Abstract
Currently, due to limited long-term evidence, there remains some controversy surrounding the recommended postoperative monitoring strategy for primary low-risk gastrointestinal stromal tumors (GISTs). This study recruited a total of 532 patients diagnosed with very low-risk and low-risk GISTs who underwent endoscopic resection from 2015 to 2021, including 460 very low-risk patients and 72 low-risk patients. Descriptive statistical analysis was used to evaluate the clinical and pathological characteristics of GIST patients, and Kaplan–Meier methods were employed for survival analysis. The results showed that the 5-year recurrence-free survival rates for very low-risk and low-risk patients were 98.5% and 95.9%, respectively. The 5-year disease-specific survival rates for both groups were 100%. Additionally, the 5-year overall survival rates were 99.7% for very low-risk patients and 100% for low-risk patients (P = 0.69). Therefore, it is suggested that routine follow-up monitoring, including endoscopic surveillance and imaging, may not be necessary for very low-risk and low-risk GISTs after endoscopic resection.
Similar content being viewed by others
Introduction
Gastrointestinal stromal tumors (GISTs) are considered to be the most common mesenchymally derived tumors of the digestive tract and are mainly derived from Cajal cells or their precursor cells, the biological behavior of GIST is diverse, manifests as benign, potentially malignant or malignant to varying degrees, and the majority of GIST have either KIT or platelet derived growth factor receptor alpha (PDGFRA) as the mutation type1,2,3. GIST can occur anywhere in the gastrointestinal tract but most commonly in the stomach (60–65%), followed by the small intestine (20–25%), and less commonly the colorectum, esophagus and other areas3,4,5. At present, The most commonly used classification system is the modified NIH risk classification criteria, which classifies the risk of GIST recurrence into very low-risk, low-risk, intermediate-risk and high-risk groups according to the tumor size, mitotic rate, tumor location and tumor rupture6. For localized GIST, the standard therapeutic approach is complete surgical resection2,3. Particularly for GIST with a diameter > 2 cm, given their inherent malignant potential, surgical intervention is recommended7. Although small GISTs with a diameter of < 2 cm can be followed up according to the patient's wishes, there is now a view that surgical resection of small GISTs is recommended to avoid unnecessary follow-up8,9. With the continuous advancements in endoscopic techniques, endoscopic resection has become widely incorporated into the therapeutic landscape of GISTs. Research indicates that the overall survival of GIST patients post-ER is comparable to that of those treated with surgical intervention10,11,12. Despite the expanding application of endoscopic resection in GIST management, our understanding of its long-term prognostic implications remains somewhat limited.
We have incorporated the information about the NCCN guidelines recommending follow-up intervals of every 3–6 months in the initial five years, noting potential flexibility for low-risk or very low-risk GISTs cases13. For GIST patients post-endoscopic resection (ER), periodic endoscopic monitoring is recommended to evaluate wound healing and recurrence status. However, due to a lack of pertinent data research, guidelines specifying the optimal timing intervals for follow-up remain ambiguous2. We recognize that existing clinical data suggest that patients with very low-risk and low-risk GISTs postoperatively exhibit notable recurrence-free survival (RFS) and overall survival (OS) periods, potentially obviating the need for routine postoperative surveillance14. Repetitive imaging follow-ups may entail unnecessary radiation exposure, while excessive gastrointestinal endoscopic monitoring could inflict additional injury to the patient's digestive tract and impose undue economic burdens. Consequently, this study aims to analyze and evaluate the postoperative outcomes of ER for very low-risk and low-risk GISTs based on authentic clinical data, exploring postoperative recurrence and survival conditions.
Methods
Population selection
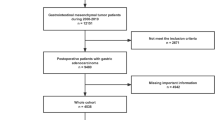
We retrospectively collected clinical data from patients with very low-risk and low-risk GIST who underwent endoscopic resection at the Digestive Department of the First Affiliated Hospital of Nanchang University between 2015 and 2021. Considering that very low-risk and low-risk gastrointestinal stromal tumors usually have a good prognosis and the economic burden of patients on genetic testing, our hospital has not performed routine genetic testing on such patients2,5. We set up the inclusion and exclusion criteria for this study. Inclusion criteria were as follows: (1) Primary very low-risk and low-risk gastrointestinal GIST diagnosed by postoperative pathology from 2015 to 2021, including esophagus, stomach, duodenum and colorectal; (2) Patients aged 18 years or older; (3) Endoscopic resection: including endoscopic submucosal dissection (ESD), endoscopic full-thickness resection (EFTR), submucosal tunneling endoscopic resection (STER), among others; (4) Imaging indicating no metastasis. Exclusion criteria: (1) Patients with a history of concurrent malignancies; (2) Patients with severely deficient pathological data; (3) Patients with incomplete follow-up information. Based on the established inclusion and exclusion criteria, we ultimately included 532 cases for study analysis, comprising 460 cases in the very low-risk group and 72 cases in the low-risk group (Fig. 1).
