Abstract
Chimeric antigen receptor (CAR) T cells are effective against hematological cancers, but are less effective against solid tumors such as non-small cell lung cancer (NSCLC). One of the reasons is that only a few cell surface targets specific for NSCLC cells have been identified. Here, we report that CD98 heavy chain (hc) protein is overexpressed on the surface of NSCLC cells and is a potential target for CAR T cells against NSCLC. Screening of over 10,000 mAb clones raised against NSCLC cell lines showed that mAb H2A011 bound to NSCLC cells but not normal lung epithelial cells. H2A011 recognized CD98hc. Although CAR T cells derived from H2A011 could not be established presumably due to the high level of H2A011 reactivity in activated T cells, those derived from the anti-CD98hc mAb R8H283, which had been shown to lack reactivity with CD98hc glycoforms expressed on normal hematopoietic cells and some normal tissues, were successfully developed. R8H283 specifically reacted with NSCLC cells in six of 15 patients. R8H283-derived CAR T cells exerted significant anti-tumor effects in a xenograft NSCLC model in vivo. These results suggest that R8H283 CAR T cells may become a new therapeutic tool for NSCLC, although careful testing for off-tumor reactivity should be performed in the future.
Similar content being viewed by others
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common causes of cancer deaths worldwide1,2. Although immune checkpoint blockade therapy has largely improved the prognosis of NSCLC3,4, advanced NSCLC remains incurable in most cases. New therapeutic options, including CAR T cell therapy, are therefore urgently needed for patients with NSCLC. CAR T cell therapy has shown tremendous efficacy in the treatment of hematological cancers5,6. Although recent reports have demonstrated that CAR T cells exert an anti-tumor effect against some types of solid tumors7,8,9,10, they are still less effective against solid tumors, including NSCLC, than against hematological cancers. One of the major reasons is the lack of cell surface target antigens that are specific for tumor cells. Several cell surface antigens, such as epidermal growth factor receptor11,12, mesothelin10, prostate stem cell antigen13, mucin 113, human epidermal growth factor receptor 214, carcinoembryonic antigen15,16, programmed death-ligand 117, and receptor tyrosine-kinase-like orphan receptor18,19,20, have been tested as targets for CAR T cells intended to treat NSCLC, although these target molecules are not completely tumor specific. Identifying additional target antigens specific for NSCLC is important to develop effective and safe CAR T-cell therapy.
Expression levels of messenger RNA were previously shown to lack sufficient correlation with the abundances of their corresponding proteins21. In addition, cancer-specific conformational epitopes formed by post-translational events such as glycosylation or conformational changes may have been missed in screening using transcriptome analysis22. Previously, we thoroughly screened for multiple myeloma (MM)-specific monoclonal antibodies (mAbs) among large numbers of mAbs raised against MM cells, and identified two novel mAbs recognizing MM-specific antigens that could not be found by transcriptome analysis23,24. The first was an mAb that specifically recognized the activated integrin β7, which is constitutively overexpressed in MM cells23. The second was R8H283, which had been shown to lack reactivity with CD98hc glycoforms expressed on normal hematopoietic cells and also with some normal tissues24. In this study, we applied the same strategy to identify NSCLC-specific cell surface targets and found that H2A011, which recognizes the CD98hc protein, reacted specifically with a subset of NSCLC samples, but not with normal lung epithelial cells. Although T cells transduced with H2A011-derived CAR could not be expanded in vitro, T cells transduced with another anti-CD98hc mAb that we previously reported24 could be expanded and exerted anti-tumor activity in vitro and in vivo.
Results
H2A011 reacted with NSCLC cells but not with normal lung epithelial cells
We immunized mice with one of five NSCLC cell lines (A549, H1792, H1975, H2228, or HCC827) and generated approximately 10,000 clones of mAbs that bound to the cell line used for immunization. Among them, we selected 573 hybridomas that produced mAbs lacking reactivity to Ep-CAM+ lung epithelial cells obtained from normal regions of resected lung tissues. Then, Ep-CAM+ NSCLC cells from tumor regions of resected specimens were stained with the candidate mAbs and subjected to flow cytometry analysis. We identified 12 candidate mAbs that bound to Ep-CAM+ NSCLC cells in at least one sample. Among them, we focused on H2A011, which reacted most frequently with NSCLC cells (Fig. 1A). Distinct binding of H2A011 to NSCLC cells was observed in four of the five patients with NSCLC, while H2A011 did not react with any of the five samples of normal lung epithelial cells (Fig. 1B,C, Supplementary Fig. S1A and B, Supplementary Table S1). H2A011 also reacted with all NSCLC cell lines tested (Supplementary Fig. S1C).
An anti-CD98hc mAb, H2A011, reacts with NSCLC cells but not with normal lung epithelial cells. (A), Strategy for identification of the NSCLC-specific mAb H2A011. (B) and (C), Flow cytometry analyses of H2A011 reactivity against CD45−Ep-CAM+ normal lung epithelial cells (B) and CD45−Ep-CAM+ tumor cells (C) from resected NSCLC tissues. Analyses of live (propidium iodide-negative) cells are shown. Results of staining with isotype control instead of anti-Ep-CAM mAb were used to draw the gate for Ep-CAM+ cells. The analysis of cells from patient UPN4 is shown as an example. The results of other patients are shown in Supplementary Fig. S1. (D), Strategy for identifying the antigen recognized by H2A011. (E), Flow cytometry plots showing the process of enriching H2A011+ cells in expression cloning of the H2A011 antigen. (F), Flow cytometry analysis of the binding of H2A011 or MEM-108 (a known anti-CD98hc mAb) to wild-type (WT) or CD98hc-deficient (CD98hc KO) A549 cells.
