Abstract
Chromodomain helicase DNA-binding protein (CHD) gene family, an ATP (adenosine triphosphate) -dependent chromatin remodeler family, is involved in multiple developmental process and tumor development. However, there have been none pan-cancer analyses of this family. The expression levels, survival profiles, mutation profiles and immune infiltration of the CHD family genes from TCGA and TARGET database were analyzed using online tools or R packages. Interestingly, all types of CHD gene expressions were associated with the prognosis of Neuroblastoma, Acute lymphoblastic leukemia-Phase 3 and Acute Myeloid Leukemia (All P < 0.05). Knock down of CHD7 and CHD9 in K562 (human erythromyeloblastoid leukemia) and HEC-1-B (human endometrial adenocarcinoma) cells significantly inhibit cell proliferation and migration (P < 0.05). Proliferation, colony formation and migration assays were performed in CHD7 and CHD9 knockdown K562 and HBC-1-B cell lines. Mechanisms were also analyzed by PPI and GO ontology for our experiments. Histone modification, especially the methylation of H3K4, might be involved in CHD7 and CHD9 related oncogenesis. Through bioinformatic analysis, we showed CHD genes significantly affected the prognosis of different tumor types, including childhood tumor. Our findings provide new insights into the function and mechanism of CHD gene family, especially in CHD7 and CHD9.
Similar content being viewed by others
Introduction
The CHD (chromosome domain helicase DNA binding protein) gene family is categorized within the adenosine triphosphate (ATP)-dependent chromatin remodeler family, overseeing chromatin remodeling, as indicated by its nomenclature. This family comprises ATP-dependent chromatin remodelers that play indispensable roles in diverse developmental processes. ATP-dependent chromatin remodeling complexes fall into four subfamilies: switching/sucrose nonfermenting (SWI-SNF), imitation switch (ISWI), CHD, and inositol-requiring 80 (INO80). These subfamilies share a conserved ATPase-containing domain, utilizing ATP hydrolysis to modulate histone-DNA interactions, thereby contributing significantly to organ development and cancer pathogenesis1. The CHD gene family exerts its functional impact by binding to chromatin, influencing transcription activation, and modulating the epigenome through chromatin remodeling processes.
While substantial investigations have been conducted on the CHD gene family across diverse cancer types, a comprehensive pan-cancer analysis of the entire CHD gene family is lacking. Despite the considerable volume of recent cancer studies, the mechanisms by which the CHD gene family influences tumorigenesis and progression, particularly for newly discovered members, remained insufficiently elucidated. For instance, CHD1, identified in 1993, plays crucial roles in various tumors, notably prostate and breast cancers2,3,4. In contrast, CHD9, reported since 2005, has received limited attention regarding its functions in cancer5. Moreover, the mechanisms of the CHD family are diverse, encompassing distinct cell fates and signal pathways. For example, CHD1 in ovarian cancer interacts with miR-30a-5p, inhibiting the Wnt/β-catenin signaling pathway6. CHD4 promotes tumor stemness by regulating SOX2 transcription or the TGF-β signaling pathway in breast cancer7,8,9. CHD5 could bind to H3 histones through the PHD domain to inhibit tumors10. CHD7 could regulate the repair of DNA double-strand breaks by binding to 53BP1, and in medulloblastoma, it modulates the ERK signaling pathway with BMI111,12,13. CHD8 is able to regulate the cell cycle by transcriptionally activating E2F transcription factors and inhibits the Wnt/β-catenin signaling pathway in gastric cancer14,15,16. Furthermore, the CHD family has been closely associated with organ development17. And it made the functions of these family members in childhood tumors most important. Therefore, a timely and systematic pan-cancer analysis is imperative to provide comprehensive insights into CHD family members, serving as supportive evidence for future tumor treatments.
Here, in this study, we present a systematic pan-cancer analysis of the CHD gene family, encompassing childhood tumors. This analysis enhances the understanding of the functions of CHD gene family in tumors, providing robust evidence of their associations with cancer. Ultimately, our findings open avenues for potential tumor treatments by targeting the CHD gene family.
Results
Pan-cancer expression profiles of CHD gene family
Initially, an exhaustive analysis of the expression levels of the CHD gene family was conducted across all 33 cancers within TCGA (The Cancer Genome Atlas) database in comparison to control samples. Gene expression data and DEGs (Differentially Expressed Genes) between cancers and normal tissues were shown using the parameter of log2FC > 1, q value cut-off = 0.01 with ANOVA method. Strikingly, among these cancers, the expression of CHD5 exhibited a very low level (Figure S1A). Further scrutiny focused on cancer types with more than five normal controls in TCGA (Figure S1B-J). Utilizing the GEPIA2 tools, which merges normal controls from TCGA and the GTEx database as its input controls, generated preferable gene expression data for the CHD gene family (Fig. 1). In-depth examination revealed distinctive expression patterns for each member. For instance, CHD1 showed significant up-regulation in THYM (Thymoma) but down-regulation in LUSC (Lung Squamous Cell Carcinoma), OV (Ovarian Cancer), TGCT (Testicular Cancer), UCEC (Uterine Corpus Endometrial Cancer) and UCS (Uterine Carcinosarcoma) (Fig. 1a). Similarly, CHD2 displayed parallel trends, being up-regulated in THYM and down-regulated in LUSC, OV, SKCM (Melanoma), UCEC and UCS (Fig. 1b). CHD3 exhibited up-regulation in LAML (Acute Myeloid Leukemia), PAAD (Pancreatic Cancer) and THYM, contrasted by down-regulation in CESC (Cervical Cancer), COAD (Colon Cancer), GBM (Glioblastoma), KICH (Kidney Chromophobe), OV, READ (Rectal Cancer), TGCT, THCA (Thyroid Cancer), UCEC and UCS (Fig. 1c). CHD4, conversely, demonstrated significant up-regulation in DLBC (Large B-cell Lymphoma), GBM, LGG (Lower Grade Glioma), PAAD and THYM (Fig. 1d). Noteworthy was the down-regulation of CHD5 in GBM, LGG and TGCT (Fig. 1e). CHD6 showcased up-regulation in ESCA (Esophageal Cancer), LGG and THYM but down-regulation in UCEC (Fig. 1f). Similarly, CHD7 displayed up-regulation in GBM, LGG, OV and STAD (Stomach Cancer), counterbalanced by down-regulation in ACC (Adrenocortical Cancer) (Fig. 1g). CHD8 exhibited up-regulation in DLBC, LAML, PAAD and THYM (Fig. 1h), while CHD9 displayed up-regulation in DLBC, GBM, LGG, PAAD and THYM, but down-regulation in UCEC and UCS (Fig. 1i). Notably, the majority of CHD gene family members were significantly up-regulated in THYM, and several members displayed significant up-regulation in neural tumors GBM and LGG, with concurrent down-regulation in UCEC and UCS. These findings underscored a closer association of the CHD gene family with these five tumor types relative to other cancer categories.