We collected essential patient data, including basic information, tumor characteristics, endoscopic features, pathological findings, NIH grading, and recurrence status throughout their clinical course. Prior to any endoscopic procedure, informed consent was obtained from all patients following institutional protocols. All patient did not take aspirin and other non-steroidal anti-inflammatory drugs at least one week before ER. We confirm that all methods were carried out in accordance with relevant guidelines, and all included cases were recorded in the Human Genetic Resources Center of the First Affiliated Hospital of Nanchang University. This study protocol has been approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University.
Endoscopic resection
In our retrospective study, a total of 532 patients with very-low and low risk GIST who underwent endoscopic resection were screened, including 497 (93.4%) cases of ESD, 32 (6.0%) cases of EFTR, and 3 (0.6%) cases of STER. ESD is the main surgical resection. All ER procedures were performed by experienced gastrointestinal endoscopist using a single-channel endoscope. The procedures were performed with the patient under intravenous anesthesia.
Endoscopic submucosal dissection (ESD)
After the lesion was directly identified under endoscopy (Fig. 2A), the lesion was marked by electrocoagulation. Several milliliters of mixture (including 250 mL of glycerol fructose, 2–3 mL of indigo carmine, and 1 mL of 1:10,000 epinephrine) was injected into the submucosa to lift the overlying mucosa with a syringe needle (Fig. 2B). High-frequency electric knife (Dual, hook, IT) was used to perform circumferential mucosal incision around the marker point, and then the tumor was gradually separated (Fig. 2C). Than, the tumor was completely removed by snare and high-frequency electric knife (Fig. 2D), and the wound was closed by titanium clip (Fig. 2E)15. Finally, the cut tumor would be carefully measured in size (Fig. 2F).
Endoscopic submucosal dissection of gastrointestinal stromal tumors in the lesser curvature of the gastric body. (A) Endoscopic manifestations of GIST in the lesser curvature of the stomach (a small ulcer can be seen on the surface). (B) Submucosal injection around the tumor. (C) Circumferential incision. (D) Stripping and completely exposing the tumor. (E) titanium clips were used to close the wound. (F) The diameter of the resected specimen was about 2.4 cm.
Endoscopic full-thickness resection (EFTR)
Similar to the ESD procedure, after endoscopic identification of the lesion (Fig. 3A), electrocoagulation was used to mark the lesion (Fig. 3B), and then a syringe was used to inject the mixture into the submucosa to bulge around the lesion (Fig. 3C). The high-frequency electric knife (hook, gold, woodpecker, IT or Dual) was used to cut the surface mucosa and then stripped. The snare and high-frequency electric knife were used for full-thickness resection and complete dissection of the lesion (Fig. 3D). Finally, titanium clips were used to close the wound and the cut tumor would be carefully measured in size (Fig. 3E and F).
Endoscopic full-thickness resection of gastric fundus gastrointestinal stromal tumors. (A) Endoscopic manifestations of GIST in the gastric fundus (depressed ulcer formation at the top). (B) Hook knife marked lesions. (C) Submucosal injection around the tumor. (D) stripping the tumor and full-thickness resection. (E) titanium clips were used to close the wound. (F) The diameter of the resected specimen was about 3.4 cm
Submucosal tunneling endoscopic resection (STER)
Upon direct identification of the lesion under endoscopy (Fig. 4A), a appropriate entry point was selected for submucosal injection, and the high-frequency electric knife (woodpecker, Dual or IT) was used to cut the mucosa and establish the tunnel entrance (Fig. 4B). Subsequently, a high-frequency electric knife was used to gradually separate the submucosa and muscle layer along the direction of the tumor to establish a tunnel below the tumor (Fig. 4C), stripped and removed the tumor (Fig. 4D), and the lesion was completely removed using a snare (Fig. 4E,F). Finally, the tunnel opening was clamped with a titanium clip and the size of the excised tumor would be measured (Fig. 4G,H).