H2A011 recognized CD98hc
The antigen recognized by H2A011 was identified by expression cloning using retroviruses25 (Fig. 1D). Specifically, retroviruses carrying a cDNA library generated from A549 cells (H2A011 positive) were used to infect Ba/F3 cells (H2A011 negative), and then cells labeled with H2A011 were enriched by FACS. After the third round of cell sorting, most cells were H2A011 positive. Sequencing of the inserted cDNA revealed that H2A011 recognized CD98hc (also known as SLC3A2) (Fig. 1E). Consistent with this, H2A011 reactivity was absent in CD98hc knockout (KO) A549 cells that were established using the CRISPR-Cas9 system and confirmed as CD98hc deficient by lack of staining with the known CD98hc-reactive antibody MEM-108 (Fig. 1F).
In transcriptome analyses comparing CD45−CD31− epithelial cell adhesion molecule (Ep-CAM)+ cells26 from tumor regions with those from normal regions of lung tissues resected from three patients with NSCLC (Supplementary Fig. S2A, B, Supplementary Table S1), CD98hc (SLC3A2) mRNA expression was comparable in two of three patients (Supplementary Fig. S2C). In addition, we confirmed the expression of CD98hc in publicly available single-cell RNA sequencing data. We extracted the data from Laughney et al.,27 which included the samples from eight lung NSCLC cells and four normal lungs from the Human Lung Cell Atlas dataset28. The expression of CD98hc was not significantly different between NSCLC cells and normal lung epithelial cells (Supplementary Fig. S3).
Successful development of CAR T cells derived not from H2A011, but from another anti-CD98hc mAb, R8H283, that lacks reactivity with CD98hc glycoforms expressed on normal hematopoietic cells.
Four CAR constructs derived from the variable region of H2A011 were established using either CD28 or 4-1BB as a co-stimulatory molecule (Fig. 2A). T cells were transduced with each CAR construct and cultured in vitro. However, after 10 d of culture, T cells expressing each CAR were scarcely detected (Fig. 2B). High levels of H2A011 reactivity in activated T lymphocytes (Fig. 2C,D) may be a cause of loss of H2A011 CAR T cells.
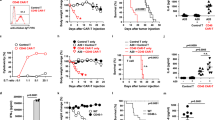
Successful development of CAR T cells derived not from H2A011, but from another anti-CD98hc mAb, R8H283, that lacks reactivity with CD98hc glycoforms expressed on normal hematopoietic cells. (A), Constructs for the CAR derived from H2A011. (B), Flow cytometry analysis of H2A011 CAR transduction efficiencies 7 d after CAR transduction. (C, D), Flow cytometric analysis of R8H283 or H2A011 reactivity against phytohemagglutinin P (PHA)-activated T cells (C) and CD3/CD28-stimulated T cells (D). A549 cells were simultaneously stained as a positive control. (E), Flow cytometry analysis of the binding of H2A011 or R8H283 to wild-type (WT) or GnTI-deficient (GnTI−) 293 cells. F, Construct for the CAR derived from R8H283. (G), Growth of R8H283 CAR T cells during in vitro culture. The data are presented as means ± standard error of the mean (SEM). *: p < 0.05. (H), Representative flow cytometry analysis data of R8H283 CAR transduction efficiencies and CD4/CD8 expression in CAR T cells 7 d after CAR transduction.
Another anti-CD98hc mAb, R8H283, was previously shown to bind myeloma cells but not normal tissues due to differences in CD98hc N-glycosylation24. Consistently, R8H283, but not H2A011, reactivity was significantly increased in GnTI-deficient 293cells, which do not have N-acetylglucosaminyltransferase I (GnTI) activity and therefore lack complex N-glycans (Fig. 2E). A CAR construct derived from the variable region of R8H283 was established using CD28 as a co-stimulatory molecule (Fig. 2F). T cells were transduced with the CAR construct and cultured in vitro. CAR T cells expressing the R8H283-derived CAR could be expanded, although the expansion of R8H283 CAR T cells was reduced compared to that of control T cells (Fig. 2G,H). Reactivity of R8H283 was detected in activated T cells but was much lower than that of H2A011 (Fig. 2C,D). R8H283 CAR T cells spontaneously produced small amounts of cytokines even in the absence of antigen stimulation (Supplementary Fig.S4).
R8H283 reacted with NSCLC cells in a subset of patients
R8H283 reacted with CD45−CD31−Ep-CAM+ NSCLC cells in six of 15 patients (Fig. 3A,B, Supplementary Fig. S5A), but did not react with any of the normal lung epithelial cells from ten patients (Fig. 3C and D and Supplementary Fig. S5B). These results indicate that R8H283 reactivity is specific for NSCLC cells in a subset of patients. Five of the six NSCLC samples that reacted with R8H283 were squamous cell carcinomas (Fig. 3A and B, Supplementary Fig. S5A, Supplementary Table S1). In the samples that we were able to analyze in pairs (tumor vs normal epithelial cells), R8H283 reacted with tumor cells but not with normal lung epithelial cells (UPN 7, 10, 11). The results of MEM108 (pan-CD98hc mAb) staining showed that CD98hc protein was expressed at significantly higher levels on NSCLC cells than on normal epithelial cells, hematopoietic cells, and endothelial cells (Fig. 3E). To further explore the basis for the NSCLC specificity of R8H283, we compared CD98hc in normal lung epithelial cells and NSCLC cells. Whole cell lysates of normal lung epithelial cells or NSCLC cells from a patient were electrophoresed and immunoblotted with polyclonal anti-CD98 antibody (Supplementary Fig. S6). The mobility of CD98hc was different in the NSCLC cells compared to normal lung epithelial cells. We also found that the electrophoretic mobilities of the CD98hc species in NSCLC cells and normal lung epithelial cells were still different after the removal of N-glycans by PNGase F treatment. Thus, it was unclear whether the difference in electrophoretic mobility reflected from the difference in glycosylation or the expressed spliced forms.
