosomes (without DIL dye) served as the negative control.
Annexin V staining apoptosis analysis
The apoptosis-mediated cell death of cancer cells was evaluated using an Annexin V/PI double staining assay, following the manufacturer’s instructions (Invitrogen, USA). MCF7 cells (3 × 105 cells/well) were seeded in 6-well plates and incubated overnight at 37 °C with 5% CO2. The cells were treated (MTX, CQDs, Ex, MTX@CQDs, and Ex@MTX-CQDs) at their respective IC50 concentrations. After treatment, the cells were detached, rinsed with ice-cold PBS, and centrifuged at 1500×g for 5 min. Subsequently, 5 μL of Annexin V-FITC and 5 μL of PI staining solution were added to the cell suspension, and the mixture was incubated in the dark for 5 min at 25 °C. Flow cytometry analysis was then performed using an Annexin V-FITC apoptosis detection kit (Ebioscience, USA) on a FACS Calibur flow cytometer (USA).
Statistics
Statistical evaluations were carried out on collected data expressing them as mean ± SD from a minimum of three independent experiments. Statistical significance was determined using one-way ANOVA, with p values of < 0.05 or < 0.001 considered significant. Data analysis was conducted using Graph Pad Prism version 9.4.1 (trial version).
Conclusion
The present study successfully developed a nanocarrier using CQDs derived from FA and encapsulated with Ex, which addresses the issue of imprecise targeting in BC therapy, potentially leading to clinical cytotoxicity. The approach involved loading MTX into FA-based CQDs, which were then coated with Ex isolated from MCF-7 cancer cells to create Ex@MTX-CQDs. The research confirmed the formation and characteristics of the CQDs and demonstrated that the Ex maintained its structural and functional properties within the system. Through the use of Ex and FA, the targeted delivery of MTX to cancer cells through HSPG receptors was enhanced, resulting in improved therapeutic effectiveness. The study indicated that Ex@MTX-CQDs exhibited precise targeting and increased cellular uptake, ultimately inducing cell death in breast cancer cells through apoptosis. These findings underscore the potential of using folate/exosome-specific targeting for MTX delivery as an effective intervention for breast cancer. The utilization of Ex and FA-CQDs as nanocarriers with co-receptors offers advantages such as precise targeting, enhanced therapeutic effectiveness, and reduced cytotoxicity, thereby facilitating the development of a novel approach to biologically sourced nanocarriers for cancer targeting.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Abbreviations
- MTX:
-
Methotrexate
- CQDs :
-
Carbon quantum dots
- FA-CQDs :
-
Folic acid-based quantum dots
- Ex :
-
Exosomes
- Ex@MTX-CQDs :
-
Exosome-encapsulated methotrexate-loaded carbon quantum dots
- HSPG:
-
Heparan sulfate proteoglycan
- FA:
-
Folic acid
- FR:
-
Folate receptor
- BC:
-
Breast cancer
References
Sharma, M. et al. Recent updates on innovative approaches to overcome drug resistance for better outcomes in cancer. J. Control. Release 346, 43–70 (2022).
Jafari, S. H. et al. Breast cancer diagnosis: Imaging techniques and biochemical markers. J. Cell. Physiol. 233, 5200–5213 (2018).
Sun, Z. et al. Progress in the research of nanomaterial-based exosome bioanalysis and exosome-based nanomaterials tumor therapy. Biomaterials 274, 120873 (2021).
Fang, X. et al. Highly efficient exosome isolation and protein analysis by an integrated nanomaterial-based platform. Anal. Chem. 90, 2787–2795 (2018).
Tiwari, P. et al. Surface modification strategies in translocating nano-vesicles across different barriers and the role of bio-vesicles in improving anticancer therapy. J. Control. Release 363, 290–348 (2023).
Gilligan, K. E. & Dwyer, R. M. Engineering exosomes for cancer therapy. Int. J. Mol. Sci. 18, 1122 (2017).