Expression profiles of CHD gene family from GEPIA2. (a–i) CHD1-CHD9 expression across the 33 cancers from TCGA database, with controls from GTEx database. The expression levels are visually represented, where red indicates significantly higher expression and green denotes significantly lower expression. CHD5 gene expression (e) is presented in log10TPM due to its low expression. PCPG, Pheochromocytoma & Paraganglioma.
Subsequently, the expression data of the CHD gene family were analyzed in seven childhood cancers from the TARGET (Therapeutically Applicable Research to Generate Effective Treatments) database. Consistent with TCGA results, only CHD5 exhibited diminished expression (Figure S2A). In WT (Wilms tumor), CHD1, CHD2, CHD4, CHD6, CHD7 and CHD8 demonstrated significant up-regulation, while CHD3 and CHD5 experienced substantial down-regulation (Figure S2B-I). These findings accentuated the close association of the CHD gene family with Wilms tumor.
Additionally, a heatmap derived from TCGA data illustrated the most significant up-regulation of CHD3 in CHOL (Bile Duct Cancer) and the most pronounced down-regulation of CHD5 in GBM, corroborating results obtained from GEPIA2 (Figure S3A). Lastly, an investigation into the interrelation between the expression levels of each CHD gene family member across all cancer types in TCGA and the TARGET database was conducted. While the overall correlation of CHD gene family expression in TCGA was not robust, the expression correlation within the TARGET database exhibited greater strength, with all members, except CHD5, displaying positive correlations (Figure S3B & S3C).
Survival analysis of CHD family
To assess the potential prognostic significance of the CHD gene family, survival profiles were systematically examined using K-M (Kaplan–Meier) plots and forest plots across 33 tumors from TCGA and six tumors from TARGET (Fig. 2, Figure S4-S6). The analysis revealed distinct associations between CHD gene family expression and survival outcomes across various cancer types.
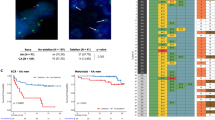
Survival analysis for CHD gene family by K-M plot. (a–s) K-M plot of significant differential results of CHD1-CHD9 gene from TCGA in our analysis which were consistent with results from GEPIA2. Group cut-offs were set at the median, creating high and low expression groups. Cancer types and survival time (years) were indicated at the top left and the bottom of each panel, respectively. Only those results with P < 0.05 were shown and P value was indicated above the abscissa axis.
For CHD1, higher expression correlated with improved survival in CHOL, SKCM and THYM, while lower expression was associated with better survival in LGG and KIRP (Kidney Papillary Cell Carcinoma) (Fig. 2a, b & Figure S4A, B, C). In LGG and NBL (Neuroblastoma), higher CHD2 expression indicated better survival (Fig. 2c & Figure S5A). Lower CHD3 expression was linked to better survival in BLCA (Bladder Cancer), LAML, THCA and AML (Acute myeloid leukemia), whereas higher CHD3 expression was associated with improved survival in LGG, PAAD, UCEC and NBL (Fig. 2d–g & Figure S4D, E & Figure S5B, C). Lower CHD4 expression predicted better survival in KICH, SKCM, UVM (Ocular melanomas), ALL-P3 (Acute lymphoblastic leukemia-Phase 3) and AML, with higher CHD4 expression correlating with improved survival solely in THYM (Figure S4F-H & Figure S5D, E). CHD5 exhibited a dual role, with lower expression indicating better survival in BRCA (Breast Cancer), COAD, UCEC and ALL-P3, while higher expression was associated with better survival in KICH, LGG, PAAD and NBL (Fig. 2h–l & Figure S4J & Figure S5F, G). Higher CHD6 expression correlated with better survival in LAML, LUAD (Lung Adenocarcinoma) and KIRC (Kidney Clear Cell Carcinoma), whereas lower expression was linked to improved survival in LIHC (Liver Cancer) and AML (Fig. 2m,n & Figure S4K, L & Figure S5H). CHD7 expression displayed tissue-specific associations; lower expression was favorable in MESO (Mesothelioma), UVM, SARC (Sarcoma), UCEC and AML, while higher expression correlated with better survival in LGG, one of the common neural tumor (Fig. 2o,p & Figure S4M-O & Figure S5I). For CHD8, lower expression indicated better survival in KICH and UVM, while higher expression correlated with improved survival in KIRC and LGG (Fig. 2q,r & Figure S4P-R). Higher CHD9 expression was associated with better survival in CHOL, KIRC and NBL, but lower expression was linked to improved survival in PRAD (Prostate Cancer) and AML (Fig. 2s & Figure S4S & Figure S5J, K).
Analysis across 33 tumors from TCGA revealed that, overall, higher expression of most CHD gene family members was associated with better survival in CHOL, KIRC, PAAD and THYM, while lower expression correlated with improved survival in UCEC and UVM, except for CHD3. However, divergent associations were noted in KICH, LAML and LGG, highlighting tumor-specific prognostic variations.
Notably, in survival analysis across six cancer types from the TARGET database, a consistent pattern emerged, indicating that higher expression of all CHD gene family members in NBL and lower expression in ALL-P3 and AML were consistently associated with better survival rates (All P < 0.05) (Figure S5). These results of NBL were consistent with the situation of LGG, which suggest the role CHD gene family plays in neurotumors, whether in adults or kids. Meanwhile, the results of AML were quite different from the situation of LAML, indicating possible different roles of CHD gene family in adults and children. These results underscored the diverse prognostic implications of the CHD gene family across different tumor types, emphasizing the need for a nuanced understanding of their roles in cancer prognosis. The summarized expression and survival profiles were presented in Table S3.