R8H283 reacted with NSCLC cells in a subset of patients. (A), Flow cytometry analyses of R8H283 reactivity against CD45− CD31−Ep-CAM+ tumor cells in the tumor region of lung tissues resected from a patient with NSCLC (UPN5). (B), Representative results of flow cytometry analyses of R8H283 reactivity against CD45− CD31−Ep-CAM+ tumor cells in the tumor regions of lung tissues resected from patients with NSCLC. Analyses of the other samples are shown in Supplementary Fig. S5A. (C), Flow cytometry analysis of R8H283 reactivity against CD45−CD31−Ep-CAM+ lung epithelial cells in normal regions of resected lung tissues from a patient with NSCLC (UPN10). (D), Representative results of flow cytometry analyses of R8H283 reactivity against CD45−CD31−Ep-CAM+ lung epithelial cells in unaffected regions of resected lung tissues. Analyses of the other samples are shown in Supplementary Fig. S5B. (E), Flow cytometric analysis of R8H283 or H2A011 reactivity against each cell subset in the normal and tumor regions of the resected lung tissue. The analysis of UPN 7 is shown as a representative of three tested samples.
CAR T cells derived from R8H283 specifically recognized and killed NSCLC cells
CAR T cells derived from R8H283, but not those derived from a CD19 antibody (used as a control because they are specific for an irrelevant target), secreted IFN-γand IL-2, and exhibited cytotoxic activity when co-cultured with A549 lung cancer cells, but not when co-cultured with CD98hc-deficient A549 cells established using CRISPR-Cas9 (Fig. 4A,B). R8H283 CAR T cells produced minimal amounts of cytokines upon co-culture with normal lung epithelial cells purified from normal regions of resected lung tissues of patients with NSCLC (Fig. 4C–E).
CAR T cells derived from R8H283 specifically recognize and kill NSCLC cells. (A), Secretion of IFN-γ and IL-2 by R8H283 CAR T cells or CD19 CAR T cells (a control cell type targeting an irrelevant antigen) after co-culture with WT or CD98hc-deficient (CD98hc KO) A549 NSCLC cells. (B), 51Cr release assay for measurement of specific lysis of WT or CD98hc KO A549 cells by R8H283 CAR T cells or CD19 CAR T cells. E/T, effector/target ratio. C, Experimental design for D and (E. D), Representative flow cytometry analysis of normal lung tissue. CD45-CD31-Ep-CAM+ normal lung epithelial cells were purified and subjected to the assay. (E), Secretion of IFN-γ and IL-2 by R8H283 CAR T cells after co-culture with normal lung epithelial cells or A549 NSCLC cells. (F), Experimental design for G-I. IVIS, in vivo imaging system; i.v., intravenous. (G), Bioluminescence imaging of mice infused with either R8H283 or CD19 CAR T cells. (n = 6 per group). Min, minimum. (H), Quantification of whole-body luminescence. Avg., average; p, photons; s, second; sr, steradian. (I), Survival curves of mice infused with either R8H283 or CD19 CAR T cells. The data are presented as means ± SEM. *P < 0.05 and **P < 0.01 were calculated using two-tailed Student’s t-test (H) and the generalized Wilcoxon test (I).
In a lung cancer xenograft model established by intravenous injection of luciferase-expressing A549 cells into NOG mice29, infusion of R8H283 CAR T cells, but not CD19 CAR T cells, significantly decreased the tumor burden as determined by bioluminescence imaging (Fig. 4F–H) and enhanced mouse survival (Fig. 4I). No unexpected side effects were observed in mice injected with R8H283 CAR T cells. In a mouse that relapsed after R8H283 CAR T cell infusion, R8H283 reactivity to tumor cells was reduced compared to untreated tumor cells (Supplementary Fig. S7).
Discussion
In this study, by thoroughly screening for NSCLC-specific mAbs from among approximately 10,000 mAbs raised against NSCLC cell lines, we found that the CD98hc-specific mAb H2A011 distinctly bound to most NSCLC cells but not to normal lung epithelial cells. Consistently, overexpression of CD98hc protein was previously demonstrated by immunohistochemistry in a subset of NSCLC samples30,31,32. CD98hc mRNA was not overexpressed in purified NSCLC cells compared with normal lung epithelial cells in two of three samples examined. In addition, according to the Cancer Genome Atlas, a gene expression database, CD98hc mRNA is also expressed in normal lung tissues at levels comparable with lung cancer tissues33. Furthermore, the analysis of publicly available single-cell RNAseq data showed that CD98hc mRNA expression did not differ between normal lung epithelial cells and NSCLC cells. These results showed that tumor-specific antigens that cannot be discovered by transcriptome analysis can be identified by thoroughly screening for tumor-specific mAbs among large numbers of mAbs raised against tumor cells, as we previously reported23,24,34, although the targets identified by the mAb discovery strategy are mostly conformational epitopes in proteins that are also expressed in normal tissues and on-target/off-tumor effects must be very carefully excluded.
The CD98 heterodimer is composed of CD98hc that is disulfide-linked with a light chain. The heavy chain binds to the cytoplasmic tails of integrin-β chains35,36,37 and mediates adhesive signals that control cell spreading, survival, and growth37,38,39,40. The CD98 light chains (lcs) function in amino acid transport41,42 and play important roles in the survival and growth of various cells43,44. Overexpression of CD98lc L-type amino acid transporter 1 (LAT1) in NSCLC45,46 may enhance cell surface expression of CD98hc/lc heterodimers. The mechanisms of CD98hc protein overexpression on the surface of NSCLC cells should be clarified in future studies.
H2A011 CAR-transduced T cells failed to survive after 10 days of in vitro culture. While the loss of H2A011 CAR T cells could be caused by fratricide, ligand-dependent suboptimal CAR signaling could cause apoptosis of H2A011 CAR T cells as shown in a previous study47. In contrast, T cells transduced with the CAR derived from another anti-CD98hc mAb R8H283 could be expanded, although the in vitro expansion of R8H283 CAR T cells was not as good as that of control T cells. R8H283 reactivity in activated T cells may cause partial loss of R8H283 CAR T cells during in vitro culture.
