Abstract
Although mangrove forests are great carbon sinks, they also release carbon dioxide (CO2) from soil, plants, and water through respiration. Many studies have focused on CO2 effluxes only from soils, but the role of biogenic structures such as pneumatophore roots has been poorly studied. Hence, CO2 effluxes from pneumatophores were quantified at sediment-air (non-flooded sediment) and water–air (flooded sediment) interfaces along a salinity gradient in three mangrove types (fringe, scrub, and basin) dominated by Avicennia germinans during the dry and rainy seasons in Yucatan, Mexico. Pneumatophore abundance explained up to 91% of CO2 effluxes for scrub, 87% for fringe, and 83% for basin mangrove forests at the water–air interface. Overall, CO2 effluxes were inversely correlated with temperature and salinity. The highest CO2 effluxes were in the fringe and the lowest were in the scrub mangrove forests. Flooding decreased CO2 effluxes from the dry to the rainy season in all mangrove forests. These results highlight the contribution of pneumatophores to mangrove respiration, and the need to include them in our current carbon budgets and models, but considering different exchange interfaces, seasons, and mangrove ecotypes.
Similar content being viewed by others
Introduction
Mangrove forests are highly productive ecosystems with great potential as a blue carbon reservoir, particularly in the soil1,2. This is why these forests have been recognized as nature-based allies in climate change mitigation3,4. In soils, roots and microorganisms’ respiration contributes to the main carbon output, as carbon dioxide (CO2) efflux of forest ecosystems5,6.
Plants, through respiration, utilize stored energy for growing and completing their lifecycle. Therefore, assessing plant respiration can deepen our understanding of mangrove tree physiology and their contribution to the ecosystem's carbon budget. In mangrove soils, several factors influencing CO2 effluxes have been documented, such as temperature, salinity, and flooding7,8,9,10. However, many studies have focused on the quantification of soil CO2 fluxes only, and a few have considered biogenic structures such as pneumatophores (root snorkels) and crab burrows, which could potentially increase CO211,12,13,14,15 and methane emissions16,17,18,19,20. Pneumatophores have an internal pathway for gas flow, or aerenchyma21,22,23, through which soil-produced gases can be emitted. Because of the presence of photosynthetic tissues and microalgae, they can carry out photosynthesis24,25, which can lead to an additional source of oxygen for the anoxic soils. Pneumatophores represent a very important component of the carbon budget in mangrove forests, and their contribution to greenhouse gas emissions has already been reported15,16,18,26.
Although mangrove forests are flooded at different times depending on regional seasonality and tidal regimes, CO2 efflux at the water–air interface has been estimated in some cases13,27, indicating that CO2 effluxes are not negligible, especially during receding tides. Studies on these below-canopy CO2 emissions should also consider the different mangrove ecological types, which vary in structure, composition, and carbon storage capacity. Also, because salinity and hydroperiod are the main drivers of mangrove zonation28,29,30,31, high salinities and long inundations decrease organic matter decomposition, which can ultimately be reduced by root respiration. In this study, we proposed quantifying CO2 fluxes from soils and waters, with pneumatophores, along a salinity gradient in three different mangrove ecotypes (fringe, scrub, and basin) dominated by the black mangrove Avicennia germinans (L.) L. in the Yucatan Peninsula (Fig. 1), a region that has 60% of the total mangrove area in Mexico32 and represents about 62% of all mangrove carbon stored in this country31. We hypothesized that CO2 efflux magnitudes would be greater at high pneumatophore abundance because of the increased amount of living tissue that carries out respiration and of the aerenchyma that mobilizes the gases produced in soils. Also, effluxes would be higher at the sediment-air interface than at the water–air interface exchange because, in the former, aerobic microorganism respiration is also contained, and, in the latter, the water column exerts a physical barrier for gas diffusion from the sediment. Similarly, due to the sensitivity of CO2 efflux to temperature and salinity33,34, these CO2 effluxes would be expected to increase at higher temperatures and lower salinities.
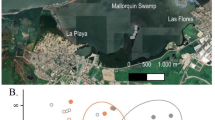
Location of the study sites in the Yucatan Peninsula. PVC tubes with pneumatophores where CO2 efflux measurements were carried out (A–C), Mangrove ecotypes (D–F), and location of the mangrove ecotypes within the Ramsar sites (G, H). SRCMCNY = State Reserve Ciénagas y Manglares de la Costa Norte de Yucatán. Satellite images were obtained from Google Earth Pro 7.3 (https://earth.google.com) and maps were generated using Procreate Raster Graphics Program 5.3.9 (Savage Interactive Pty Ltd., https://procreate.com/procreate).