Correlation Between Immune Infiltration and CHD gene family
Previously, the association between the CHD gene family and immune infiltration had received limited attention. In this study, we systematically investigated the relationship between CHD gene family expression and various immune cell types, including B cells, T cells, monocytes, macrophages, neutrophils and NK cells, utilizing TIMER2.0 (Fig. 3 & Figure S7-S10). Notably, due to low expression levels in tumors, CHD5 exhibited no significant correlation with immune infiltration (Figure S8A). However, other members of the CHD gene family consistently displayed a positive association with CD4+ T cells and a negative association with CD4 + Th1 cells across multiple tumor types. Additionally, CHD1, CHD4, CHD6, CHD8 and CHD9 exhibited positive correlations with neutrophil cells, particularly notable in the MCPCOUNTER algorithm (Fig. 3a, Figure S7B, Figure S8B, Figure S9B & Figure S10). Apart from these shared trends, each CHD gene member demonstrated distinct correlations with specific immune cell types in individual cancer types. For instance, CHD1 expression was negatively correlated with macrophages in THYM and positively correlated with CD4+ and CD8+ T cells (Fig. 3a). The correlation coefficient was shown in Table S4.
The correlation between the expression of CHD gene family and immune infiltration. (a) The correlations between CHD1 expression and immune infiltration in B cells, T cells, monocytes, macrophages, neutrophils and NK cells with various analyzing methods. (b) The correlations between CHD2 expression and immune infiltration in B cells, T cells, monocytes, macrophages, neutrophils and NK cells, employing various methods. Cancer types and sample number were indicated on the left. Cell types and analyzing methods were indicated above. The results are represented in correlation matrices, highlighting positive (red rectangle) and negative (blue rectangle) partial correlations (P < 0.05).
Furthermore, we focused on exploring the correlation between CHD7 and CHD9 expression and immune infiltration in UCEC, UCS and LAML individually. Utilizing CIBERSORT, we identified specific immune cell types showing significant correlations with CHD7 and CHD9 expression in each cancer type (Figure S11–S14). In UCEC, CHD7 expression significantly correlated with immune infiltration in activated NK cells, CD4+ T memory resting cells, CD8+ T cells, macrophages M0, follicular helper T cells and T regulatory cells (Figure S11A, C-H). In UCS, CHD7 expression correlated with memory B cells, plasma cells and CD4+ T memory resting cells (Figure S11B, I, J, K). In LAML, CHD9 expression significantly correlated with immune infiltration in activated naive B cells, monocytes, neutrophils, CD4 + T memory resting cells, CD4+ naive T cells, CD8+ T cells and gamma delta T cells (Figure S12A-K). Moreover, in UCEC, CHD9 expression significantly correlated with immune infiltration in memory B cells, eosinophils, macrophages M0, neutrophils, activated NK cells, resting NK cells, plasma cells, CD4 + activated memory T cells, CD4+ memory resting T cells, CD8+ T cells and T regulatory cells (Figure S13A-L). In UCS, CHD9 expression was significantly correlated with immune infiltration in macrophages M0, resting mast cells, T follicular helper cells and T regulatory cells (Figure S14A-L).
Additionally, we assessed the expression of the CHD gene family in different immune subtypes (C1-C6) within LAML, UCEC and UCS (Figure S15A-C). The results demonstrated significant differences in the expression of CHD gene family members across immune subtypes in LAML and UCEC, indicating a heterogeneous immune infiltration pattern associated with CHD gene family expression. Conversely, in UCS, no significant differences were observed in the expression of CHD family members across immune subtypes.
In summary, our findings highlighted the nuanced and tumor-specific relationships between the CHD gene family and immune infiltration, shedding light on the potential immunomodulatory roles of CHD gene family members in distinct cancer types.
Stromal and immune cell score, DNA methylation and stemness score of CHD gene family
Immune infiltration is intricately influenced by the tumor microenvironment and stemness. Concordantly, analyses assessing stromal cells and immune cells demonstrated a notable correlation within the CHD gene family, as evidenced by scores obtained for stromal and immune cell infiltration (Figure S16A, B). Across various cancers, particularly in GBM and LGG, the expression of the CHD gene family exhibited a consistent negative relationship with stromal and immune cell scores. This observation suggested that heightened expression of the CHD gene family in GBM and LGG might suppress immune cell functions, aligning with the finding that elevated expression in these tumors correlated with poorer prognosis (Fig. 2B, C, F, K & Figure S4M, R). Examining pan-cancer DNA methylation and RNA expression scores revealed associations between CHD gene family expression and tumor stemness (Figure S16C, D). In OV, excluding low CHD5 expression, all other CHD gene family members exhibited a robust negative correlation with DNA methylation, a pattern not observed in other tumors. Consequently, the impact of CHD gene family expression on DNA methylation and stemness appeared inconspicuous, except in ovarian cancer. Detailed analyses of LAML, UCEC and UCS provided insights into the relationships between CHD gene family expression, tumor microenvironment and stemness scores. In LAML, CHD4, CHD5 and CHD6 displayed significant negative correlations with tumor microenvironment and stemness scores. In UCEC, the expression of CHD7 and DNA methylation exhibited notable increases and significant correlations with all scores, while CHD4, CHD5 and CHD6 were significantly negatively correlated with the tumor microenvironment score. In UCS, only CHD4 exhibited a significant negative correlation with the microenvironment immune score. Collectively, CHD4 emerged as significantly correlated with the tumor microenvironment and stemness scores in these three tumors (Figure S17, 18A, B).
Mutation profiles of CHD gene family
Mutations occurring at specific loci within certain genes often contribute to various developmental disorders or are implicated in tumor progression. In our exploration of the mutation landscape of the CHD gene family across diverse cancer types, we employed the cBioPortal. Notably, the CHD gene family exhibited the highest mutation frequency in UCEC, surpassing 10%, with CHD4 mutations exceeding 20% in UCS (Figure S19B). In contrast, the mutation frequency of the CHD gene family in other cancer types was notably lower than that observed in UCEC. Intriguingly, no mutations in the CHD gene family were detected in UVM (Fig. 4, Figures S19–S22). Examining the distribution of amino acid mutations within the CHD gene family, we observed a generally even distribution across the entire protein, except for CHD6, CHD8 and CHD9, where the most frequent mutations clustered within specific protein domains (Figure S23 & S24). Notably, among the mutations observed in CHD4, CHD6 and CHD7, there was a significantly higher occurrence of gain and amplification mutations compared to other family members, which predominantly exhibited deletions, including deep deletions (Figure S25D & Figure S26A). Further analysis of the relationship between copy number alterations and mRNA expression levels revealed minimal variations across the five types of mutations (deep deletion, shallow deletion, diploid, gain and amplification), with mean values differing by less than a fourfold magnitude (Figure S27 & S28).