R8H283, which has been shown to lack reactivity with CD98hc glycoforms expressed on normal hematopoietic cells and some normal tissues, reacted with NSCLC cells in a subset of patients. In the samples that we were able to analyze in pairs (tumor vs normal epithelial cells), R8H283 reacted with tumor cells but not with normal lung epithelial cells (UPN 7, 10, 11), although paired analysis of more samples should be performed in the future. In a previous report, we showed that R8H283 did not react with normal lymphocytes, monocytes, or non-hematopoietic cells such as intestinal epithelial cells or skin epidermal cells, although CD98hc protein is expressed on these cells24. We demonstrated that CAR T cells derived from R8H283 exerted a significant anti-tumor effect in an in vivo xenograft model. These results suggest that R8H283 CAR T cells have the potential to specifically target NSCLC cells without damaging normal cells in a subset of NSCLC patients, while the possibility of immune escape of tumor cells with low R8H283 reactivity should be carefully evaluated. Most of R8H283-reactive tumors in this study were squamous cell carcinomas, suggesting that R8H283-derived therapies will be useful in patients with squamous cell carcinoma, although a larger number of NSCLC samples should be analyzed in the future.
Although CD98hc protein expression on the surface of tumor cells was detected in most patients with NSCLC, reactivity to R8H283 was observed in only six of the 15 patients examined in this study. R8H283, which is expected to have a lower affinity for CD98hc than MEM108, may only react with NSCLC cells that express high levels of CD98hc. While the reactivity of R8H283 is certainly affected by alterations in the N-glycosylation of CD98hc, it remains unclear whether the NSCLC-specific reactivity of R8H283 is associated with alterations in the glycosylation of CD98hc in NSCLC cells.
A number of mAbs targeting CD98hc have been described in the context of cancer therapy48,49,50,51,52,53,54,55, and some have been tested in clinical trials. Since CD98hc is expressed by several normal tissues, including normal lymphocytes, on-target off-tumor toxicity in normal tissues is always a concern when CD98hc is used as a therapeutic target. Although we showed that R8H283 reactivity was not detected in the normal human tissues that were available for testing24, it is difficult to completely exclude the possibility that under some conditions, the epitope recognized by R8H283 is formed in normal tissues expressing CD98hc. While the low levels of cytokine production by R8H283 CAR T cells co-cultured with normal lung epithelial cells is likely to reflect the spontaneous secretion from R8H283 cells, we could not completely exclude the possibility that R8H283 CAR T cells may be weakly reactive with normal lung epithelial cells. Since R8H283 does not react with mouse CD98hc, it is impossible to analyze the toxicity of R8H283 CAR T cells against normal cells in mouse xenograft models. Therefore, we must carefully examine the off-tumor reactivity of R8H283 before initiating a clinical study. In addition, it may be beneficial to develop a logic-gated CAR56,57,58 that recognizes only cells expressing both the R8H283 antigen and another NSCLC-specific antigen, for example mesothelin.
Methods
Clinical samples
Lung tissue specimens from patients diagnosed with adenocarcinoma, squamous cell carcinoma, or pleomorphic carcinoma and who underwent surgical resection were used after written informed consent was obtained. This study conformed to the ethical guidelines outlined in the Declaration of Helsinki, and was approved by the institutional review boards of the Osaka University School of Medicine, Osaka Toneyama Medical Center, Osaka International Cancer Institute, Takarazuka City Hospital, Toyonaka Municipal Hospital, Suita Municipal Hospital, Minoh City Hospital, Kinki-Chuo Chest Medical Center, and Osaka Fukujuji Hospital.
Cell lines
The A549, H1792, H1975, H2228, and HCC827 cell lines were purchased from the American Type Culture Collection (ATCC). The SP2/0 mouse myeloma cell line was kindly gifted by I. Weissman (Stanford University). The Expi293 and Expi293 GnTI-deficient cell lines were purchased from Thermo Fisher Scientific. A549 cells expressing green fluorescent protein (GFP) and firefly luciferase (A549-GFP-luc) were established by retroviral transduction. Following gene transduction, GFPhigh cells were enriched by fluorescence-activated cell sorting (FACS) on a BD FACS Aria II (Becton Dickinson). CD98-deficient A549 cells were established using CRISPR-Cas9, as previously reported24.
Flow cytometry and cell sorting
To prepare single-cell suspensions from lung tissues, samples were dissociated using the Human Tumor Dissociation Kit (Miltenyi Biotech) and gentleMACS Octo Dissociator with Heaters (Miltenyi Biotech). After tissue dissociation, cell suspensions were filtered through a cell strainer (Corning) and red blood cells were lysed using ACK Lysing Buffer (Gibco). Cells were stained with the indicated mAbs after incubation with Human Serum AB (GeminiBio) and FcR Blocking Reagent Human (Miltenyi Biotech). The following antibodies were used: anti-human CD326 (Ep-CAM)-PE/Cyanine7 (9C4, BioLegend), anti-human CD45-APC (HI130, BioLegend), anti-human CD45-FITC (HI130, BioLegend), anti-human CD31-APC (WM-59, Invitrogen), anti-human CD3-FITC (SK7, BioLegend), anti-human CD19-APC/Cyanine7 (HIB19, BioLegend), and anti-human CD14-APC (M5E2, BioLegend), goat anti-mouse IgG, F(ab’)2 Fragment Specific-Alexa Fluor 647 (115–605-072, Jackson ImmunoResearch), Goat anti-mouse IgG-PE (405,307, BioLegend, Poly4053). H2A011 (mouse IgG1) and R8H283 (mouse IgG2a) were purified from hybridoma supernatants with Protein G Sepharose 4 Fast Flow (GE Healthcare) and used for staining at a concentration of 10 µg/ml and 50 µg/ml, respectively. Flow cytometry analysis and cell sorting were performed using a BD Canto II and Aria II (Becton Dickinson).
