Abstract
Thyroid-associated ophthalmopathy (TAO) is a hallmark autoimmune condition, and the treatment of TAO requires a multidisciplinary approach. Radiation therapy (RT) is a viable treatment option for active TAO, IMRT is a more precise technology in radiation oncology. This study aims to evaluate the efficacy, feasibility, and safety of orbital intensity-modulated radiation therapy (IMRT) in the treatment of TAO. A single-center retrospective analysis was conducted, including patients diagnosed with moderate to severe active TAO at the Department of Radiation Oncology, Peking University Third Hospital, from October 2020 to October 2023, who had poor responses to corticosteroid treatment. These patients subsequently received IMRT treatment, followed by a period of follow-up and retrospective analysis. The study focused on the outcomes of treatment efficacy, safety, and acute toxic reactions induced by radiation therapy. Improvements in clinical activity score (CAS) at 4 and 12 months were considered as primary and secondary study endpoints, respectively, along with the incidence rate of adverse events. The median follow-up period was 12 months. The median follow-up time after radiation therapy was 12 months. There was no statistically significant difference in CAS between before and 4 months after radiation therapy (CAS: 5.53 ± 2.07 vs.4.68 ± 2.62; R squared: 0.21; 95% CI: − 1.01–0.02; P = 0.054). However, there was a significant reduction in CAS 12 months post-treatment compared to pre-treatment (CAS: 5.53 ± 2.07 vs. 3.06 ± 2.38; R squared: 0.66; 95% CI: 3.42 − 1.52; P < 0.001). The CAS showed a progressively decreasing trend at both 4 months and 12 months post-treatment. In the combined radiotherapy with glucocorticoid treatment group, a statistically significant difference was found between the CAS before treatment and 12 months after radiotherapy (CAS: 6.38 ± 2.00 vs. 3.88 ± 2.85; R squared: 0.66; 95% CI − 4.11 to 0.89; P = 0.008). In the radiotherapy alone group, a statistically significant difference was found between the CAS before treatment and 12 months after radiotherapy (CAS: 4.78 ± 1.92 vs. 2.33 ± 1.73; R squared: 0.66; 95% CI − 3.89 to 1.00; P = 0.005). A few patients experienced Grade I periorbital edema, conjunctival congestion, and dry eye syndrome, but no adverse events such as cataracts, radiation retinopathy, or radiation-induced optic neuropathy were observed by the end of the follow-up period. Orbital IMRT is an effective treatment modality for moderate to severe active TAO, demonstrating significant efficacy even in patients who had not achieved success with previous treatments such as corticosteroids. This retrospective study was approved by the Ethics Committee of Peking University Third Hospital. The permit number was M2024220 and data of registration was April I, 2024.
Similar content being viewed by others
Introduction
Thyroid-associated ophthalmopathy (TAO), also known as Graves’ ophthalmopathy (GO), represents the most common extrathyroidal manifestation of Graves’ disease (GD). Approximately 20–25% of patients with GD develop clinically significant TAO, and 3–5% advance to severe TAO1. TAO is a hallmark autoimmune condition, primarily characterized by enlargement and inflammation of the extraocular muscles (EOM). The interaction between the thyroid-stimulating hormone receptor (TSHR) and the insulin-like growth factor 1 (IGF-1) receptor forms a functional complex that triggers an inflammatory cascade. This cascade interacts with orbital fibroblasts, leading to the secretion and deposition of collagen and glycosaminoglycans behind the eye, and consequently, an increase in intraorbital pressure2,3. The primary symptoms of TAO include proptosis, eyelid redness and swelling, eyelid retraction, impaired eye movements, diplopia, and in severe cases, compressive optic neuropathy, significantly impacting patients’ quality of life.