Mutation profiles of CHD gene family from cBioPortal. (a) Mutation frequency of CHD1 in pan-cancer studies. (b) Mutation frequency of CHD2 in pan-cancer studies. The mutation types of mutation, structural variant, amplification, deep deletion and multiple alternations were indicated in green, purple, red, blue and brown, respectively. Cancer types and origin of data were indicated below the abscissa axis.
Function of CHD7 and CHD9 in cancer cell lines
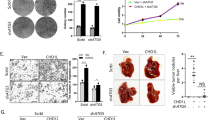
To validate the aforementioned findings, we selected two members of the CHD gene family for further experimental investigation. As shown in Fig. 1i & Figure S2O, CHD7 significantly influenced patient survival in UCEC, while CHD9 was significantly up-regulated in LAML. For in vitro experiments, the HEC-1-B cell line was employed as a model for UCEC/UCS, and the K562 cell line was used to simulate LAML. Initially, short hairpin RNAs (shRNAs) were employed to knockdown CHD7 and CHD9, with the knockdown efficiency validated by RT-qPCR (Fig. 5a). Subsequently, colony formation assays demonstrated a significant decrease in the colony-forming ability of K562 cells upon CHD9 knockdown (Fig. 5b). The impact of CHD7 or CHD9 knockdown on cell proliferation was assessed using the CCK-8 kit, revealing a significant inhibition in the proliferation of both K562 and HEC-1-B cells (Fig. 5c & Table S5). These results were further corroborated through EdU labeling assays after CHD7 or CHD9 knockdown, demonstrating a substantial reduction in the percentage of EdU-positive cells (Fig. 5d). Specifically, the percentage of EdU-positive cells decreased to 55.96% ± 4.56% and 48.33% ± 3.76% of the control upon CHD9 knockdown in K562 and HEC-1-B cells, respectively (shRNA #1: 36.96% ± 1.02%, 29.93% ± 4.89% and shRNA #2: 35.91% ± 7.75%, 35.86% ± 2.22%) (shRNA #1: P = 0.027 and shRNA #2: P = 0.041 for K562 cell line; shRNA #1: P = 0.099 and shRNA #2: P = 0.006 for HEC-1-B cell line). Similarly, the percentage of EdU-positive cells decreased to 53.76% ± 6.72% of the control upon CHD7 knockdown in HEC-1-B cells (30.12% ± 3.65% and 38.64% ± 4.29% for shRNA #1 and shRNA #2, respectively) (shRNA #1: P = 0.015 and shRNA #2: P = 0.140) (Fig. 5e). Lastly, wound healing assays were conducted in HEC-1-B cell lines, starting with a 2 mm gap. Both CHD9 and CHD7 knockdown resulted in a significant reduction in migration, with the gap width after 24 h measured as (1.00 ± 0.05 mm and 1.13 ± 0.06 mm) of the control compared to (1.39 ± 0.04 mm, 1.43 ± 0.04 mm, 1.45 ± 0.06 mm, 1.48 ± 0.05 mm) upon CHD9 or CHD7 knockdown, respectively (shRNA #1: P = 0.0005 and shRNA #2: P = 0.003 for CHD9; shRNA #1: P = 0.041 and shRNA #2: P = 0.003 for CHD7) (Fig. 5f). Knock down of CHD7 and CHD9 inhibited the progression of tumor cells, which suggested the role as tumor promoters for CHD7 and CHD9 in these cell lines.
Phenotypic changes after knock down of CHD7 and CHD9 in K562 and HEC-1-B cell lines. (a) RT-qPCR results for validation of knock down efficiency of CHD7 and CHD9. (b) Colony formation assay for knock down of CHD9 in K562 cell line. (c) CCK-8 proliferation assay for knock down of CHD7 and CHD9. (d) EdU labeling assay for knock down of CHD7 and CHD9. (e) Statistics of positive signal counting in (d). (f) Wound healing assay for knock down of CHD7 and CHD9. All experiments were performed with two effective shRNAs. All P value were calculated comparing with control. Ctrl, shControl; *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
The molecular function of CHD7 and CHD9 in LAML, UCEC and UCS
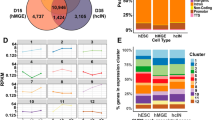
As there is limited research on the molecular mechanisms of CHD7 and CHD9 in these cancers, we employed protein interaction information from the String database to construct a network of top 100 genes closely interacting with CHD7 or CHD9 (Fig. 6a,b). Then those genes and the DEGs (Differentially Expressed Genes) in LAML, UCEC and UCS (Fold change > 2, P < 0.05) were intersected as shown in Venn diagram (Fig. 6c–f, Table S1). GO analysis were conducted to these obtained intersected genes with up-regulated genes and down-regulated genes separately. Due to few intersected DEGs, GO analysis could not be analyzed with up-regulated DEGs of CHD7 and CHD9 in UCEC/UCS as well as down-regulated DEGs of CHD9 in LAML. The rest DEG were analyzed by BP (biological process), CC (cellular component) and MF (molecular function) sub-ontology (Fig. 6g–j, Figure S30). Of notes, the results in UCEC and UCS were almost the same in CHD7 or CHD9. Notably, in UCEC and UCS, CHD7 was associated with histone modifications related to transcriptional regulation, indicating a role in chromatin remodeling and epigenetic regulation. It suggested that CHD7 mainly regulates gene transcription and functions through chromatin remodeling and epigenetic regulation in UCEC and UCS (Fig. 6g,h & Figure S30A). In LAML, the significantly up-regulated DEGs that interacted closely with CHD9 were very similar to the significantly down-regulated DEGs in UCEC and UCS itself. In LAML, CHD9 was linked to fatty acid metabolism, hormone response pathways and similar histone modification functions (Fig. 6i,j & Figure S30B, C). These results were also consistent with the significantly up-regulated expression of CHD9 in LAML and significantly down-regulated expression of CHD9 in UCEC and UCS, respectively. It also indicated that the genes regulated by CHD9 in these three tumors were very similar, and implied a non-tissue specific, conservative mechanism regulated by CHD9 in these cancers (Table S2).