Phytohemagglutinin P (PHA)-activated T cells were generated by culturing human PBMC in the presence of 3ug/ml PHA (Sigma) for 72 h, stained with R8H283 or H2A011, then with goat anti-mouse IgG-PE, and analyzed on flow cytometry. Peripheral blood mononuclear cells were activated with anti-CD3 (OKT3, eBioscience) and anti-CD28 (CD28.2, eBioscience) mAbs and cultured in X-VIVO 15 (Lonza) supplemented with 5% human Sserum AB (GeminiBio) for 24 h, stained with biotinylated R8H283 or H2A011, then with streptavidin-PE (BioLegend), and analyzed on flow cytometry. R8H283 or H2A011 was biotinylated using biotin labeling kit (Dojindo).
RNA sequencing
NSCLC cells and unaffected lung epithelial cells were purified by FACS. Total RNA was extracted using TRIzol Reagent (Thermo Fisher Scientific) and the RNeasy Mini Kit (QIAGEN). Full-length cDNA was generated using the SMART-Seq HT Kit (Takara Bio). Each library was prepared using a Nextera XT DNA Library Prep Kit (Illumina). Whole-transcriptome sequencing was performed on RNA samples using an Illumina HiSeq 3000 platform (Illumina) in 100-base single-end mode. Sequenced reads were mapped to human reference genome sequences (hg19) using TopHat v2.0.13 in combination with Bowtie2 ver. 2.2.3 and SAMtools ver. 0.1.19. The number of fragments per kilobase of exon per million mapped fragments was calculated using Cufflinks ver. 2.2.1. RNA sequencing data concerning this study have been deposited in the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE226774). The data sets were analyzed using Ingenuity Pathway Analysis (Ingenuity Systems Inc).
Single-cell RNA sequencing
To confirm the expression of CD98hc (SLC3A2), publicly available single-cell RNA sequencing data was re-analyzed. We extracted the data of Laughney et al., which included the samples from eight lung NSCLC cells and four normal lungs from the human lung cell atlas dataset. We evaluated the gene expression changes in epithelial cells between the NSCLC tissues and normal lung tissues using the Wilcoxon rank-sum test, employing the Seurat FindMarkers function. We then checked the CD98hc data from the output. The P-value was corrected using the Bonferroni method for all genes expressed in the epithelial cells.
Generation of anti-NSCLC mAbs
Six- to eight-week-old BALB/cAJcl mice (CLEA Japan) were immunized by footpad injection with human NSCLC cell lines (A549, H1792, H1975, H2228, or HCC827). Lymphocytes from popliteal lymph nodes were fused with SP2/0 mouse myeloma cells in PEG solution (Roche Applied Science). To identify hybridoma clones producing mAbs that reacted with NSCLC cells, NSCLC cells were first incubated with hybridoma supernatants, then incubated with PE-conjugated anti-mouse IgG antibody and analyzed by flow cytometry. Hybridoma clones producing mAbs that reacted with NSCLC cells were selected and stocked for further analyses.
Expression cloning
Expression cloning was performed as previously reported25. A cDNA library was generated from A549 cells using the Superscript Choice System (Invitrogen) and linked with a BstXI adaptor. cDNA fragments ranging from 2.0 to 5.0 kb were selected on a CHROMA SPIN column (Takara Bio), purified by agarose gel electrophoresis, and then cloned into retrovirus vector pMX (a kind gift from T. Kitamura, Tokyo University). The A549 cDNA library was subjected to screening by transduction into Ba/F3 cells. Ba/F3 cells with which H2A011 reacted were enriched by FACS, then subjected to PCR cloning of the inserted cDNA.
Development of CAR T cells
cDNA of the variable region of H2A011 or R8H283 was obtained by 5’-RACE PCR with a Smarter RACE PCR Kit (Takara Bio), then sequenced. The isolated cDNAs of the κ light and heavy chain variable regions were fused to CD28 (Uniprot P10747 aa.114–220) and CD3ζ(Uniprot P20963 aa.52–164) cDNAs by overlapping PCR. Sequences of the leader peptides, linker, and variable regions of the κ light and the heavy chains of H2A011 are listed in Supplementary Table S2. The sequences of the variable regions of the κ light and the heavy chains of R8H283 are shown in the patent (WO2017026497A1). The resultant H2A011 or R8H283 CAR constructs were inserted into pMSCV retroviral vectors. The CD19 CAR was constructed according to the reported sequences of the anti-CD19 mAb59,60. Then, 293 T cells were co-transfected with retroviral vector, gag-pol, and VSV-G envelope plasmids with Lipofectamine 2000 reagent (Thermo Fisher Scientific). Supernatants containing the retrovirus were collected 48 h and 72 h later. Activated T cells were infected with retrovirus carrying the H2A011 or R8H283 CAR. Briefly, peripheral blood mononuclear cells were activated with anti-CD3 (OKT3, eBioscience) and anti-CD28 (CD28.2, eBioscience) mAbs and cultured in X-VIVO 15 (Lonza) supplemented with 5% Human Serum AB (GeminiBio). The next day, recombinant human IL-2 (Shionogi Pharma) was added to the culture at a final concentration of 100 IU/ml. Cells were harvested 2 d after activation, then subjected to retroviral transduction with RetroNectin (Takara Bio). After transduction, the cells were cultured in the presence of 100 IU/ml IL-2 for 7 d. Dasatinib (1 µM) was added to the culture medium beginning 4 d after CAR transduction to prevent T-cell exhaustion, as previously described61. The transduction efficiency of each CAR was measured by staining cells with goat anti-mouse F(ab′)2-Alexa Fluor 647 mAb.
Cytokine release assays
R8H283 CAR T cells or mock-transduced (control) T cells were tested for reactivity in cytokine release assays. Cytokine concentrations were measured using an ELISA kit (IFN-γ and IL-2; R&D Systems). Effector cells and target cells (1.0 × 105 cells each) were co-cultured for 16 h. Co-culture was performed in technical-triplicate wells. Cytokine secretion was measured in culture supernatants diluted to fall within the linear range of the assay.
