Abstract
Metal implants play a significant role in orthopedics, commonly used for treating fractures, joint replacement surgeries, spinal procedures, and more. Chromium (Cr), crucial in these implants, may raises health concerns. However, the relationship between metal implants and urine Cr levels remains uncertain. We aimed to evaluate this relationship. We conducted a cross-sectional study on 1419 individuals aged 40 years or older using data from the National Health and Nutrition Examination Survey (NHANES) spanning the years 2017 to 2020. Multivariate linear regression models and subgroup analysis were applied to assess associations between metal implants and urine Cr levels. Among the 1419 participants, 402 [28.3%] self-reported having metal objects in their bodies. After adjusting for potential confounding factors, metal implants were positively correlated with the accumulation of urine Cr (β = 0.41, 95% CI 0.04–0.77, p = 0.028). However, the positive correlation of metal implants with urine Cr was only present in females (β = 0.81, 95% CI 0.08–1.53, p = 0.029), but not in males. Our study revealed higher urine Cr levels in individuals with metal implants, with noticeable gender differences. Additionally, those with metal implants exhibited a more pronounced elevation in urine Cr levels with increasing age compared to individuals without implants.
Similar content being viewed by others
Introduction
Metal exposure is ubiquitous1, and people can be exposed to metals through food, drinking water1,2, and even surgery. Metal implants play an important role in the medical field, especially in orthopedics. Metal implants such as plates, screws, artificial joints are commonly used to stabilize fractures, support the spine, and replace damaged joints3. Although these implants are often designed to treat injuries or improve function, metal ion release from these materials is becoming a major cause of concern. The potential health hazards associated with this release have raised concerns among some individuals, who fear it could trigger allergic reactions, metal poisoning, or other health problems.
Metal implants are typically made of metal alloys and may need to remain in the body for extended periods. It is well-known that cobalt-chromium-molybdenum (CoCrMo) alloy is one of the most commonly used metal materials in total hip replacement due to its excellent biocompatibility, mechanical strength, and corrosion resistance4,5. Nonetheless, over time, physical wear, electrochemical corrosion, and inflammation can cause these metal implants to release metal ions into the body, where they can circulate, metabolize, and potentially cause local or systemic harm6. Chromium (Cr) is an essential component in metal implants but may pose potential health risks. On one hand, trivalent Cr (III) is considered essential for nutrition and plays a role in glucose metabolism7. On the other hand, hexavalent Cr (VI) is highly biologically toxic and has been listed as one of the top 20 toxic pollutants8.
As most bioavailable Cr is excreted in urine, the concentration of Cr in urine is considered a reliable biological indicator for monitoring Cr exposure9,10. Recent studies have shown a correlation between serum Cr levels and metal implants2,11, but there's a lack of studies on the relationship between urine Cr levels and metal implants. Further research in this field could contribute to a deeper understanding of the impact of metal implants on human Cr metabolism and the potential link between implants and Cr exposure. Therefore, this cross-sectional study aims to explore the relationship between metal implants and urine Cr levels in US adults using data from the National Health and Nutrition Examination Survey (NHANES). We hypothesize that participants with metal implants will have significantly higher urine Cr levels than those without metal implants.
Materials and methods
Study population
The NHANES is a research project designed to assess the health and nutritional status of American adults and children using a stratified multistage probability sampling technique12,13. The NHANES research protocols received approval from the National Center for Health Statistics (NCHS) Research Ethics Review Board, with written informed consent has been obtained from all participants. All methods of this study was performed in accordance with the principles outlined in the Declaration of Helsinki.
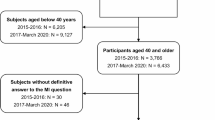
In this study, we analyzed NHANES data from 2017 to 2020 and selected participants aged 40 years or older who responded to the questionnaire regarding the presence of metal implants in their bodies (n = 6433). We excluded individuals with missing data on "Any metal objects inside your body?" (n = 46), urine Cr levels (n = 4458), and other covariates (n = 510). Ultimately, a total of 1419 individuals were included in the final analysis. The participant selection process is illustrated in Fig. 1.