Submucosal tunnel endoscopic resection of esophageal gastrointestinal stromal tumors. (A) Endoscopic manifestations of esophageal GIST. (B) Establish a tunnel portal. (C) Establish a tunnel below the tumor. (D) Stripping and completely exposing the tumor. (E), (F) snare complete resection of the tumor. (G) The appearance of the wound after the titanium clip closed the tunnel mouth. (H) The diameter of the resected specimen was about 3cm.
Risk stratification and follow-up
The GIST risk stratification was conducted according to the 2008 modified NIH consensus. GISTs were categorized as 'very low-risk' if they had a tumor diameter < 2 cm and ≤ 5 mitoses per 50 high-power fields (HPFs), and as 'low-risk' if the tumor size ranged between 2.1 and 5.0 cm with ≤ 5 mitoses per 50 HPFs (Table 1)6. All enrolled patients were recommended to undergo gastrointestinal endoscopic follow-up within the first year postoperatively, followed by annual gastrointestinal endoscopy or imaging examinations. The most recent follow-up date was recorded as the date of the latest imaging examination, gastrointestinal endoscopy, or telephone follow-up. Survival and recurrence serve as the endpoints for this study. Recurrence was categorized as local recurrence, metastatic recurrence, or combined recurrence. Local recurrence was defined as GIST pathologically confirmed to have developed anew on or near the prior surgical scar16. Metastatic recurrence was identified by imaging findings coupled with pathological confirmation of visceral metastatic GIST. Combined recurrence referred to the confluence of local and visceral metastatic recurrence. Recurrence-free survival (RFS) was defined as the interval from endoscopic resection to recurrence (either local and/or distant), with patients without recurrence censored at the latest follow-up date. Disease-specific survival (DSS) was delineated from the date of diagnosis to the date of death attributable to GIST or the most recent follow-up date. Overall survival (OS) was characterized from the diagnosis date to the patient's death or the most recent follow-up date. The last follow-up date was recorded as November 2023.
Statistical analysis
Categorical variables were expressed as frequencies and percentages, while continuous variables were presented as mean ± standard deviation (SD) or median and interquartile range (IQR). The Kaplan–Meier method was employed to estimate the recurrence-free survival (RFS) and disease-specific survival (DSS) of patients with very low-risk and low-risk GISTs. Group comparisons were conducted using the Log-Rank test. Statistical analyses were performed using SPSS version 25.0 and R version 4.3.0. P < 0.05 was considered statistically significant.
Ethical approval
We confirm that all methods were carried out in accordance with relevant guidelines, and all included cases were recorded in the Human Genetic Resources Center of the First Affiliated Hospital of Nanchang University. This study protocol has been approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University. Given the retrospective nature of the study, the need for informed consent was waived by the institutional review boards.
Results
Patient characteristics
Based on the established inclusion and exclusion criteria, we ultimately included 532 cases for study analysis, comprising 460 cases in the very low-risk group and 72 cases in the low-risk group (Fig. 1). Basic information and tumor characteristics are summarized in Table 2. The average age of the entire cohort was 55.50 (± 9.55) years, with a median tumor size of 1.00 (IQR: 0.70–1.5) cm. Regarding the primary tumor locations, there were 2 cases in the esophagus, 313 cases in the cardia and gastric fundus, 206 cases in the gastric body, angle, and antrum, 7 cases in the duodenum, and 4 cases in the rectum. In our retrospective study, a total of 532 patients with very-low and low risk GIST who underwent endoscopic resection were screened, including 497 (93.4%) cases of ESD, 32 (6.0%) cases of EFTR, and 3 (0.6%) cases of STER. Postoperative adverse events predominantly included 44 cases of fever (8.3%), 6 cases of bleeding (1.1%) (with 2 cases being delayed bleeding), and 1 case (0.2%) of perforation (imaging indicated subdiaphragmatic free gas). A total of 522 cases (98.1%) underwent En bloc resection, while 10 cases (1.9%) underwent piecemeal resection.