Cytotoxicity assay
The cytotoxic ability of CAR T cells was evaluated by 51Cr release assay. Briefly, target cells were labeled for 90 min at 37ºC with 25 μCi of [51Cr] sodium chromate (PerkinElmer). Labeled target cells (1.0 × 104) were incubated with effector cells for 4 h at the indicated effector/target ratios. 51Cr release in harvested supernatants was counted with a gamma counter. Total and spontaneous 51Cr release was determined by incubation of 1.0 × 104 labeled target cells in either 1% Triton X-100 or culture medium. The percentage of specific lysis was calculated as ([specific 51Cr release − spontaneous 51Cr release] / [total 51Cr release − spontaneous 51Cr release]) × 100.
Immunoblotting
Total cell lysate was prepared in lysis buffer [10 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% NP-40, and protease inhibitor cocktail (Nacalai Tesque)]. Cell lysates from normal lung epithelial cells or NSCLC cells were run on a 4–12% NuPAGE gel system (Invitrogen) under reducing (0.7 M 2ME) or non-reducing conditions. To remove N-glycans attached to proteins, cell lysates were incubated at 37 °C for 30 min with 1,000 units of PNGase F PRIME (N-Zyme Scientifics), and then subjected to SDS-PAGE. Western blotting was carried out with anti-CD98 polyclonal Ab (pAb) (#15,193–1-AP, ProteinTech) and subsequently with HRP-conjugated donkey anti–rabbit IgG (#NA934V, GE Healthcare). Imaging of blots was performed using the LAS system (GE Healthcare).
In vivo xenograft mouse models
Female NOD/SCID/IL-2Rγcnull (NOG) mice aged 6–8 weeks (In-Vivo Science) were injected intravenously via the tail vein with 2.0 × 105 A549-luc/GFP tumor cells. Two days after tumor inoculation, the mice were intraperitoneally infused with VIVOGlo Luciferin (Promega, 150 mg/kg body weight), anesthetized with isoflurane, and imaged using an in vivo imaging system (IVIS) (PerkinElmer). The mice were then injected intravenously with CD19 or R8H283 CAR T cells (5.0 × 106 cells/mouse). Mice were reanalyzed with the IVIS every week. To minimize suffering and distress, mice were subjected to inhaled anesthesia (isoflurane) before cell injection. The health status of the mice was carefully examined three times per week by a veterinarian. Mice were euthanized when moribund or as recommended by a veterinarian. Investigators were not blinded.
Animal experiments
All mouse experiments in this study were approved by the administrative panel on Laboratory Animal Care at Osaka University (Ethical Approval ID 03–071 (for mAb production), 28–054 and 03–045 (for xenograft models)). Mice were euthanized by CO2 asphyxia. This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and is reported in accordance with ARRIVE guidelines.
Statistical analyses
Statistical analyses for significant differences between two groups were conducted using unpaired two-tailed Student’s t-test. The generalized Wilcoxon test was used to compare survival differences between the two groups. P < 0.05 was considered to indicate a significant difference. Statistical analyses were performed in GraphPad Prism 9.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Hirsch, F. R. et al. Lung cancer: Current therapies and new targeted treatments. Lancet 389, 299–311. https://doi.org/10.1016/s0140-6736(16)30958-8 (2017).
Chen, Z., Fillmore, C. M., Hammerman, P. S., Kim, C. F. & Wong, K. K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 14, 535–546. https://doi.org/10.1038/nrc3775 (2014).
Herbst, R. S., Morgensztern, D. & Boshoff, C. The biology and management of non-small cell lung cancer. Nature 553, 446–454. https://doi.org/10.1038/nature25183 (2018).
Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F. & Heist, R. S. Lung cancer. Lancet 398, 535–554. https://doi.org/10.1016/s0140-6736(21)00312-3 (2021).
June, C. H. & Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 379, 64–73. https://doi.org/10.1056/NEJMra1706169 (2018).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365. https://doi.org/10.1126/science.aar6711 (2018).
Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941. https://doi.org/10.1038/s41586-022-04489-4 (2022).
Quintarelli, C., Del Bufalo, F. & Locatelli, F. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. Reply. N. Engl. J. Med. 388, 2303–2304. https://doi.org/10.1056/NEJMc2305296 (2023).
Qi, C. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med 28, 1189–1198. https://doi.org/10.1038/s41591-022-01800-8 (2022).
Adusumilli, P. S. et al. A Phase I Trial of Regional Mesothelin-Targeted CAR T-cell Therapy in Patients with Malignant Pleural Disease, in Combination with the Anti-PD-1 Agent Pembrolizumab. Cancer Discov. 11, 2748–2763. https://doi.org/10.1158/2159-8290.Cd-21-0407 (2021).
Feng, K. et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci. China Life Sci. 59, 468–479. https://doi.org/10.1007/s11427-016-5023-8 (2016).
Li, H. et al. Antitumor activity of EGFR-specific CAR T cells against non-small-cell lung cancer cells in vitro and in mice. Cell. Death Dis. 9, 177. https://doi.org/10.1038/s41419-017-0238-6 (2018).
Wei, X. et al. PSCA and MUC1 in non-small-cell lung cancer as targets of chimeric antigen receptor T cells. Oncoimmunology https://doi.org/10.1080/2162402x.2017.1284722 (2017).
Gao, Q. et al. Cancer-cell-secreted CXCL11 promoted CD8(+) T cells infiltration through docetaxel-induced-release of HMGB1 in NSCLC. J. Immunother. Cancer 7, 42. https://doi.org/10.1186/s40425-019-0511-6 (2019).
Chmielewski, M. & Abken, H. CAR T Cells Releasing IL-18 Convert to T-Bet(high) FoxO1(low) Effectors that Exhibit Augmented Activity against Advanced Solid Tumors. Cell Rep. 21, 3205–3219. https://doi.org/10.1016/j.celrep.2017.11.063 (2017).