Variables
The presence of metal implants within the body was defined as an affirmative response to the following question: “Do you have any artificial joints, pins, plates, metal suture material, or other types of metal objects in your body?”. Moreover, the mentioned metal implants exclude piercings, crowns, dental braces or retainers, shrapnel, or bullets, and should not be visible on the outside of the body or in the mouth.
The urine concentrations of Cr were measured using a specific analytical process. After acidification and dilution with nitric acid, the Cr content in the urine sample was measured using inductively coupled plasma mass spectrometry. During Cr detection, ammonia gas was introduced into the Universal Cell Technology to eliminate polyatomic interferences. Quantification of Cr was achieved by comparing the blank-subtracted counting rate of Cr from the sample, adjusted to an internal standard, to the blank-subtracted counting rate of matrix-matched external calibrators, also adjusted to an internal standard, within the same analytical run. A detailed description of the laboratory methods can be found in the Laboratory Method Files section (https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/P_UCM.htm). To minimize statistical errors caused by variations in urinary Cr concentrations due to drinking habits, kidney function, and other factors, we also normalized the urinalysis results to creatinine content. Specifically, we calculated the urine Cr/creatinine (Cr/Cre) ratio. However, because the resulting values were too small for effective statistical analysis, we multiplied the ratio by a factor of 10 before including it in our statistical analysis. This adjustment ensures that the data is manageable for statistical evaluation without affecting the relative differences or accuracy of our findings. Consequently, this adjustment increased the reliability of our data and ensured the validity of our statistical analysis, further confirming the stability of our results.
Based on previous studies2,14, the potential covariates considered in this study included age, gender, marital status, race/ethnicity, educational level, family income, smoking status, drinking status, serum hemoglobin, tap water intake, shellfish intake, fish intake, hypertension, diabetes, and coronary heart disease. Marital status was categorized as married, living with a partner, or living alone. Race/ethnicity included classifications such as non-Hispanic white, non-Hispanic black, Mexican American, or other races. Educational attainment was classified as less than 9 years, 9 to 12 years, and more than 12 years. Family income, based on the poverty income ratio (PIR), was grouped as low (PIR ≤ 1.3), medium (1.3 < PIR ≤ 3.5), and high (PIR > 3.5) according to a US government report15. Referring to previous literature16,17, smoking status was categorized as never, former, or current smoker, while drinking status was categorized as never, former, mild, moderate, or heavy drinker. The definition of tap water, shellfish, and fish intake was based on dietary interview inquiries about “tap water source”, “shellfish eaten during past 30 days”, and “fish eaten during past 30 days”, respectively. The definition of hypertension, diabetes, and coronary heart disease was based on questionnaire inquiries about whether participants had been informed by their doctor of these conditions in the past. The detailed definitions of the covariates are available at https://wwwn.cdc.gov/nchs/nhanes/.
Statistical analysis
A secondary analysis was conducted on publicly available datasets, with categorical variables represented as percentages (%) and continuous variables expressed as means (SD) or median (IQR) based on the data distribution. To assess differences between groups, we utilized analysis of variance, Kruskal–Wallis, and chi-squared tests. Additionally, multivariate linear regression models were employed to evaluate the association between metal implants and urine Cr. Model 1 was adjusted for sociodemographic characteristics, including age, gender, race/ethnicity, marital status, education level, and family income. Model 2 was further adjusted for smoking status and drinking status, in addition to the factors included in model 1. Model 3 was adjusted for the factors included in model 2 and serum hemoglobin.Model 4 was adjusted for the factors included in model 3 and tap water source, shellfish intake, fish intake. Model 5 achieved full adjustment, encompassing factors from Model 4 and incorporating hypertension, diabetes, coronary heart disease.
The subgroup analysis was conducted using stratified multivariate regression analysis. Additionally, to detect trends, smooth curve fittings were utilized to explore the relationship between age and urine Cr in different groups.