The treatment of TAO requires a multidisciplinary approach involving endocrinology, ophthalmology, radiology, and radiation oncology. The selection of an appropriate treatment plan necessitates consideration of various factors such as thyroid function, and the severity and activity of the disease. While mild TAO may resolve spontaneously, moderate to severe and active TAO necessitates aggressive treatment. Corticosteroids are the first-line treatment for moderate to severe active TAO, but approximately 20–40% of patients do not respond adequately to corticosteroid therapy4. Immunomodulators and immunosuppressants have also been proven to be effective in patients with moderate to severe active TAO5,6. Radiation therapy (RT) is a viable treatment option for active TAO7,8, offering nonspecific anti-inflammatory effects. It inhibits the activation of radiation-sensitive lymphocytes, reduces fibroblast proliferation and glycosaminoglycan secretion, thereby alleviating orbital pressure and local symptoms9.
With the advancement of radiation therapy techniques, intensity-modulated radiation therapy (IMRT) has emerged as a primary modality. It enables precise energy delivery to kill tumor cells while minimizing damage to surrounding healthy tissue. Large sample randomized controlled trials on RT for moderate to severe active TAO are scarce, and studies exhibit significant heterogeneity. The efficacy and safety of RT in Chinese patients warrant further exploration. This article presents a retrospective study conducted in a real-world setting, sharing the experiences gained in treating moderate to severe active TAO with IMRT at our institution. It aims to assess the treatment outcomes, safety, and feasibility of an 18 Gy radiation therapy regimen (administered in 10 fractions of 1.8 Gy each) in Chinese patients with TAO.
Materials and methods
Study participants and inclusion criteria
This center’s retrospective study enrolled patients with moderate to severe active TAO who were treated with IMRT at Peking University Third Hospital's Department of Radiation Oncology from October 2020 to October 2023. The diagnosis and clinical staging of TAO were based on the Bartley criteria, the Clinical Activity Score (CAS) system, orbital MRI or CT imaging, the European Group on Graves’ Orbitopathy (EUGOGO) assessment criteria, and the NOSPECS classification system of the American Thyroid Association. Inclusion criteria were patients aged 18–75 years with moderate to severe active TAO, who had poor response or were intolerant to corticosteroid therapy before radiation treatment, and who had a follow-up period of 12 months or longer. Exclusion criteria included: patients under 18 or over 75 years of age, those with mild inactive TAO, patients with psychiatric disorders, those who had received radiation therapy previously, and patients who had undergone TAO-related surgical treatment prior to radiation therapy.
Radiation therapy protocol and radiobiological assessment
A computed tomography (CT) scan with a slice thickness of 3 mm was performed for localization, and the image data were transferred to the radiation therapy planning system. During localization, patients were positioned supine and immobilized using a thermoplastic head mask to ensure stability. To further ensure the accuracy of radiation therapy, a pre-treatment plan for orbital CT was conducted. Detailed instructions were provided to patients during the CT scan, simulation, and the entire treatment process to maintain stability.
The CT dataset was imported into the Varian radiation therapy planning system. The clinical target volume (CTV) planned target volume (PTV), and normal structures (such as organs at risk) were precisely delineated following the standards of the International Commission on Radiation Units and Measurements (ICRU)10,11. Based on this, dose calculation was performed using the Eclipse (Varian Associates, Palo Alto, CA) or PLATO (Nucletron, Netherlands) system. Additionally, an IMRT plan targeting the contents of both orbits was developed for each patient. Detailed contours of the eyeball, lens, extraocular muscles, and brain were described for each patient, with special emphasis on protecting sensitive areas such as the anterior chamber of the eye, the lens, as well as the brain, pituitary gland, and lacrimal glands.
The goal was to ensure full coverage of all thickened eye straight muscles and retro-orbital tissues while minimizing the radiation dose to the lens. The treatment dose was controlled to be within 95–107% of the prescribed dose, using wedge compensators to ensure uniform dose distribution across the target area. The QUANTEC trial standards were referenced to better assess dose limitations for normal tissues12. RT treatment was conducted once daily, 2 Gy per session, five times a week for a total of 10 days. Post-treatment, an evaluation of various parameters including the average dose, median dose, and maximum dose was performed. To ensure accuracy during treatment, cone beam computed tomography (CBCT) images based on large-area amorphous silicon digital X-ray detectors were registered weekly.