Zhu, Y.-S., Tang, K. & Lv, J. Peptide–drug conjugate-based novel molecular drug delivery system in cancer. Trends Pharmacol. Sci. 42, 857–869 (2021).
Saw, P. E. & Song, E.-W. Phage display screening of therapeutic peptide for cancer targeting and therapy. Protein Cell 10, 787–807 (2019).
Liu, J. et al. Strong penetration-induced effective photothermal therapy by exosome-mediated black phosphorus quantum dots. Small 17, 2104585 (2021).
Zhang, L. et al. NSCs migration promoted and drug delivered exosomes-collagen scaffold via a bio-specific peptide for one-step spinal cord injury repair. Adv. Healthc. Mater. 10, 2001896 (2021).
Prieto-Montero, R., Katsumiti, A., Cajaraville, M. P., López-Arbeloa, I. & Martínez-Martínez, V. Functionalized fluorescent silica nanoparticles for bioimaging of cancer cells. Sensors 20, 5590 (2020).
Khoshnood, A. et al. N doped-carbon quantum dots with ultra-high quantum yield photoluminescent property conjugated with folic acid for targeted drug delivery and bioimaging applications. J. Photochem. Photobiol. A Chem. 444, 114972 (2023).
Zahed, Z. et al. Recent advances in fluorescence nanoparticles “quantum dots” as gene delivery system: A review. Int. J. Biol. Macromol. 254, 127802 (2024).
Sattariazar, S., Ebrahimi, S. N., Arsalani, N. & Kazeminava, F. Encapsulation of thymol and menthol loaded N/S co-doped carbon dots derived from a mixture of herbal extracts as theranostic agents with anticancer properties. Colloids Surf. B Biointerfaces 232, 113603 (2023).
Pei, M., Jia, X. & Liu, P. Design of Janus-like PMMA-PEG-FA grafted fluorescent carbon dots and their nanoassemblies for leakage-free tumor theranostic application. Mater. Des. 155, 288–296 (2018).
Lai, C.-W., Hsiao, Y.-H., Peng, Y.-K. & Chou, P.-T. Facile synthesis of highly emissive carbon dots from pyrolysis of glycerol; gram scale production of carbon dots/mSiO2 for cell imaging and drug release. J. Mater. Chem. 22, 14403–14409 (2012).
Zhao, A. et al. Recent advances in bioapplications of C-dots. Carbon 85, 309–327 (2015).
Li, G., Pei, M. & Liu, P. pH/Reduction dual-responsive comet-shaped PEGylated CQD-DOX conjugate prodrug: Synthesis and self-assembly as tumor nanotheranostics. Mater. Sci. Eng. C 110, 110653 (2020).
Ziaee, N. et al. Dual targeting of Mg/N doped-carbon quantum dots with folic and hyaluronic acid for targeted drug delivery and cell imaging. Biomed. Pharmacother. 164, 114971 (2023).
Poursadegh, H., Amini-Fazl, M. S., Javanbakht, S. & Kazeminava, F. Magnetic nanocomposite through coating mannose-functionalized metal-organic framework with biopolymeric pectin hydrogel beads: A potential targeted anticancer oral delivery system. Int. J. Biol. Macromol. 254, 127702 (2024).
Zuber, M. et al. Methotrexate related cutaneous adverse drug reactions: A systematic literature review. J. Basic Clin. Physiol. Pharmacol. 33, 549–565 (2021).
Haustein, U. F. & Rytter, M. Methotrexate in psoriasis: 26 years’ experience with low-dose long-term treatment. J. Eur. Acad. Dermatol. Venereol. 14, 382–388 (2000).
Abolmaali, S. S., Tamaddon, A. M. & Dinarvand, R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother. Pharmacol. 71, 1115–1130 (2013).
Tiwari, P. et al. Dacarbazine-primed carbon quantum dots coated with breast cancer cell-derived exosomes for improved breast cancer therapy. J. Control. Release 365, 43–59 (2024).