Statistical analyses were conducted using R software version 4.2.1 and Free Statistics software version 1.9.2. Significance was declared using P < 0.05 (two-tailed).
Results
From 2017 to 2020, a total of 1419 individuals aged 40 years or older were successfully enrolled in this study, consisting of the without metal implants group (n = 1017) and the with metal implants group (n = 402) (Fig. 1). Table 1 shows the baseline characteristics of participants stratified according to the presence or absence of metal implants in the body. Compared to participants without metal implants, those with metal implants were more likely to be older, non-Hispanic White, former smokers, former drinkers, and have hypertension or diabetes. Moreover, individuals with metal implants tended to have higher levels or concentrations of Cr in their urine.
Table 2 presents the results of multivariate liner regression analyses. In the unadjusted model, there is a positive correlation between the presence of metal implants and urinary Cr levels (β = 0.43, 95% CI 0.09–0.77, p < 0.05). Even after adjusting for confounding factors, this positive correlation persists in model 1 (β = 0.37, 95% CI 0.01–0.72, p = 0.042), model 2 (β = 0.38, 95% CI 0.03–0.74, p = 0.034), model 3 (β = 0.37, 95% CI 0.02–0.73, p = 0.038), model 4 (β = 0.39, 95% CI 0.03–0.74, p = 0.033), and model 5 (β = 0.41, 95% CI 0.04–0.77, p = 0.028). In all models, the presence of metal implants is positively associated with urine Cr levels, and the association remained statistically significant. Additionally, metal implants were positively correlated with the urinary Cr/Cre ratio in all five models. Individuals with metal implants had a 0.03 higher urinary Cr/Cre ratio than those without metal implants (Table 2).
As shown in Table 3, in subgroup analysis stratified by gender, the positive correlation between metal implants and urinary Cr levels was only observed in females (β = 0.81, 95% CI 0.08–1.53, p = 0.029). In supplementary Table 1, our subgroup analysis stratified by gender reveals that metal implants are positively correlated with urinary Cr/Cre ratio only in females.
As indicated by the smoothed curve in Fig. 2, participants with metal implants show a more pronounced increase in urinary Cr levels with advancing age compared to those without metal implants.
Stratified analyses are displayed in Fig. 3. We observed significant interactions between individuals of different genders (p = 0.03 for interaction). The positive correlation between metal implants and urinary Cr levels was only observed in females (β = 0.81, 95% CI 0.08–1.53, p = 0.029). Aside from these findings, no significant interactions were observed in analyses stratified by age.
Stratified analysis of the relationship between metal implants and urinary chromium levels. Except for the stratification component itself, each stratification factor was adjusted for all other variables (age, gender, marital status, race/ethnicity, educational level, family income, smoking status, drinking status, serum hemoglobin, tap water intake, shellfish intake, fish intake, hypertension, diabetes, coronary heart disease.). CI confidence interval.
Discussion
The findings of our study suggest a significant correlation between the presence of metal implants and elevated Cr levels in urine samples, with differences observed between genders. Previous studies have highlighted gender disparities in blood Cr ion levels among patients with metal implants, revealing a positive correlation exclusively in females and not in males2. Additionally, research on metal-on-metal total hip arthroplasty has demonstrated varying levels of metal ion release between men and women, with women generally exhibiting higher Cr ion levels in their blood compared to men18. However, the specific mechanisms underlying these gender differences remain unclear. Currently, there is no literature reporting on gender differences in Cr ion levels in urine among patients with metal implants. It is noteworthy that individuals with metal implants show a more significant increase in urinary Cr levels as they age, compared to those without metal implants.