Efficacy evaluation and follow-up
All patients were jointly evaluated by physicians from the Department of Radiation Oncology, Ophthalmology, and Endocrinology. Evaluations were scheduled before treatment, weekly during treatment, monthly within six months after treatment, bi-monthly from six months to one year, and quarterly thereafter. The 2021 European Guidelines for the Clinical Management of Graves’ Ophthalmopathy state that the Clinical Activity Score (CAS) comprises seven criteria: ① spontaneous retrobulbar pain, ② pain on eye movement, ③ eyelid erythema, ④ eyelid edema, ⑤ conjunctival injection, ⑥ caruncular edema, and ⑦ chemosis, with each criterion scoring 1 point. The 2022 Chinese Guidelines for the Diagnosis and Treatment of Thyroid-Associated Ophthalmopathy13 recommend adding three additional criteria for the follow-up and observation of TAO patients beyond the aforementioned seven: an increase in proptosis ≥ 2 mm, a reduction in eye movement of more than 8°, and a decrease in visual acuity by 1 line or more.Follow-up assessments included CAS (on a scale of 10), diplopia status, eyelid retraction, symptoms of corneal exposure, and the occurrence of side effects within the eye and radiation field (including eyelids, conjunctiva, cornea, lens, retina, skin, hair, etc.).The adverse reactions occurring within 90 days after the start of IMRT were defined as acute (early) reactions, while those occurring three months after treatment were defined as chronic (late) reactions. Acute and late radiation toxicities were assessed using the EORTC/RTOG scoring criteria. Improvements in CAS and symptom scores at 4 months were considered primary study endpoints, while improvements at 12 months and the incidence rate of adverse events were secondary endpoints, with a median follow-up time of 12 months (range: 10–15 months).
Statistical analysis methods
Data analysis was performed using GraphPad Prism version 9.0. Results are expressed as mean ± SD for numerical data and mean + SEM for graphical data. Differences between multiple groups were analyzed using one-way ANOVA or the non-parametric LSD-t test, and comparisons between two groups were conducted using the paired sample t-test. Patient age and follow-up duration are presented as median ± interquartile range. The analysis of the correlation between Δ symptom scores before and after radiation therapy and age was performed using Pearson’s test, while the correlation analysis of other variables was conducted using Spearman’s test. A P-value < 0.05 was considered statistically significant, and P < 0.01 was considered highly significant.
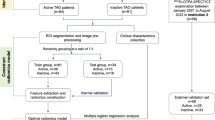
This retrospective study was approved by the Ethics Committee of Peking University Third Hospital and all methods were performed in accordance with the relevant guidelines and regulations. The permit number was M2024220 and data of registration was April I, 2024. Informed Consent was waived by the Ethics Committee of Peking University Third Hospital due to the following justifications: (1) the absence of informed consent will not have any detrimental impact on the health and rights of the participants; (2) adequate measures are implemented to safeguard the privacy and confidentiality of personal information pertaining to the subjects; (3) waiving informed consent does not pose any adverse effects on the rights and interests of the subjects. The flow chart is shown in Fig. 1.
Results
Patient clinical characteristics
This study included 17 patients with moderate to severe active TAO. Table 1 describes the clinical characteristics of the enrolled patients. The median age was 50 years (range: 29–60), with a higher proportion of females (64.7%), bilateral eye involvement (94.1%), and non-smokers (58.8%). A majority of patients had hyperthyroidism (88.2%), while hypothyroidism and euthyroid status were each present in 5.9% of patients. Patients with TAO symptoms persisting for ≤ 6 months before radiation therapy accounted for 58.8%, and 47% of patients received concurrent corticosteroid treatment during the radiation therapy period. The pre-radiotherapy intravenous glucocorticoid regimen is as follows:
-
1.