Details of recurrent patients
Throughout the entire follow-up period of the cohort, only four patients were diagnosed with local recurrence (0.75%), and the number needed to follow up to detect one recurrence was 133 individuals. There was only one patient who succumbed to pancreatic cancer, with no fatalities attributed to Gastrointestinal Stromal Tumors (GISTs). The median recurrence-free survival (RFS) for the four recurrent patients was 24.88 [9.74, 38.65] months (Table 3). Among the recurrent cases, one patient underwent surgical resection post-recurrence with a low-risk NIH pathology classification, another patient opted for laparoscopic resection with a low-risk NIH pathology classification, and the remaining two patients underwent ESD post-recurrence, all achieving an very low-risk NIH classification after surgery (Table 4). Follow-up examinations, including gastrointestinal endoscopy and imaging, conducted up to November 2023, revealed no evidence of GIST recurrence in these four recurrent patients over the past two years.
Recurrence-free, disease-specific survival and overall survival
According to the definition of RFS, the median follow-up time for the entire cohort was 20.53 [7.06, 42.11] months. Within this cohort, the median follow-up time for the very low-risk group was 20.87 [7.44, 42.11] months, and for the low-risk group, it was 19.47 [6.62, 42.83] months.
As shown in Fig. 5, the RFS of patients with GIST categorized into very low-risk and low-risk groups. Among the entire cohort, 331 cases (62.22%) had follow-up periods exceeding one year, while 177 cases (33.27%) extended beyond three years. The 3-year and 5-year RFS rates for the entire cohort were 99.4% and 98.2%, respectively. Specifically, in the very low-risk group, the 3-year and 5-year RFS rates were 100% and 98.5%, respectively; in the low-risk group, the rates were 95.9% for both 3-year and 5-year RFS (P = 0.03).
As displayed in Fig. 7, the OS of patients with GIST categorized into very low-risk and low-risk groups. The median follow-up time for the entire cohort was 39.13 [16.93, 65.15] months. Specifically, the median follow-up time for the very low-risk group was 39.13 [16.29, 65.15] months, while for the low-risk group, it was 38.92 [23.23, 65.68] months. Throughout the follow-up period, only one patient succumbed to pancreatic cancer. Both the 3-year and 5-year DSS rates for both the very low-risk and low-risk groups stood at 100% (Fig. 6) (P = 1.00). The 3-year and 5-year OS rates were 99.7% for the very low-risk group and 100% for the low-risk group (Fig. 7). There was no significant difference observed between the two risk groups (P = 0.69).
Discussion
Our analysis indicates that GIST patients classified into very low-risk and low-risk groups based on the 2008 NIH risk stratification (Table 1) exhibit favorable 5-year survival outcomes post-endoscopic resection. There was no statistically significant difference in overall survival between the two groups. Consequently, this study suggests that post-endoscopic resection follow-up examinations, including imaging and gastrointestinal endoscopies, may not offer added benefits in monitoring disease recurrence.
As of today, there remains insufficient evidence regarding the optimal postoperative follow-up strategy for GISTs, including both surgical and endoscopic resections. Various guidelines lack a unified consensus on the postoperative follow-up approach for GISTs. For very low-risk GISTs, guidelines consistently recommend against routine follow-up. In the case of low-risk GISTs, ESMO guidelines suggest performing CT or MRI scans every 6–12 months for a duration of 5 years2, while GEIS guidelines recommend annual CT examinations for up to 10 years, with the option to extend further to a maximum of 10 years3. The natural history of GIST after ER surgery is not clear. Although there are guidelines recommending endoscopic review at 6–12 months after surgery and then within 2–3 years8, clinical studies on the follow-up of GIST after ER surgery are still lacking. Additionally, for intermediate to high-risk GIST patients requiring adjunctive therapy with imatinib and similar drugs, genomic differences may play a more significant role in prognosis17,18. Therefore, this study focuses on the post-ER follow-up outcomes of very low-risk and low-risk GIST patients, analyzing and discussing the results.