The current research mainly focuses on the impact of metal implants on Cr concentration in the blood2,19,20. However, urinary Cr levels, recognized as an acceptable biomarker of Cr exposure9,10,21,22, should receive equal attention for monitoring to better assess the long-term potential impacts of metal implants on kidney function or overall health23,24. Metal implants undergo a process of frictional corrosion within the body, leading to the generation of metal debris and the release of metal ions and particles25. These ions and particles can be absorbed by surrounding tissues, deposited within tissues, released into the bloodstream, or excreted from the body through urine26,27,28,29. Studies on renal handling indicate that Cr undergoes slow urinary excretion30. A prospective study on urinary metal excretion in patients with metal-on-metal (MoM) prostheses revealed that Cr levels peaked approximately 1–2 years after surgery and subsequently exhibited a slight decline31. Evidence exists indicating that Cr can impair renal function and lead to tubular necrosis in both humans and animals32,33.
Metal implants release free Cr ions that precipitate in local tissues in the form of Cr phosphate, but do not form organometallic complexes in serum34,35,36,37,38. However, other studies suggest that Cr released from metal implants preferentially distributes into the serum, with Cr in the blood existing in a non-toxic trivalent state39. Trivalent Cr is considered essential for glucose metabolism and has a wide safety range, with no reports of high-dose toxicity in nutritional studies40. Some experimental studies and small-scale clinical trials suggest that supplementing with Cr can reduce oxidative stress41,42. However, hexavalent Cr is toxic and has a tendency to enter blood cells43, classified by the World Health Organization as a Group 1 human carcinogen44. Some studies indicate that while Cr levels rise in patients with metal implants, they do not reach levels high enough to cause disease45.
This study has several limitations to consider. Firstly, being a cross-sectional study, it cannot establish causal relationships among dependent, independent, and covariate variables. Therefore, it is impossible to determine the temporal relationship between metal implants and Cr levels in urine. Secondly, considering privacy concerns, the NHANES database does not provide specific geographical locations or related occupations of participants. This limitation prevents the study from assessing the influence of geographical environment and occupational exposure on urine Cr levels. Additionally, the NHANES database lacks detailed records of metal implants, making it impossible to determine the type, quantity, and duration of these implants in the body. The function and biomechanical load of metal implants vary greatly, and some implants may not contain chromium. The effects of load-bearing and non-load-bearing joints on implants may differ. Various factors play crucial roles in the degradation of metal implant materials, particularly joint replacement implants, which are associated with chromium release. This results in a highly heterogeneous group, lacking clinical specificity. In future research, we will aim to refine the details regarding metal implants and consider their potential confounding effects. Finally, it is not possible to determine information about individual Cr compounds, such as trivalent or hexavalent Cr, from the total urinary Cr levels. Addressing this limitation requires the initiation of future prospective studies. Moreover, it is essential to recognize that complete elimination of residual confounding effects from unmeasured or unknown factors could not be achieved.
Conclusion
In summary, our study revealed higher urinary Cr levels in individuals with metal implants, with noticeable gender differences. Additionally, those with metal implants exhibited a more pronounced elevation in urinary Cr levels with increasing age compared to individuals without implants. Further large-scale prospective investigations are warranted to validate and expand upon our findings.
Data availability
The datasets generated or analysed during the current study are available from the corresponding author on reasonable request.
References
Vogel, N. et al. Lead, cadmium, mercury, and chromium in urine and blood of children and adolescents in Germany Human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Int. J. Hyg. Environ. Health 237, 113822 (2021).
He, J., Li, J., Wu, S., Wang, J. & Tang, Q. Accumulation of blood chromium and cobalt in the participants with metal objects: Findings from the 2015 to 2018 National Health and Nutrition Examination Survey (NHANES). BMC Geriatr. 3, 23 (2023).
Bhandari, M. et al. Total hip arthroplasty or hemiarthroplasty for hip fracture. N. Engl. J. Med. 381, 2199–2208 (2019).
Manthe, J. et al. Hip implant modular junction: The role of CoCrMo alloy microstructure on fretting-corrosion. J. Mech. Behav. Biomed. Mater. 134, 105402 (2022).
Kaur, M. & Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 102, 844–862 (2019).
Zhong, Q. et al. Prosthetic metals: Release, metabolism and toxicity. Int. J. Nanomed. 19, 5245–5267 (2024).