Protein interaction and Gene Ontology analysis of CHD7 and CHD9 in LAML, UCEC and UCS. (a) PPI networks of top 100 proteins interacting with CHD7. (b) PPI networks of top 100 proteins interacting with CHD9. (c) Venn diagram highlighted overlaps between top 100 proteins interacting with CHD7 and up-regulated DEGs in UCEC and UCS. (d) Venn diagram highlighted overlaps between top 100 proteins interacting with CHD7 and down-regulated DEGs in UCEC and UCS. (e) Venn diagram among top 100 proteins interacting with CHD9 and up-regulated DEGs in LAML, UCEC and UCS. (f) Venn diagram among top 100 proteins interacting with CHD9 and down-regulated DEGs in LAML, UCEC and UCS. (g) Biological progress (BP) of GO analysis for down-regulated DEGs interacting with CHD7 in UCEC. (h) Biological progress (BP) of GO analysis for down-regulated DEGs interacting with CHD7 in UCS. (i) Biological progress (BP) of GO analysis for up-regulated DEGs interacting with CHD9 in LAML. (j) Biological progress (BP) of GO analysis for down-regulated DEGs interacting with CHD9 in UCEC.
Clinical parameter analysis of CHD7 and CHD9 in LAML, UCEC and UCS
Cancer development is frequently influenced by clinical characteristics such as age and sex. Considering this, we conducted an analysis of the CHD gene family expression in LAML, UCEC and UCS based on individual clinical parameters (Figure S29). Excluding CHD5 with low expression, the expression of the CHD gene family exhibited no significant differences across age and sex subgroups in LAML (Figure S29A, B). In UCEC, the expression of CHD3, CHD4 and CHD5 displayed significant differences between subgroups stratified by age (below and above 65 years old) and among subgroups with different clinical stages (Figure S29C, D). Conversely, in UCS, the expression of the CHD gene family demonstrated no significant differences across age and clinical stage subgroups (Figure S29E, F). Overall, this suggests that, specifically in UCEC, CHD3, CHD4 and CHD5 may function at varying levels based on the individual patient's state.
Discussion
In this comprehensive investigation, we conducted a pan-cancer analysis focusing on CHD family members, yielding crucial insights into the functional roles of these genes. Overall, we observed that the expression of most family members across various cancers was generally moderate, with the exception of CHD5, which consistently exhibited low expression in almost all cancer types. Notably, in THYM, most CHD gene family members were prominently up-regulated, while in GBM and LGG, several members showed significant up-regulation, and in UCEC and UCS, down-regulation was prevalent (Fig. 1). These findings suggest that the CHD gene family may play pivotal roles in the mentioned five tumor types. Survival analysis results exhibited inconsistency in the impact of CHD families across different tumors, indicating the diverse nature of tissue-specific properties. Intriguingly, analysis of childhood tumors revealed consistent patterns in CHD families, suggesting crucial functions in NBL, ALL-P3 and AML (Figure S5). However, the CHD gene family demonstrated no strong association with immune infiltration. Nevertheless, these families displayed a high mutation frequency across various tumors, particularly in UCEC, highlighting the potential significance of CHD genes in cancer. In conclusion, this pan-cancer analysis sheds light on diverse aspects of the CHD gene family, offering critical insights into their functions and potential as therapeutic targets in specific tumors.
As shown in Fig. 1i, the expression of CHD9 in the mentioned three cancer types was not negligible. This suggests a potentially conservative function for CHD9 across different tumors. Notably, CHD9 exhibited significant up-regulation in LAML and down-regulation in UCEC and UCS. Given the limited existing studies on CHD9 in cancers, we selected K562 and HEC-1-B cell lines to conduct preliminary functional analyses. Concurrently, the prognostic significance of CHD7 in UCEC prompted further investigation. Our experiments revealed that the knockdown of CHD7 and CHD9 significantly inhibited cell proliferation and migration, consistent with improved prognosis in the lower expression group. These findings align with previous studies, such as the study of LINC01410/miR-23c/CHD7 in endometrial cancer indicated the promoting effects of CHD7 on cell growth and aggressiveness in endometrial cancer cells, and the study in gastric cancer tissues and cells showed inhibition of CHD9 was positively correlated with CircPDZD8 suppressed the proliferation and migration12,18.
To explain the molecular functions of CHD7 and CHD9, we performed GO analysis. Due to our strict criteria, we didn’t get enough genes to conduct GO analysis in up-regulating group of genes in UCEC and UCS for CHD7 or CHD9 and down-regulating group of genes in LAML for CHD9. Some of the DEGs which couldn’t be analyzed with GO analysis were still discussed individually below. For example, in a small number of proteins in UCEC and UCS on interaction with CHD9, SMARCA4 is a chromatin remodeling factor as well as a tumor suppressor gene19. GRHL1 has two-sided, being highly expressed in lung cancer and promoting cell cycle progression through the EGFR-ERK pathway, whereas in neuroblastoma it is regulated by MYCN in association with HDAC3 to act as a tumor suppressor20,21. TRIB3, on the other hand is a typical pro-oncogene, which can enhance the stemness of tumors by inhibiting the degradation of FOXO1 while enhancing the expression of SOX2 in breast cancer, STAT1-CXLCL10 in colorectal cancer to down-regulated the immune infiltration of CD8 + T cells, and also being able to promote tumor progression by binding to EGFR in lung cancer22,23,24. MDK is also a pro-oncogene reported in several types of cancers including lung cancer25,26. In contrast, TRIB3, GRHL1, MDK all emerge as genes down-regulated upon interaction with CHD9 in LAML. This was also consistent with the finding that the expression of CHD9 was significantly up-regulated in LAML and down-regulated in UCEC/UCS. In addition to the aforementioned genes, TXNRD1 was associated with iron death27. FDFT1, on the other hand, is able to regulate mTOR/Akt/HIF1α pathway in colorectal cancer28. The up-regulated DEGs interacting with CHD7 in UCEC and UCS were similar to CHD9, but the number of DEGs was more. Among those genes, RAD51 played key roles in double-strand break repair, replication stress and meiosis29. MAL2 is a well-studied gene which promoted cancer progression in breast cancer30,31. As mentioned above, several DEGs interacting with CHD7 and CHD9 exhibited both tumor-suppressing and promoting characteristics, underscoring the complexity of the regulatory mechanisms associated with these genes. Based on the GO analysis results and previous studies, it is indicated that CHD7 and CHD9 may function as chromatin remodeler to regulate transcription, including histone modification, and regulate target genes such as TRIB3 or FDFT1, affecting important pathways or immune infiltration in an indirect way. Besides, CHD9 may regulate fatty acid metabolism, hormone response pathways to promote cancer progression.