Cumulative dose of 4.5 g: Methylprednisolone 0.5 g administered via intravenous infusion once a week for 6 weeks, followed by Methylprednisolone 0.25 g administered via intravenous infusion once a week for 6 weeks.
-
2.
Cumulative dose of 4.5 g: Methylprednisolone 0.5 g administered via intravenous infusion once a week for 6 weeks, followed by Methylprednisolone 0.25 g administered via intravenous infusion once a week for 6 weeks.
Patients received a cumulative intravenous dose ranging from 3.25 to 7.5 g before radiotherapy. Among the 17 patients, 4 received oral glucocorticoids, 1 received a biological agent (tocilizumab), and 1 received an immunosuppressive agent (mycophenolate mofetil).
Analysis of treatment efficacy
CAS before and after IMRT
Patients were monitored before, during, and after radiation therapy, with a median follow-up duration of 12 months post-treatment (as detailed in Table 2). Among the various components of CAS, the most common issues pre-treatment and up to four months post-treatment were reduced monocular eye movement. Twelve months post-treatment, the highest incidence was observed in proptosis. Comparing CAS and symptom scores pre-treatment and at 4- and 12-months post-treatment revealed that the difference in CAS between pre-treatment and 4 months post-treatment was not statistically significant (CAS:5.53 ± 2.07 vs.4.68 ± 2.62; R squared: 0.21; 95%CI − 1.01 to 0.02; P = 0.054. However, there was a significant reduction in CAS 12 months post-treatment compared to pre-treatment (CAS:5.53 ± 2.07 vs. 3.06 ± 2.38; R squared: 0.66; 95%CI 3.42–1.52; P < 0.001) (Fig. 2).
Among the 17 patients, the baseline examinations before radiotherapy showed that the incidences of diplopia, elevated intraocular pressure, and widened palpebral fissure were 82.4%, 58.8%, and 88.2%, respectively. Four months after the completion of RT, 35.7% of the patients with diplopia showed improvement, and 53.3% of the patients with elevated intraocular pressure also showed improvement. Among patients with widened palpebral fissures, 30.0% of the patients showed improvement. Twelve months after RT, 64.3% of the patients with diplopia showed improvement, and 80.0% of the patients with elevated intraocular pressure also showed improvement, and 70.0% of the patients with widened palpebral fissures showed improvement.
An analysis was conducted comparing the two subgroups: radiotherapy alone and combined radiotherapy with glucocorticoid treatment. The CAS showed a progressively decreasing trend at both 4 months and 12 months post-treatment (Fig. 3). In the combined treatment group, there was no statistically significant difference in CAS before treatment and 4 months after radiotherapy (CAS: 6.38 ± 2.00 vs. 6.25 ± 1.98; R squared: 0.13; 95% CI − 0.42 to 0.17; P = 0.351). However, a statistically significant difference was found between the CAS before treatment and 12 months after radiotherapy (CAS: 6.38 ± 2.00 vs. 3.88 ± 2.85; R squared: 0.66; 95% CI − 4.11 to 0.89; P = 0.008) (Fig. 4A).
Changes in CAS before and after RT. (A) CAS of patients who received RT in combination with steroids before RT, 4 months and 12 months after RT were statistically analyzed. (B) CAS of patients who received RT before RT, 4 months and 12 months after RT were statistically analyzed. n = 17, *P < 0.05, **P < 0.01, ***P < 0.001.
In the radiotherapy alone group, there was no statistically significant difference in CAS before treatment and 4 months after radiotherapy (CAS: 4.78 ± 1.92 vs. 3.89 ± 2.26; R squared: 0.32; 95% CI − 1.94 to 0.16; P = 0.086). However, a statistically significant difference was found between the CAS before treatment and 12 months after radiotherapy (CAS: 4.78 ± 1.92 vs. 2.33 ± 1.73; R squared: 0.66; 95% CI − 3.89 to 1.00; P = 0.005) (Fig. 4B).