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B. & Beeregowda, K. N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 7, 60–72 (2014).
Yu, Y. T., Yuan, Y. N., Yang, S. J. & Wu, F. Comment on “synergetic transformations of multiple pollutants driven by Cr(VI)-sulfite reactions”. Environ. Sci. Technol. 50, 6107–6108 (2016).
Langård, S. & Costa, M. Chromium. In Handbook on the Toxicology of Metals (eds Nordberg, G. F. et al.) 717–742 (Elsevier, 2015).
Leng, G. Chrom und seine Verbindungen BAT Value Documentation in German language. In The MAK-Collection for Occupational Health and Safety 1–7 (Wiley, 2012).
Mathew, S. E. et al. Are serum ion levels elevated in pediatric patients with metal implants?. J. Pediatr. Orthop. 42, 162–168 (2022).
Zipf, G. et al. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat. 1, 1–37 (2013).
Huyett, P., Siegel, N. & Bhattacharyya, N. Prevalence of sleep disorders and association with mortality: Results from the NHANES 2009–2010. Laryngoscope 131, 686–689 (2021).
Norwood, W. P., Borgmann, U. & Dixon, D. G. Saturation models of arsenic, cobalt, chromium and manganese bioaccumulation by Hyalella azteca. Environ. Pollut. 143, 519–528 (2006).
Agricultural Rural Research Service; US Department of Agriculture. What We Eat in America: Data Tables. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/. Accessed March 7 (2021).
Liu, H., Wang, L., Chen, C., Dong, Z. & Yu, S. Association between dietary niacin intake and migraine among American adults: National Health and Nutrition Examination Survey. Nutrients 14, 3052 (2022).
Chen, H. Y. et al. Life’s essential 8 and mortality in US adults with chronic kidney disease. Am. J. Nephrol. 54, 516–527 (2023).
Moroni, A., Savarino, L., Hoque, M., Cadossi, M. & Baldini, N. Do ion levels in hip resurfacing differ from metal-on-metal THA at midterm?. Clin. Orthop. Relat. Res. 469, 180–187 (2011).
Walter, L. R., Marel, E., Harbury, R. & Wearne, J. Distribution of chromium and Co ions in various blood fractions after resurfacing hip arthroplasty. J. Arthroplast. 23, 814–821 (2008).
Smolders, J. M. et al. Metal ion interpretation in resurfacing versus conventional hip arthroplasty and in whole blood versus serum. How should we interpret metal ion data. Hip Int. 21, 587–595 (2011).
Kerger, B. D., Paustenbach, D. J., Corbett, G. E. & Finley, B. L. Absorption and elimination of trivalent and hexavalent chromium in humans following ingestion of a bolus dose in drinking water. Toxicol. Appl. Pharmacol. 141, 145–158 (1996).
Bernard, A. Biomarkers of metal toxicity in population studies: Research potential and interpretation issues. J. Toxicol. Environ. Health A 71, 1259–1265 (2008).
Newton, A. W., Ranganath, L., Armstrong, C., Peter, V. P. & Roberts, N. B. Differential distribution of cobalt, chromium, and nickel between whole blood, plasma and urine in patients after metal-on-metal (MoM) hip arthroplasty. J. Orthop. Res. 30, 1640–1646 (2012).
Merritt, K., Crowe, T. D. & Brown, S. A. Elimination of nickel, Co and chromium following repeated injections of high dose metal salts. J. Biomed. Mater. Res. 23, 845–862 (1989).
Sinnett-Jones, P. E., Wharton, J. A. & Wood, R. J. K. Micro-abrasion-corrosion of a CoCrMo alloy in simulated artificial hip joint environments. J. Wear. 259, 898–909 (2005).
Cooper, H. J., Urban, R. M., Wixson, R. L., Meneghini, R. M. & Jacobs, J. J. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J. Bone Joint Surg. Am. 95, 865–872 (2013).