Yang, J. et al. Engineered exosome-mediated cobalt sulfide quantum dot targeted delivery for photothermal and chemodynamic anticancer therapy. J. Drug Deliv. Sci. Technol. 83, 104441 (2023).
Kazeminava, F. et al. Gentamicin-loaded chitosan/folic acid-based carbon quantum dots nanocomposite hydrogel films as potential antimicrobial wound dressing. J. Biol. Eng. 16, 1–13 (2022).
Zeng, Q. et al. Carbon dots as a trackable drug delivery carrier for localized cancer therapy in vivo. J. Mater. Chem. B 4, 5119–5126 (2016).
Sun, H. et al. Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer. Adv. Funct. Mater. 27, 1604300 (2017).
Rao, L. et al. Cancer cell membrane-coated nanoparticles for personalized therapy in patient-derived xenograft models. Adv. Funct. Mater. 29, 1905671 (2019).
Álvarez-González, B., Rozalen, M., Fernández-Perales, M., Álvarez, M. A. & Sánchez-Polo, M. Methotrexate gold nanocarriers: Loading and release study: Its activity in colon and lung cancer cells. Molecules 25, 6049 (2020).
Javanbakht, S., Nabi, M. & Shaabani, A. Graphene quantum dots-crosslinked gelatin via the efficient Ugi four-component reaction: Safe photoluminescent implantable carriers for the pH-responsive delivery of doxorubicin. Materialia 20, 101233 (2021).
Sharifi, M. et al. An updated review on EPR-based solid tumor targeting nanocarriers for cancer treatment. Cancers 14, 2868 (2022).
Javanbakht, S., Khodkari, V., Nazeri, M. T. & Shaabani, A. Efficient anchoring of CuO nanoparticles on Ugi four-component-functionalized graphene quantum dots: Colloidal soluble nanoplatform with great photoluminescent and antibacterial properties. React. Chem. Eng. 7, 1210–1218 (2022).
Rasmussen, M. K., Pedersen, J. N. & Marie, R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat. Commun. 11, 2337 (2020).
Wang, X., Tian, L., Lu, J. & Ng, I.O.-L. Exosomes and cancer—Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 11, 54 (2022).
Shukla, R. P. et al. Development of putrescine anchored nano-crystalsomes bearing doxorubicin and oleanolic acid: Deciphering their role in inhibiting metastatic breast cancer. Biomater. Sci. 9, 1779–1794 (2021).
Liu, H. et al. Synthesis of luminescent carbon dots with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability. Sci. Rep. 8, 1086. https://doi.org/10.1038/s41598-018-19373-3 (2018).
Li, R. & Xie, Y. Nanodrug delivery systems for targeting the endogenous tumor microenvironment and simultaneously overcoming multidrug resistance properties. J. Control. Release 251, 49–67 (2017).
Ding, C. & Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C 76, 1440–1453 (2017).
Biswas, A., Ghosh, T., Gavel, P. K. & Das, A. K. PEG functionalized stimuli responsive self-healable injectable dynamic imino-boronate G-quadruplex hydrogel for the delivery of doxorubicin. ACS Appl. Bio Mater. 3, 1052–1060 (2020).
Amjadi, S., Hamishehkar, H. & Ghorbani, M. A novel smart PEGylated gelatin nanoparticle for co-delivery of doxorubicin and betanin: A strategy for enhancing the therapeutic efficacy of chemotherapy. Mater. Sci. Eng. C 97, 833–841 (2019).
Battaglia, L. et al. Solid lipid nanoparticles by coacervation loaded with a methotrexate prodrug: Preliminary study for glioma treatment. Nanomedicine 12, 639–656 (2017).
Chen, Z. et al. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 370, 153–164 (2016).
Huang, Y., Cole, S. P., Cai, T. & Cai, Y. Applications of nanoparticle drug delivery systems for the reversal of multidrug resistance in cancer. Oncol. Lett. 12, 11–15 (2016).