Despite these findings, our study has limitations, primarily due to the diverse nature of pan-cancer analysis, making it challenging to identify commonalities among the multitude of genes analyzed. Moreover, the available data limits our ability to conduct detailed functional analyses of these genes. Future studies with a focus on basic research are warranted to enhance our understanding of the functions of CHD genes in different cancers.
In conclusion, as mentioned at the beginning, the function of the CHD gene family itself was pivotal, not only in ontogeny but during early mammalian development as well, combined with analyzing results of childhood tumors presented here, the CHD gene family had the potential to be a therapeutic target such as NBL in children. In addition to this, individual members of the CHD family had the potential to be therapeutic targets for individual tumors, such as CHD7 for GBM. It is possible that this study might provide some help and evidences for the treatment of childhood tumors in the future.
Methods
Data collection
The transcription sequencing matrix and clinical data of 33 cancers were downloaded from UCSC Xena Explorer (https://xenabrowser.net/datapages/, cohort: GDC TARGET (https://ocg.cancer.gov/programs/target) and GDC TCGA). Publications by the TCGA Research Network and the TARGET Program was originally published by the National Cancer Institute. The results published here are in whole or part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga and the Therapeutically Applicable Research to Generate Effective Treatments (https://www.cancer.gov/ccg/research/genome-sequencing/target) initiative, phs000218. The data used for this analysis are available at the Genomic Data Commons (https://portal.gdc.cancer.gov). Gene expression data and DEGs (Differentially Expressed Genes) between cancers and normal tissues were acquired from GEPIA2 (http://gepia2.cancer-pku.cn) based on TCGA and GTex database using the parameter of log2FC > 1, q value cut-off = 0.01 with ANOVA method32. The cancer cohort database used were listed below as Table 1.
Data analysis
TCGA and TARGET datasets from UCSC XENA were analyzed to generate results for mRNA expression pattern. The relationship of gene expression between any two CHDs were shown in heatmaps. K-M (Kaplan–Meier) overall survival condition and cox regression analysis, immune infiltration and stemness score was calculated by R scripts. For cox regression analysis, it was considered that CHDs was the positive factor to promote death when the hazard ratio > 1. P value < 0.05 was considered to be significant. The survival analysis was performed through R package “survival” (version 3.12–13) and “survminer” (version 0.4.9). The immune infiltration was analyzed with CIBERSORT method. For CHD7 and CHD9, mRNA expression based on clinical parameters (age, gender and various pathological stages) were also studied based on clinical data from TCGA, respectively. Stromal and immune cell score, DNA methylation and stemness score were generated from clinical data downloaded from TCGA.
Immune infiltration analysis
Tumor Immune Estimation Resource 2.0 (TIMER2.0; http://timer.cistrome.org/) was used to investigate the interactions between tumor and immune infiltration33. “Immune Gene” module was used to evaluate the relationship between CHD gene levels and infiltrating levels in specific immune cells (including B cells, T cells, monocytes, macrophages, neutrophils and NK cells) in all these 33 cancers from TCGA.
Mutation Profiles of CHD gene family
The cBioPortal for cancer genomics (http://www.cbioportal.org) was applied to generate the mutation profiles of each CHD gene34,35. TCGA datasets and part of CCLE database were collected to generate the mutation sites and their frequency, as well as the relationship between mRNA expression and types of genomic alternation.
Cell culture
K562 cells and HEC-1-B cells were purchased from NCAC (National Collection of Authenticated Cell Cultures, Shanghai, China). K562 cells were cultured in RPMI-1640 medium (Gibco, 11875093) with 10% FBS while HEC-1-B cells were cultured in DMEM (HyClone, SH30243.01) with 10% FBS in 37 °C incubator containing 5% CO2 following the manufacturer’s culture method. Each cell line was passaging after cultured for 2 ~ 3 days.
shRNA transfection and stable knock-down cell lines construction
Briefly, seed sequences of shCtrl, shCHD7 and shCHD9 were cloned into pLKO.1 plasmid (Addgene, #10878). First, the cells were seeded into 6-well plates at a density of 1 × 105 cells per well. When 293 T cells reached 70% confluency, they were transfected with pLKO.1 and packaging plasmids by Lipofectamine 3000 reagent (Invitrogen, L3000008) according to the manufacturer’s protocols. The seed sequences for shRNA were: shCtrl: ACGTGACACGTTCGGAGAA, shCHD7 #1: CCCTACTTACACTGTTGATAT, shCHD7 #2: GCCTATCAGCGCAGCTATAAA, shCHD9 #1: GATCAATCCAGGGACTATAAA, shCHD9 #2: GATCAATCCAGGGACTATAAA. Then the supernatant was collected 48 h after transfection and purified. After infection by purified lentivirus, the stably transfected cells were obtained by treating with 2 μg/ml of puromycin for 3 days and proceeded to later experiments.
Proliferation assay
Cell proliferation was performed by CCK-8 kit (Beyotime, C0038, Shanghai, China) followed the manufacturer’s instruction with four technical replicates every 24 h. The absorption spectrum was measured at 560 nm to calculate the proliferation stage of cells. Besides, proliferation was detected by Edu kit (Beyotine, C0071S, Shanghai, China) following the manufacturer’s instructions. Finally, cells were incubated with 0.1 μg/mL 4′,6-Diamidino-2-Phenylindole Dihydrochloride (DAPI) before observed under microscope.
Wound healing
Briefly, HEC-1-B cells were plated in a 6-well plate until a confluent monolayer was formed. Then mitomycin C was added for a few hours before making the scratch. A sterile 200ul micropipette tip was used to create a straight-edged zone about 1 cm of width without cells across the whole well. After scratching, the debris was totally removed and set in an incubator at 37 °C containing 5% CO2 for 24 h. Results were collected by microscopes and wound area were analyzed with ImageJ software.