When comparing the combined treatment group with the radiotherapy alone group, there was no statistically significant difference in ΔCAS between the two groups at 4 months post-treatment (ΔCAS: 0.13 ± 0.35 vs. 0.89 ± 1.36; R squared: 0.14; 95% CI − 0.30 to 1.83; P = 0.146). Similarly, no statistically significant difference was found in ΔCAS between the two groups at 12 months post-treatment (ΔCAS: 2.50 ± 1.93 vs. 2.44 ± 1.88; R squared: 0.00; 95% CI − 2.03 to 1.91; P = 0.953) (Fig. 5).
Correlation analysis between patient factors and CAS
To further explore the relationship between various clinical characteristics of patients and their CAS, detailed results are presented in Table 3. Initially, the normality of data such as patient age, duration of illness, changes in CAS (ΔCAS) post-treatment were assessed. The correlation between changes in CAS post-treatment and patient age was analyzed using Pearson’s test, while the correlation between other variables was evaluated using Spearman’s test. The analysis indicated that there was no significant correlation between patient age, gender, duration of illness, smoking history, and concurrent hormone therapy with CAS (P > 0.05).
Safety analysis
Transient Grade I periorbital edema and conjunctival congestion were observed in 17.6% of patients, with symptoms gradually resolving 1–2 months after treatment. Dry eye syndrome was experienced by 17.6% of patients, who were treated symptomatically with artificial tears, and symptoms gradually resolved 2 months after the completion of radiotherapy. Additionally, 11.7% of patients experienced Grade I skin reactions in the irradiated area, which resolved within 3 weeks to 1 month post-radiotherapy. Up to the end of the follow-up period, no adverse events such as hair loss, cataracts, radiation retinopathy, or radiation-induced optic neuropathy were observed.
Discussion
TAO is characterized by involvement of the extraocular muscles and orbital fat, with severe TAO potentially leading to vision-threatening complications, such as corneal disorders and dysthyroid optic neuropathy, resulting in decreased quality of life, permanent disfigurement, and loss of vision. The selection and strategy of treatment should be carefully evaluated based on the activity and severity of the disease, as well as the presence of any comorbidities. Most TAO patients also suffer from hyperthyroidism, though a minority may have other thyroid conditions, such as Hashimoto's thyroiditis, or no thyroid-related diseases at all. While normalization of thyroid function is key to controlling the progression of TAO, corticosteroids and orbital radiotherapy often serve as first-line treatments for moderate to severe TAO, especially in patients with impaired eye muscle function1. However, long-term and high-dose corticosteroid therapy is often associated with a variety of systemic side effects, particularly pronounced in patients with underlying conditions such as diabetes or hypertension. Furthermore, long-term use of corticosteroids may also increase the risk of liver function abnormalities14.
In contrast, radiation therapy offers TAO patients a relatively safe and long-lasting treatment option, especially during the active phase of the disease14. The efficacy of radiation therapy for TAO has been reported with great variability, with overall success rates around 60% and contradictory reports on the effectiveness on symptoms such as soft tissue involvement15,16, proptosis, eye movement, and optic neuropathy. In our study, no statistical difference in CAS was observed between pre-treatment and 4 months post-radiation therapy, but a significant difference was noted at 12 months post-treatment, affirming the role of IMRT in TAO treatment and suggesting that symptom improvement continues beyond 4 months post-treatment. In the subgroup analysis, there was no statistically significant difference in the changes in CAS between the combined treatment group and the radiotherapy-only group at either 4 months or 12 months post-treatment. This indicates that, in this study, no significant difference was observed in the improvement of CAS between radiotherapy combined with glucocorticoid treatment and radiotherapy alone. This may be related to the clinical tendency to adopt the combined treatment regimen for patients with more severe symptoms and higher CAS. Additionally, the small sample size of this retrospective clinical study may also be a contributing factor. Furthermore, the inherent characteristics of TAO, which has a certain degree of self-limitation, might also be a partial reason for the reduction in CAS.