Thistlethwaite, F. C. et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 66, 1425–1436. https://doi.org/10.1007/s00262-017-2034-7 (2017).
Liu, M. et al. Targeting PD-L1 in non-small cell lung cancer using CAR T cells. Oncogenesis 9, 72. https://doi.org/10.1038/s41389-020-00257-z (2020).
Wallstabe, L. et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight https://doi.org/10.1172/jci.insight.126345 (2019).
Srivastava, S. et al. Immunogenic Chemotherapy Enhances Recruitment of CAR-T Cells to Lung Tumors and Improves Antitumor Efficacy when Combined with Checkpoint Blockade. Cancer Cell. 39, 193-208.e110. https://doi.org/10.1016/j.ccell.2020.11.005 (2021).
Berger, C. et al. Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol. Res. 3, 206–216. https://doi.org/10.1158/2326-6066.Cir-14-0163 (2015).
Vogel, C. & Marcotte, E. M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 13, 227–232. https://doi.org/10.1038/nrg3185 (2012).
Posey, A. D. Jr. et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 44, 1444–1454. https://doi.org/10.1016/j.immuni.2016.05.014 (2016).
Hosen, N. et al. The activated conformation of integrin beta7 is a novel multiple myeloma-specific target for CAR T cell therapy. Nat. Med. 23, 1436–1443. https://doi.org/10.1038/nm.4431 (2017).
Hasegawa, K. et al. Selective targeting of multiple myeloma cells with a monoclonal antibody recognizing the ubiquitous protein CD98 heavy chain. Sci. Transl. Med https://doi.org/10.1126/scitranslmed.aax7706 (2022).
Kitamura, T. et al. Efficient screening of retroviral cDNA expression libraries. Proc. Natl. Acad. Sci. U S A 92, 9146–9150. https://doi.org/10.1073/pnas.92.20.9146 (1995).
Baeuerle, P. A. & Gires, O. EpCAM (CD326) finding its role in cancer. Br. J. Cancer 96, 417–423. https://doi.org/10.1038/sj.bjc.6603494 (2007).
Laughney, A. M. et al. Regenerative lineages and immune-mediated pruning in lung cancer metastasis. Nat. Med. 26, 259–269. https://doi.org/10.1038/s41591-019-0750-6 (2020).
Sikkema, L. et al. An integrated cell atlas of the lung in health and disease. Nat. Med. 29, 1563–1577. https://doi.org/10.1038/s41591-023-02327-2 (2023).
Li, H. et al. Targeting brain lesions of non-small cell lung cancer by enhancing CCL2-mediated CAR-T cell migration. Nat. Commun. 13, 2154. https://doi.org/10.1038/s41467-022-29647-0 (2022).
Kaira, K. et al. CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann. Surg. Oncol. 16, 3473–3481. https://doi.org/10.1245/s10434-009-0685-0 (2009).
Kaira, K. et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in stage I pulmonary adenocarcinoma. Lung. Cancer 66, 120–126. https://doi.org/10.1016/j.lungcan.2008.12.015 (2009).
Kaira, K. et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in early stage squamous cell carcinoma of the lung. Cancer Sci. 100, 248–254. https://doi.org/10.1111/j.1349-7006.2008.01029.x (2009).
Xia, P. & Dubrovska, A. CD98 heavy chain as a prognostic biomarker and target for cancer treatment. Front Oncol. 13, 1251100. https://doi.org/10.3389/fonc.2023.1251100 (2023).
Nakagawa, T. et al. Identification of glioblastoma-specific antigens expressed in patient-derived tumor cells as candidate targets for chimeric antigen receptor T cell therapy. Neurooncol. Adv. https://doi.org/10.1093/noajnl/vdac177 (2023).
Miyamoto, Y. J., Mitchell, J. S. & McIntyre, B. W. Physical association and functional interaction between beta1 integrin and CD98 on human T lymphocytes. Mol. Immunol. 39, 739–751. https://doi.org/10.1016/s0161-5890(02)00255-9 (2003).
Prager, G. W., Féral, C. C., Kim, C., Han, J. & Ginsberg, M. H. CD98hc (SLC3A2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling. J. Biol. Chem. 282, 24477–24484. https://doi.org/10.1074/jbc.M702877200 (2007).
Zent, R. et al. Class- and splice variant-specific association of CD98 with integrin beta cytoplasmic domains. J. Biol. Chem. 275, 5059–5064. https://doi.org/10.1074/jbc.275.7.5059 (2000).
Fenczik, C. A., Sethi, T., Ramos, J. W., Hughes, P. E. & Ginsberg, M. H. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 390, 81–85. https://doi.org/10.1038/36349 (1997).
Feral, C. C. et al. CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. U S A 102, 355–360. https://doi.org/10.1073/pnas.0404852102 (2005).
Rintoul, R. C. et al. Cross-linking CD98 promotes integrin-like signaling and anchorage-independent growth. Mol. Biol. Cell 13, 2841–2852. https://doi.org/10.1091/mbc.01-11-0530 (2002).
Devés, R. & Boyd, C. A. Surface antigen CD98(4F2): Not a single membrane protein, but a family of proteins with multiple functions. J. Membr. Biol. 173, 165–177. https://doi.org/10.1007/s002320001017 (2000).
Verrey, F. System L: Heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 445, 529–533. https://doi.org/10.1007/s00424-002-0973-z (2003).
Reynolds, B., Laynes, R., Ogmundsdóttir, M. H., Boyd, C. A. & Goberdhan, D. C. Amino acid transporters and nutrient-sensing mechanisms: New targets for treating insulin-linked disorders?. Biochem. Soc. Trans. 35, 1215–1217. https://doi.org/10.1042/bst0351215 (2007).