In the latest 2020 World Health Organization (WHO) classification for soft tissue and bone tumors, it is explicitly noted that all GIST are categorized as malignant tumors19. Unlike the TNM grading used for other malignant tumors, the risk stratification for GIST most commonly employs the modified 2008 NIH classification. This classification is based on tumor size, location, mitotic index, and tumor rupture status, categorizing them into very low-risk, low-risk, intermediate-risk, and high-risk groups6.The primary treatment modalities for GIST include surgical intervention and adjuvant drug therapy. Imatinib is a frontline drug for treating advanced-stage GIST, effectively prolonging patient survival4,20. Nevertheless, surgical resection of non-metastatic small GIST remains the only potentially curative treatment option, significantly enhancing both recurrence-free survival and overall survival rates for patients21. The widespread use of gastrointestinal endoscopy has increased the detection rate of GISTs. Concurrently, with advancements in endoscopic technology, endoscopic resection is increasingly utilized for the treatment of early localized lesions of GIST. Although the likelihood of positive margins resulting from endoscopic resection is higher than that of surgical resection and laparoscopic resection, a study indicates that the recurrence rate of GIST removed via endoscopy may not be closely associated with the tumor margin status and microscopically positive tumor margins do not seem to increase the recurrence rate of GIST removed endoscopically22.This finding suggests that endoscopic resection could serve as an alternative treatment option to surgical and laparoscopic resection.
A multicenter study from Scandinavian sarcoma centers has indicated that non-high-risk GISTs following surgical resection exhibit a considerable long-term recurrence-free survival rate, with a 5-yearRFS of 99.1%. This is particularly notable for very low and low-risk GISTs, suggesting that prolonged imaging follow-up with CT and similar modalities may not be necessary14. In a study by Zhang et al., endoscopic resection of small (≤ 4 cm) GISTs originating from the muscular layer demonstrated no recurrence or metastatic lesions during a median follow-up of 57 months 23. Park JJ et al. conducted a long-term follow-up ( 46.0 ± 28.5 months ) analysis of gastric GIST after ER and found that the recurrence rate was relatively low (2.2%)24.These findings, consistent with the results of our study, suggest that postoperative very low-risk and low-risk GISTs exhibit favorable RFS, potentially obviating the need for routine follow-up.
This study represents a pioneering endeavor to scrutinize and analyze the postoperative surveillance benefits of GISTs from the perspective of endoscopic resection. Nonetheless, the article is not devoid of limitations. Primarily, it is a single-center, retrospective cohort study, thereby harboring inherent selection biases. Secondly, given the temporal span of this research, there exists a potential recall bias concerning the exact outcomes and follow-up intervals when conducting telephonic surveillance of patients. Lastly, despite our recommendation for annual gastrointestinal endoscopy and/or imaging follow-up, there were instances of loss to follow-up. Only 62.22% of patients exceeded a one-year follow-up duration, indicating a relatively short monitoring period. Consequently, the imperative remains to embark on expansive, multicenter, prospective, randomized controlled trials in the future to elucidate the surveillance benefits for very low-risk and low-risk GIST post-endoscopic resection comprehensively.
Conclusion
In conclusion, our study is the first to explore and analyze the long-term follow-up outcomes of very low-risk and low-risk GIST from the perspective of ER surgery. Primary very low-risk and low-risk GIST of the digestive tract have excellent RFS, DSS and OS after endoscopic surgery. It is considered that routine follow-up, including imaging and gastrointestinal endoscopy, may not be required.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Blay, J.-Y., Kang, Y.-K., Nishida, T. & von Mehren, M. Gastrointestinal stromal tumours. Nat. Rev. Dis. Primers 7, 1–22 (2021).
Casali, P. G. et al. Gastrointestinal stromal tumours: ESMO–EURACAN–GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 33, 20–33 (2022).
Serrano, C. et al. 2023 GEIS Guidelines for gastrointestinal stromal tumors. Ther. Adv. Med. Oncol. 15, 1–18 (2023).
Kelly, C. M., Gutierrez Sainz, L. & Chi, P. The management of metastatic GIST: current standard and investigational therapeutics. J. Hematol. Oncol. 14, 2 (2021).
Blay, J. Y. et al. SELNET clinical practice guidelines for soft tissue sarcoma and GIST. Cancer Treat. Rev. 102, 102312 (2022).
Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Human Pathol. 39, 1411–1419 (2008).
Shaib, Y. H., Rugge, M., Graham, D. Y. & Genta, R. M. Management of gastric polyps: an endoscopy-based approach. Clin. Gastroenterol. Hepatol. 11, 1374–1384 (2013).
Deprez, P. H. et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy 54, 412–429 (2022).