Javanbakht, S., Saboury, A., Shaabani, A., Mohammadi, R. & Ghorbani, M. Doxorubicin imprinted photoluminescent polymer as a pH-responsive nanocarrier. ACS Appl. Bio Mater. 3, 4168–4178 (2020).
Ye, M. et al. A tumor-specific cascade amplification drug release nanoparticle for overcoming multidrug resistance in cancers. Adv. Mater. 29, 1702342 (2017).
Christianson, H. C., Svensson, K. J., Van Kuppevelt, T. H., Li, J.-P. & Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. 110, 17380–17385 (2013).
Khan, F. A. et al. Quantum dots encapsulated with curcumin inhibit the growth of colon cancer, breast cancer and bacterial cells. Nanomedicine 15, 969–980 (2020).
Pandey, G. et al. P-gp modulatory acetyl-11-keto-β-boswellic acid based nanoemulsified carrier system for augmented oral chemotherapy of docetaxel. Colloids Surf. B Biointerfaces 155, 276–286 (2017).
Hajipour, H. et al. A human chorionic gonadotropin (hCG) delivery platform using engineered uterine exosomes to improve endometrial receptivity. Life Sci. 275, 119351 (2021).
Dash, M., Palaniyandi, K., Ramalingam, S., Sahabudeen, S. & Raja, N. Exosomes isolated from two different cell lines using three different isolation techniques show variation in physical and molecular characteristics. Biochim. Biophys. Acta (BBA) Biomembr. 1863, 183490 (2021).
Keerthikumar, S. et al. ExoCarta: A web-based compendium of exosomal cargo. J. Mol. Biol. 428, 688–692 (2016).
Nezhad-Mokhtari, P., Arsalani, N., Javanbakht, S. & Shaabani, A. Development of gelatin microsphere encapsulated Cu-based metal–organic framework nanohybrid for the methotrexate delivery. J. Drug Deliv. Sci. Technol. 50, 174–180 (2019).
Acknowledgements
The authors acknowledged the following support: Drug Applied Research Center, Tabriz University of Medical Sciences (DARC) (grant number 65068; Ethics Code: IR.TBZMED.VCR.REC. 1399.143) and Iran National Science Foundation (INSF) (grant number 99018647). The authors would like to thank the DARC and INSF for financially supports. Also, they are grateful to Stem Cell Research Center, Tabriz University of Medical Sciences (SCRC) for technically support and staff collaboration.
Author information
Authors and Affiliations
Contributions
F.K.: Conceptualization, Investigation, Methodology, Writing-original draft. S.J.: Investigation, Methodology, Validation, Writing-review & editing. Z.L.: Methodology. M.R.: Investigation. M.A.: Investigation. B.A.: Formal analysis. M.M.: Formal analysis. A.F.: Methodology. H.H.: Validation. Z.A.: Investigation and formal Analysis. M.N.: Supervision, Project administration, Writing-review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kazeminava, F., Javanbakht, S., Latifi, Z. et al. Ultrasound-assisted encapsulating folic acid-based carbon quantum dots within breast cancer cell-derived exosomes as a co-receptors-mediated anticancer nanocarrier for enhanced breast cancer therapy. Sci Rep 14, 16941 (2024). https://doi.org/10.1038/s41598-024-67934-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-67934-6
Keywords
This article is cited by
-
Insights into extracellular vesicle-based platforms on the way to cancer treatment
Journal of Translational Medicine (2025)
-
In vitro antimicrobial and anticancer potentials of green synthesized luminescent carbon quantum dots derived from artichoke leaves
Scientific Reports (2025)
-
Apigenin-loaded exosome-like vesicles suppress triple-negative breast cancer by modulating miR-155/SOCS1/VHL, miR-146a/IRAK1/TRAF6 and reactivating STING/BRCA1
Scientific Reports (2025)