Cho, J. Y. et al. Cynaropicrin, a sesquiterpene lactone, as a new strong regulator of CD29 and CD98 functions. Biochem. Biophys. Res. Commun. 313, 954–961. https://doi.org/10.1016/j.bbrc.2003.12.026 (2004).
Takeuchi, K. et al. LAT1 expression in non-small-cell lung carcinomas: analyses by semiquantitative reverse transcription-PCR (237 cases) and immunohistochemistry (295 cases). Lung. Cancer 68, 58–65. https://doi.org/10.1016/j.lungcan.2009.05.020 (2010).
Nakanishi, K. et al. LAT1 expression in normal lung and in atypical adenomatous hyperplasia and adenocarcinoma of the lung. Virchows. Arch. 448, 142–150. https://doi.org/10.1007/s00428-005-0063-7 (2006).
Zheng, L. et al. A Humanized Lym-1 CAR with Novel DAP10/DAP12 Signaling Domains Demonstrates Reduced Tonic Signaling and Increased Antitumor Activity in B-Cell Lymphoma Models. Clin. Cancer Res. 26, 3694–3706. https://doi.org/10.1158/1078-0432.Ccr-19-3417 (2020).
Tian, X. et al. An anti-CD98 antibody displaying pH-dependent Fc-mediated tumour-specific activity against multiple cancers in CD98-humanized mice. Nat. Biomed. Eng. 7, 8–23. https://doi.org/10.1038/s41551-022-00956-5 (2023).
Montero, J. C. et al. An amino acid transporter subunit as an antibody-drug conjugate target in colorectal cancer. J. Exp. Clin. Cancer Res. 42, 200. https://doi.org/10.1186/s13046-023-02784-0 (2023).
Pellizzari, G. et al. Immunotherapy using IgE or CAR T cells for cancers expressing the tumor antigen SLC3A2. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-002140 (2021).
Bajaj, J. et al. CD98-Mediated Adhesive Signaling Enables the Establishment and Propagation of Acute Myelogenous Leukemia. Cancer Cell. 30, 792–805. https://doi.org/10.1016/j.ccell.2016.10.003 (2016).
Hayes, G. M. et al. Antitumor activity of an anti-CD98 antibody. Int. J. Cancer 137, 710–720. https://doi.org/10.1002/ijc.29415 (2015).
Cantor, J. M. & Ginsberg, M. H. CD98 at the crossroads of adaptive immunity and cancer. J. Cell. Sci. 125, 1373–1382. https://doi.org/10.1242/jcs.096040 (2012).
Papetti, M. & Herman, I. M. Controlling tumor-derived and vascular endothelial cell growth: role of the 4Ff2 cell surface antigen. Am. J. Pathol. 159, 165–178. https://doi.org/10.1016/s0002-9440(10)61683-5 (2001).
Yagita, H., Masuko, T. & Hashimoto, Y. Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation-associated cell surface antigen system in rats and humans. Cancer Res. 46, 1478–1484 (1986).
Roybal, K. T. et al. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell 164, 770–779. https://doi.org/10.1016/j.cell.2016.01.011 (2016).
Tousley, A. M. et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature 615, 507–516. https://doi.org/10.1038/s41586-023-05778-2 (2023).
Wilkie, S. et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J. Clin. Immunol. 32, 1059–1070. https://doi.org/10.1007/s10875-012-9689-9 (2012).
Kowolik, C. M. et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 66, 10995–11004. https://doi.org/10.1158/0008-5472.Can-06-0160 (2006).
Terakura, S. et al. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood 119, 72–82. https://doi.org/10.1182/blood-2011-07-366419 (2012).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science https://doi.org/10.1126/science.aba1786 (2021).
Acknowledgements
The authors thank Y. Kadota (Osaka Habikino Medical Center) and Y. Susaki (Ikeda City Hospital) for clinical samples. They also thank K. Terasaki for technical assistance, and I. Weissman (Stanford University) and T. Kitamura (Tokyo University) for providing materials.
Funding
This work was supported in part by the Japan Agency for Medical Research and Development (AMED) (23ama221318h0001 to N.H), the Japan Society for the Promotion of Science (JSPS) KAKENHI (JP19K16799 and JP21K15487 to K.H., and JP19H04810 and JP20H03710 to N.H.), and by grants from the Yasuda Kinen Medical Foundation (to N.H.), the SENSHIN Medical Research Foundation (to N.H.), KAKETSUKEN (to N.H.), the Uehara Memorial Foundation (to N.H.), the Astellas Foundation for Research on Metabolic Disorders (to N.H.), and the Takeda Science Foundation (to N.H.).
Author information
Authors and Affiliations
Contributions
M.Y., K.H., and N.H. designed the experiments; M.Y., K.H., S.I., W.T., M. Matsubara, T.H., H.U., M.T., Y.O., M.S., H.M., Y.K., H.A. and D.O. performed the experiments; M.Y., W.T., M. Matsubara, H.U., M.T., T.H., T. Kimura, K. Kawagishi, S. Kawanaka, H. Yamato, Y. Takeuchi, E.O., T. Kanzaki, J.O., I.N, S.N., A. Kobayashi, T.I., T. Tokunaga, H. Yokouchi, Y.Y, J.U., M. Mori, K. Komuta, T. Tachi, H. Kuroda, N.K., H. Kishima, S.F., Y.N., T.S., K.M., S. Koyama, H.H., Y. Takeda, and Y.S. collected and analyzed clinical samples; M.Y., K.H., M. Matsubara, Y.S., D.O., and N.H. analyzed the data; M.Y., D.O., A. Kumanogoh, and N.H. wrote the manuscript; and all authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Naoki Hosen and Atsushi Kumanogoh: a patent application has been filed on R8H283. All other authors don't have any competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yaga, M., Hasegawa, K., Ikeda, S. et al. CD98 heavy chain protein is overexpressed in non-small cell lung cancer and is a potential target for CAR T-cell therapy. Sci Rep 14, 17917 (2024). https://doi.org/10.1038/s41598-024-68779-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-68779-9