Longcroft-Wheaton, G. & Bhandari, P. Endoscopic resection of submucosal tumors. Exp. Rev. Gastroenterol. Hepatol. 9, 659–669 (2015).
Joo, M. K. et al. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointestinal Endoscopy 83, 318–326 (2016).
Kim, G. H. et al. Comparison of the treatment outcomes of endoscopic and surgical resection of GI stromal tumors in the stomach: a propensity score–matched case-control study. Gastrointestinal Endoscopy 91, 527–536 (2020).
Shen, C. et al. Endoscopic versus open resection for small gastric gastrointestinal stromal tumors. Medicine (Baltimore) 94, e376 (2015).
von Mehren, M. et al. NCCN Guidelines® insights: gastrointestinal stromal tumors, version 2.2022: featured updates to the NCCN guidelines. J. Natl. Comprehensive Cancer Netw. 20, 1204–1214 (2022).
Berndsen, M. et al. Long-term outcome after surgical resection of non-high-risk gastrointestinal stromal tumours without adjuvant therapy. British J. Surg. 110, 1857–1862 (2023).
Chen, Q. et al. Efficacy and safety of endoscopic submucosal dissection for large gastric stromal tumors. Clin. Res. Hepatol. Gastroenterol. 44, 90–100 (2020).
Ye, L.-P. et al. Safety of endoscopic resection for upper gastrointestinal subepithelial tumors originating from the muscularis propria layer: an analysis of 733 tumors. Am. J. Gastroenterol. 111, 788–796 (2016).
Dermawan, J. K. et al. Novel genomic risk stratification model for primary gastrointestinal stromal tumors (GIST) in the adjuvant therapy era. Clin. Cancer Res. 29, 3974–3985 (2023).
Gronchi, A. Risk stratification models and mutational analysis: Keys to optimising adjuvant therapy in patients with gastrointestinal stromal tumour. Eur. J. Cancer 49, 884–892 (2013).
Anderson, W. J. & Doyle, L. A. Updates from the 2020 world health organization classification of soft tissue and bone tumours. Histopathology 78, 644–657 (2021).
Klug, L. R., Khosroyani, H. M., Kent, J. D. & Heinrich, M. C. New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat. Rev. Clin. Oncol. 19, 328–341 (2022).
Akahoshi, K., Oya, M., Koga, T. & Shiratsuchi, Y. Current clinical management of gastrointestinal stromal tumor. World J. Gastroenterol. 24, 2806–2817 (2018).
Zhu, Y. et al. Microscopic positive tumor margin does not increase the rate of recurrence in endoscopic resected gastric mesenchymal tumors compared to negative tumor margin. Surg. Endosc. 34, 159–169 (2020).
Zhang, Y. et al. Long-term outcomes of endoscopic resection for small (≤ 4.0 cm) gastric gastrointestinal stromal tumors originating from the muscularis propria layer. World J. Gastroenterol. 24, 3030–3037 (2018).
Park, J.-J. Long-term outcomes after endoscopic treatment of gastric gastrointestinal stromal tumor. Clin. Endoscopy 49, 232–234 (2016).
Acknowledgements
This work was supported by the Key Laboratory Project of Digestive Diseases in Jiangxi Province (2024SSY06101) and Jiangxi Clinical Research Center for Gastroenterology (20223BCG74011).
Funding
The study was supported by grants of Jiangxi Clinical Research Center for Gastroenterology (grant No. 20223BCG74011, PI: Youxiang Chen); This study was supported by grants from the National Natural Science Foundation of China (Grant No. 82060448 and 82360112, PI: Youxiang Chen) and 2023 clinical research program of the first affiliated hospital of Nanchang University (PI: Chunyan Zeng).
Author information
Authors and Affiliations
Contributions
CZ and JG conceptualized the study. JG, ZL, and XL collected and analyzed the data. JG and ZL drafted the manuscript. XL, XS, YZ, and CZ contributed to manuscript revisions and proofreading. YC and CZ managed the project and secured funding. All authors consented to the publication of this manuscript. JG and ZL have contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, J., Liu, Z., Liu, X. et al. Follow-up analysis and research of very low-risk and low-risk gastrointestinal stromal tumors after endoscopic resection. Sci Rep 14, 17872 (2024). https://doi.org/10.1038/s41598-024-68460-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-68460-1