Colony formation
Briefly, K562 cells (250 cells/well) were seeded into six-well plates in duplicates, and cultured in media in 37 °C incubator containing 5% CO2 for 10 days. The cells were fixed in staining solution (10% v/v methanol and 0.5% w/v crystal violet in H2O). The number of colonies (> 50 cells) were counted. The experiments were reproduced for three times.
Protein–protein interaction network generation of DEGs with CHD7 and CHD9
PPI network was generated with STRING database and modified by Cytoscape software (version 3.9.1). The confidence score cutoff was set to 0.4 and the top 100 proteins interacted with CHD7 or CHD9 were chosen to intersect with DEGs (fold changes > 2 and P value < 0.01) downloaded from GEPIA2 in LAML, UCEC and UCS, respectively (Table S1). Up-regulated and down-regulated intersecting DEGs in above cancers were calculated respectively (Table S2). The intersecting DEGs were shown in Venn diagram as well and used to perform GO analysis.
Gene Ontology (GO) analysis of intersecting genes
Gene ontology analysis was performed by R package “clusterProfiler” (version 4.2.1) with identified DEGs from Venn diagram. The adjusted P value < 0.01 was thought to be significant. Biological process (BP), cellular component (CC) and molecular function (MF) sub-ontology were all enriched. Up-regulated DEGs and down-regulated DEGs were enriched separately.
Data availability
All the data used in this paper were downloaded from GDC TCGA database and GDC TARGET database (https://xenabrowser.net/datapages) 36.
Abbreviations
- ACC:
-
Adrenocortical carcinoma
- BLCA:
-
Bladder Urothelial Carcinoma
- BRCA:
-
Breast invasive carcinoma
- CESC:
-
Cervical squamous cell carcinoma and endocervical adenocarcinoma
- CHOL:
-
Cholangio carcinoma
- COAD:
-
Colon adenocarcinoma
- DLBC:
-
Lymphoid Neoplasm Diffuse Large B-cell Lymphoma
- ESCA:
-
Esophageal carcinoma
- GBM:
-
Glioblastoma multiforme
- HNSC:
-
Head and Neck squamous cell carcinoma
- KICH:
-
Kidney Chromophobe
- KIRC:
-
Kidney renal clear cell carcinoma
- KIRP:
-
Kidney renal papillary cell carcinoma
- LAML:
-
Acute Myeloid Leukemia
- LGG:
-
Brain Lower Grade Glioma
- LIHC:
-
Liver hepatocellular carcinoma
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- MESO:
-
Mesothelioma
- OV:
-
Ovarian serous cystadenocarcinoma
- PAAD:
-
Pancreatic adenocarcinoma
- PCPG:
-
Pheochromocytoma and Paraganglioma
- PRAD:
-
Prostate adenocarcinoma
- READ:
-
Rectum adenocarcinoma
- SARC:
-
Sarcoma
- SKCM:
-
Skin cutaneous melanoma
- STAD:
-
Stomach adenocarcinoma
- TGCT:
-
Testicular germ cell tumors
- THCA:
-
Thyroid carcinoma
- THYM:
-
Thymoma
- UCEC:
-
Uterine Corpus Endometrial Carcinoma
- UCS:
-
Uterine Carcinosarcoma
- UVM:
-
Uveal melanoma
- ALL-P3:
-
Acute lymphoblastic leukemia-Phase 3
- AML:
-
Acute Myeloid Leukemia
- CCSK:
-
Clear cell sarcoma of the kidney
- NBL:
-
Neuroblastoma
- OS:
-
Osteosarcoma
- RT:
-
Rhabdoid tumor
- WT:
-
Wilms tumor
References
Reyes, A. A., Marcum, R. D. & He, Y. Structure and function of chromatin remodelers. J. Mol. Biol. 433, 166929. https://doi.org/10.1016/j.jmb.2021.166929 (2021).
Delmas, V., Stokes, D. G. & Perry, R. P. A mammalian DNA-binding protein that contains a chromodomain and an SNF2/SWI2-like helicase domain. Proc. Natl. Acad. Sci. USA 90, 2414–2418. https://doi.org/10.1073/pnas.90.6.2414 (1993).
Li, H., Gigi, L. & Zhao, D. CHD1, a multifaceted epigenetic remodeler in prostate cancer. Front Oncol. 13, 1123362. https://doi.org/10.3389/fonc.2023.1123362 (2023).
Zhao, D. et al. Synthetic essentiality of chromatin remodelling factor CHD1 in PTEN-deficient cancer. Nature 542, 484–488. https://doi.org/10.1038/nature21357 (2017).
Shur, I. & Benayahu, D. Characterization and functional analysis of CReMM, a novel chromodomain helicase DNA-binding protein. J. Mol. Biol. 352, 646–655. https://doi.org/10.1016/j.jmb.2005.06.049 (2005).
Wang, X. et al. MiR-30a-5p/CHD1 axis enhances cisplatin sensitivity of ovarian cancer cells via inactivating the Wnt/beta-catenin pathway. Anticancer Drugs 33, 989–998. https://doi.org/10.1097/CAD.0000000000001397 (2022).
Li, Y. et al. CHD4 mutations promote endometrial cancer stemness by activating TGF-beta signaling. Am. J. Cancer Res. 8, 903–914 (2018).
Wang, Y. et al. CHD4 promotes breast cancer progression as a coactivator of hypoxia-inducible factors. Cancer Res. 80, 3880–3891. https://doi.org/10.1158/0008-5472.CAN-20-1049 (2020).
Zhang, J., Lv, X., Wei, B., Gong, X. & Chen, L. CHD4 mediates SOX2 transcription through TRPS1 in luminal breast cancer. Cell Signal 100, 110464. https://doi.org/10.1016/j.cellsig.2022.110464 (2022).
Paul, S. et al. Chd5 requires PHD-mediated histone 3 binding for tumor suppression. Cell Rep. 3, 92–102. https://doi.org/10.1016/j.celrep.2012.12.009 (2013).
Badodi, S. et al. Convergence of BMI1 and CHD7 on ERK signaling in medulloblastoma. Cell Rep. 21, 2772–2784. https://doi.org/10.1016/j.celrep.2017.11.021 (2017).
Lu, M., Ding, N., Zhuang, S. & Li, Y. LINC01410/miR-23c/CHD7 functions as a ceRNA network to affect the prognosis of patients with endometrial cancer and strengthen the malignant properties of endometrial cancer cells. Mol. Cell Biochem. 469, 9–19. https://doi.org/10.1007/s11010-020-03723-9 (2020).