Laura, A. D. et al. Retrieval findings of recalled dual-taper hips. J. Bone Joint Surg. Am. 100, 1661–1672 (2018).
Kim, C. H. et al. Serum metal ion levels in cementless metal-on-metal total hip arthroplasty: Long-term follow-up trends. J. Arthroplast. 34, 534–537 (2019).
Gkiatas, I. et al. Serum metal ion levels in modular dual mobility acetabular components: A systematic review. J. Orthop. 21, 432–437 (2020).
Brown, S. A., Farnsworth, L. J., Merritt, K. & Crowe, T. D. In vitro and in vivo metal ion release. J. Biomed. Mater. Res. 22, 321–338 (1988).
Daniel, J., Ziaee, H., Pradhan, C. & McMinn, D. J. W. Six-year results of a prospective study of metal ion levels in young patients with metal-on-metal hip resurfacings. J. Bone Joint Surg. Br. 91, 176–179 (2009).
Oliveira, H., Santos, T. M., Ramalho-Santos, J. & Pereira, M. L. Histopathological effects of hexavalent chromium in mouse kidney. Bull. Environ. Contam. Toxicol. 76, 977–983 (2006).
Barceloux, D. G. Chromium. J. Toxicol. Clin. Toxicol. 37, 173–194 (1999).
Shao, W. et al. Association between level of urinary trace heavy metals and obesity among children aged 6–19 years: NHANES 1999–2011. Environ. Sci. Pollut. Res. Int. 24, 11573–11581 (2017).
Jin, R. et al. Associations of renal function with urinary excretion of metals: Evidence from NHANES 2003–2012. Environ. Int. 121, 1355–1362 (2018).
Martin, J. R. et al. Increased femoral head offset is associated with elevated metal ions in asymptomatic patients with metal-on-polyethylene total hip arthroplasty. J. Arthroplast. 31, 2814–2818 (2016).
Maurer-Ertl, W. et al. Clinical results and serum metal ion concentrations following ceramic-on-metal total hip arthroplasty at a mean follow-up of 60 months. Biomed. Res. Int. 2017, 3726029 (2017).
Gilbert, J. L., Buckley, C. A. & Jacobs, J. J. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J. Biomed. Mater. Res. 27, 1533–1544 (1993).
Ho, J. H. et al. Metal-onMetal Hip joint prostheses: A retrospective case series investigating the association of systemic toxicity with serum cobalt and chromium concentrations. J. Med. Toxicol. 13, 321–328 (2017).
Anderson, R. A. Chromium as an essential nutrient for humans. Regul. Toxicol. Pharmacol. 26, S35–S41 (1997).
Jamilian, M. et al. The effects of chromium supplementation on endocrine profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 172, 72–78 (2016).
Anderson, R. A. et al. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J. Am. Coll. Nutr. 20, 212–218 (2001).
Bhamra, M. & Case, C. Biological effects of metal-onmetal hip replacements. Proc. Inst. Mech. Eng. H 220, 379–384 (2006).
IARC. Chromium, nickel and welding. IARC Monogr. Eval. Carcinog. Risks Hum. 49, 17–33 (1990).
Di, S. P. et al. Evaluation of cobalt and chromium levels following implantation of cobalt chromium coronary stents: A pilot study. Heart Lung Circ. 27, 763–766 (2018).
Acknowledgements
The authors acknowledge Huanxian Liu from the Department of Neurology, Chinese PLA General Hospital, for his contributions to study design consultations and manuscript comments.
Author information
Authors and Affiliations
Contributions
XG.Q. wrote the main manuscript text and performed the statistical analysis. JM.L. and WH.L. and XD.Q. collected data. JW.F. and JK.G. performed the statistical analysis and prepared figures and tables. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qi, X., Liu, J., Liu, W. et al. Association between metal implants and urinary chromium levels in US adults: a cross-sectional study from NHANES. Sci Rep 14, 17111 (2024). https://doi.org/10.1038/s41598-024-68049-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68049-8