Although the conventional recommended total dose for radiation therapy is 20 Gy, delivered in 10 sessions, lower doses may also be effective for certain patient groups, such as those with mild orbital soft tissue swelling without posterior globe displacement or eye muscle function impairment. This study used a total dose of 18 Gy, divided into 10 sessions, achieving satisfactory efficacy and acceptable side effects17. We also observed a positive correlation between the duration of TAO before IMRT and symptom relief, although without significant statistical difference, suggesting that the timing of radiation therapy, especially the interval from symptom onset to treatment, might influence treatment response, as reported in previous studies18. In this study, the median interval was 9 months.
Up to the observation point, we found that patients experienced Grade I acute reactions during radiotherapy, including eyelid erythema and swelling, conjunctival congestion, dry eyes, and skin redness. These symptoms gradually resolved within 3 weeks to 2 months after the completion of treatment. Long-term follow-up and monitoring are crucial for patients receiving radiation therapy for TAO, to ensure timely identification and management of potential late adverse effects of radiation therapy. Currently, although there is no definitive evidence linking radiation therapy to the development of secondary tumors, long-term safety in patients remains a concern18,19. Additionally, while the risk of radiation-induced retinopathy reported in the literature is relatively low8,20, close monitoring of patients is still recommended.
Conclusion
This study further emphasizes the value of intensity-modulated radiation therapy in the treatment of TAO. Most patients who underwent treatment experienced significant symptom improvement with minimal side effects, making it an effective treatment option for patients who do not respond well to corticosteroid therapy. Follow-up periods post-radiation therapy could be appropriately extended beyond 12 months, and radiation therapy should be considered promptly for patients with moderate to severe active TAO who do not respond well to corticosteroids. Considering the literature, IMRT offers an effective and safe strategy for the treatment of TAO, with the potential to further improve patient quality of life and disease prognosis. Due to the small sample size, retrospective nature, and potential selection bias of this study, as well as the inherent self-limiting characteristics of TAO. Prospective, large-cohort, and controlled controlled trials are needed in the future.
Data availability
Data are available on request due to privacy/ethical restrictions. Data can be obtained by contacting the corresponding author’s email (suqing.tian@bjmu.edu.cn).
References
Bartalena, L., Pinchera, A. & Marcocci, C. Management of Graves’ ophthalmopathy: Reality and perspectives. Endocr. Rev. 21, 168–199. https://doi.org/10.1210/edrv.21.2.0393 (2000).
Krieger, C. C., Neumann, S., Place, R. F., Marcus-Samuels, B. & Gershengorn, M. C. Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves’ disease immunoglobins. J. Clin. Endocrinol. Metab. 100, 1071–1077. https://doi.org/10.1210/jc.2014-3566 (2015).
Morshed, S. A. & Davies, T. F. Graves’ disease mechanisms: The role of stimulating, blocking, and cleavage region TSH receptor antibodies. Hormone Metabol. Res. 47, 727–734. https://doi.org/10.1055/s-0035-1559633 (2015).
Bartalena, L. et al. The 2016 European thyroid association/european group on graves’ orbitopathy guidelines for the management of graves’ orbitopathy. Eur. Thyroid J. 5, 9–26. https://doi.org/10.1159/000443828 (2016).
Rao, R., MacIntosh, P. W., Yoon, M. K. & Lefebvre, D. R. Current trends in the management of thyroid eye disease. Curr. Opin. Ophthalmol. 26, 484–490. https://doi.org/10.1097/icu.0000000000000203 (2015).
Victores, A. J. & Takashima, M. Thyroid eye disease: Optic neuropathy and orbital decompression. Int. Ophthalmol. Clin. 56, 69–79. https://doi.org/10.1097/iio.0000000000000101 (2016).
Grassi, P., Strianese, D., Piscopo, R., Pacelli, R. & Bonavolontà, G. Radiotherapy for the treatment of thyroid eye disease-a prospective comparison: Is orbital radiotherapy a suitable alternative to steroids?. Ir. J. Med. Sci. 186, 647–652. https://doi.org/10.1007/s11845-016-1542-3 (2017).