Rother, M. B. et al. CHD7 and 53BP1 regulate distinct pathways for the re-ligation of DNA double-strand breaks. Nat. Commun. 11, 5775. https://doi.org/10.1038/s41467-020-19502-5 (2020).
Durak, O. et al. Chd8 mediates cortical neurogenesis via transcriptional regulation of cell cycle and Wnt signaling. Nat. Neurosci. 19, 1477–1488. https://doi.org/10.1038/nn.4400 (2016).
Nishiyama, M., Skoultchi, A. I. & Nakayama, K. I. Histone H1 recruitment by CHD8 is essential for suppression of the Wnt-beta-catenin signaling pathway. Mol. Cell Biol. 32, 501–512. https://doi.org/10.1128/MCB.06409-11 (2012).
Sawada, G. et al. CHD8 is an independent prognostic indicator that regulates Wnt/beta-catenin signaling and the cell cycle in gastric cancer. Oncol. Rep. 30, 1137–1142. https://doi.org/10.3892/or.2013.2597 (2013).
Feng, W. et al. Chd7 is indispensable for mammalian brain development through activation of a neuronal differentiation programme. Nat. Commun. 8, 14758. https://doi.org/10.1038/ncomms14758 (2017).
Xia, T., Pan, Z. & Zhang, J. CircPDZD8 promotes gastric cancer progression by regulating CHD9 via sponging miR-197-5p. Aging (Albany, NY) 12, 19352–19364. https://doi.org/10.18632/aging.103805 (2020).
Mardinian, K., Adashek, J. J., Botta, G. P., Kato, S. & Kurzrock, R. SMARCA4: implications of an altered chromatin-remodeling gene for cancer development and therapy. Mol. Cancer Ther. 20, 2341–2351. https://doi.org/10.1158/1535-7163.MCT-21-0433 (2021).
Fabian, J. et al. GRHL1 acts as tumor suppressor in neuroblastoma and is negatively regulated by MYCN and HDAC3. Cancer Res. 74, 2604–2616. https://doi.org/10.1158/0008-5472.CAN-13-1904 (2014).
He, Y. et al. EGFR-ERK induced activation of GRHL1 promotes cell cycle progression by up-regulating cell cycle related genes in lung cancer. Cell Death Dis. 12, 430. https://doi.org/10.1038/s41419-021-03721-9 (2021).
Shang, S. et al. TRIB3 reduces CD8(+) T cell infiltration and induces immune evasion by repressing the STAT1-CXCL10 axis in colorectal cancer. Sci. Transl. Med. 14, eabf0992. https://doi.org/10.1126/scitranslmed.abf0992 (2022).
Yu, J. J. et al. TRIB3-EGFR interaction promotes lung cancer progression and defines a therapeutic target. Nat. Commun. 11, 3660. https://doi.org/10.1038/s41467-020-17385-0 (2020).
Yu, J. M. et al. TRIB3 supports breast cancer stemness by suppressing FOXO1 degradation and enhancing SOX2 transcription. Nat. Commun. 10, 5720. https://doi.org/10.1038/s41467-019-13700-6 (2019).
Filippou, P. S., Karagiannis, G. S. & Constantinidou, A. Midkine (MDK) growth factor: a key player in cancer progression and a promising therapeutic target. Oncogene 39, 2040–2054. https://doi.org/10.1038/s41388-019-1124-8 (2020).
Wang, Z. et al. Deciphering cell lineage specification of human lung adenocarcinoma with single-cell RNA sequencing. Nat. Commun. 12, 6500. https://doi.org/10.1038/s41467-021-26770-2 (2021).
Liu, S. et al. TXNRD1: A key regulator involved in the ferroptosis of CML cells induced by cysteine depletion in vitro. Oxid. Med. Cell Longev. 2021, 7674565. https://doi.org/10.1155/2021/7674565 (2021).
Weng, M. L. et al. Fasting inhibits aerobic glycolysis and proliferation in colorectal cancer via the Fdft1-mediated AKT/mTOR/HIF1alpha pathway suppression. Nat. Commun. 11, 1869. https://doi.org/10.1038/s41467-020-15795-8 (2020).
Bonilla, B., Hengel, S. R., Grundy, M. K. & Bernstein, K. A. RAD51 gene family structure and function. Annu. Rev. Genet. 54, 25–46. https://doi.org/10.1146/annurev-genet-021920-092410 (2020).
Fang, Y. et al. MAL2 drives immune evasion in breast cancer by suppressing tumor antigen presentation. J. Clin. Invest. 131, e140837. https://doi.org/10.1172/JCI140837 (2021).
Jeong, J. et al. MAL2 mediates the formation of stable HER2 signaling complexes within lipid raft-rich membrane protrusions in breast cancer cells. Cell Rep. 37, 110160. https://doi.org/10.1016/j.celrep.2021.110160 (2021).
Tang, Z., Kang, B., Li, C., Chen, T. & Zhang, Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47, W556–W560. https://doi.org/10.1093/nar/gkz430 (2019).
Li, T. et al. TIMER20 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 48, W509–W514. https://doi.org/10.1093/nar/gkaa407 (2020).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404. https://doi.org/10.1158/2159-8290.CD-12-0095 (2012).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 6, pl1. https://doi.org/10.1126/scisignal.2004088 (2013).
Goldman, M. J. et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38, 675–678. https://doi.org/10.1038/s41587-020-0546-8 (2020).
Acknowledgements
We thank Dr. Chuantao Fang for technical support of R scripts and Dr. Weijun Feng for advice of manuscripts. This work was supported by the Grant from National Natural Science Foundation of China (No. 82301840 to Jie Cheng).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.L. and J.C.; methodology, Y.L., J.J and Z.H.; software, X.C.; validation, J.J. and X.C; formal analysis, Z.H.; investigation, Y.L.; resources, Z.H.; data curation, Y.L.; writing—original draft preparation, Y.L.; writing—review and editing, All authors; visualization, Z.H; supervision, Z.B, X.C and J.C.; project administration, Z.B, X.C and J.C.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lu, Y., Jiang, J., He, Z. et al. Molecular characteristics and oncogenic role of CHD family genes: a pan-cancer analysis based on bioinformatic and biological analysis. Sci Rep 14, 18923 (2024). https://doi.org/10.1038/s41598-024-68644-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68644-9