Mourits, M. P. et al. Radiotherapy for Graves’ orbitopathy: Randomised placebo-controlled study. Lancet (Lond., Engl.) 355, 1505–1509. https://doi.org/10.1016/s0140-6736(00)02165-6 (2000).
Dolman, P. J. & Rath, S. Orbital radiotherapy for thyroid eye disease. Curr. Opin. Ophthalmol. 23, 427–432. https://doi.org/10.1097/ICU.0b013e3283560b2b (2012).
Jones, D. Report 50. Prescribing, recording and reporting photon beam therapy. Med. Phys. 21, 833–834. https://doi.org/10.1118/1.597396 (1994).
Wambersie, A. ICRU Report 62, Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50). ICRU News (1999).
Marks, L. B. et al. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 76, S10-19. https://doi.org/10.1016/j.ijrobp.2009.07.1754 (2010).
Oculoplastic and Orbital Disease Group of Chinese Ophthalmological Society of Chinese Medical Association; Thyroid Group of Chinese Society of Endocrinology of Chinese Medical AssociationLess. Chinese guideline on the diagnosis and treatment of thyroid-associated ophthalmopathy. Chin. J. Ophthalmol. 58, 646–668. https://doi.org/10.3760/cma.j.cn112142-20220421-00201 (2022).
Marcocci, C. & Marinò, M. Treatment of mild, moderate-to-severe and very severe Graves’ orbitopathy. Best Pract. Res. Clin. Endocrinol. Metabol. 26, 325–337. https://doi.org/10.1016/j.beem.2011.11.005 (2012).
Prummel, M. F. et al. Randomized double-blind trial of prednisone versus radiotherapy in Graves’ ophthalmopathy. Lancet (Lond. Engl.) 342, 949–954. https://doi.org/10.1016/0140-6736(93)92001-a (1993).
Olivotto, I. A., Ludgate, C. M., Allen, L. H. & Rootman, J. Supervoltage radiotherapy for Graves’ ophthalmopathy: CCABC technique and results. Int. J. Radiat. Oncol. Biol. Phys. 11, 2085–2090. https://doi.org/10.1016/0360-3016(85)90088-4 (1985).
Johnson, K. T. et al. A retrospective study on the efficacy of total absorbed orbital doses of 12, 16 and 20 Gy combined with systemic steroid treatment in patients with Graves’ orbitopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 248, 103–109. https://doi.org/10.1007/s00417-009-1214-3 (2010).
Matthiesen, C. et al. The efficacy of radiation therapy in the treatment of Graves’ orbitopathy. Int J. Radiat. Oncol. Biol. Phys. 82, 117–123. https://doi.org/10.1016/j.ijrobp.2010.08.053 (2012).
Schaefer, U. et al. A long-term follow-up study after retro-orbital irradiation for Graves’ ophthalmopathy. Int J. Radiat. Oncol. Biol. Phys. 52, 192–197. https://doi.org/10.1016/s0360-3016(01)01754-0 (2002).
Prabhu, R. S. et al. Clinical outcomes of radiotherapy as initial local therapy for Graves’ ophthalmopathy and predictors of the need for post-radiotherapy decompressive surgery. Radiat. Oncol. Lond. Engl. 7, 95. https://doi.org/10.1186/1748-717x-7-95 (2012).
Acknowledgements
This research was supported by special fund of the National Clinical Key Specialty Construction Program, P. R. China (2021).
Author information
Authors and Affiliations
Contributions
Qiman Han wrote the main manuscript text and Xinhui Mao and Suqing Tian prepared figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, Q., Mao, X. & Tian, S. A retrospective study on the effectiveness of intensity modulated radiation therapy for thyroid associated ophthalmopathy at a single institute. Sci Rep 14, 17834 (2024). https://doi.org/10.1038/s41598-024-68809-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-68809-6