Results
Air and pneumatophores characterization
Mean air temperature was slightly higher during the dry season for all mangrove forests (H = 97.64; P < 0.001), but the greatest was for the scrub ecotype (dry, 31.98 ± 1.59 °C; rainy, 31.61 ± 2.26 °C; P < 0.001), which also had the highest temperatures of pneumatophores during the dry season (Table 1). Also, pneumatophores’ dimensions (height, volume, and biomass) were slightly higher for the rainy than for the dry seasons for all mangrove types (Table 1).
Physicochemical variables
Flooding level for the basin mangrove was always higher than for the scrub and fringe mangroves (dry season, H = 56.30; P < 0.001; rainy season, H = 48.32; P < 0.001; Fig. 2A). For the fringe mangrove, flooding level was significantly higher during the rainy season than during the dry season (Fig. 2A; P < 0.001).
Flooding level (A), sediment porewater salinity (B), and temperature (C) of sediment (dry season) or surface water (rainy season) in the three mangrove ecotypes for the dry (open bars) and rainy (filled bars) season in Yucatan, Mexico. Data are means ± SE. Letters denote differences among mangrove ecotypes within each season (capital letters for dry and lowercase for rainy); and asterisks denote differences between seasons within mangrove ecotypes (P < 0.001). H-values for the Kruskal Wallis tests are given for dry (Hdry) and rainy (Hrainy) season.
During the dry season, the scrub mangrove had the highest mean porewater salinity (48.61 ± 2.43‰; H = 73.20; P < 0.001), followed by the fringe (33.69 ± 0.65‰) and the basin (17.82 ± 0.91‰) mangroves (Fig. 2B). Surface water salinity was about seven-fold higher in scrub (36.39 ± 20.01‰; H = 62.75; P < 0.001) than in fringe (4.53 ± 0.54‰) and in basin (5.93 ± 1.33‰) mangrove ecotypes. Porewater and surface water salinities had significant differences in basin (t = − 16.31; P < 0.001) and fringe (t = − 33.69; P < 0.001), but slight in scrub (t = − 2.75; P = 0.020) mangrove ecotypes.
In the rainy season, porewater and surface water salinities at the basin mangrove ecotype were significantly lower than for the fringe and the scrub mangrove ecotypes (porewater, H = 34.11; water, H = 62.75; P < 0.001; Fig. 2B; Table 1). However, no differences in salinity porewater were found between seasons for the basin and fringe mangroves; it only decreased significantly for the scrub mangrove in the rainy season (Fig. 2B; P < 0.001).
Mean temperature of the sediment was higher for the scrub mangrove than for the basin and fringe mangrove ecotypes (dry season, H = 69.36; P < 0.001; rainy season, H = 37.11; P < 0.001). This temperature was also significantly higher during the rainy season than during the dry season in the fringe mangrove ecotype (Fig. 2C; P < 0.001).
Pneumatophore CO2 effluxes
Pneumatophore abundance explained up to 91% of the variation in CO2 effluxes for the scrub, 87% for the fringe, and 83% for the basin mangrove ecotypes (Fig. 3; Table S1) during the rainy season at the water–air interface. During the dry season, when measurements were also done for the sediment-air interface, pneumatophore abundance explained up to 83% of the variation in CO2 effluxes for scrub, 77% for basin, and 65% for fringe mangrove forests (Fig. 3; Table S1). The slope of these relationships, which denote the individual contribution of pneumatophores, were also related to the mangrove ecotype, where the steepest slopes were recorded at the fringe mangrove for sediment-air (4.51 × 10−3) and the scrub mangrove for water–air (4.78 × 10−3) interfaces as compared to the lower contribution of pneumatophores in basin mangrove for sediment-air (3.04 × 10−3) and water–air (2.34 × 10−3) interfaces (Fig. 3; Table S1). This effect was greater in the dry season (4.30 × 10−3) than in the rainy season (3.49 × 10−3) as well as in the sediment-air (4.54 × 10−3) than in the water–air (3.20 × 10−3) interface (Table S1). Overall, the highest contribution of pneumatophores to CO2 effluxes was found in the fringe mangrove (6.24 × 10−3; Table S1).
Biophysical associations
The CO2 efflux at the sediment-air was mainly correlated with sediment temperature (ρ = − 0.73), porewater salinity (ρ = − 0.45), and pneumatophore abundance (ρ = 0.49; Fig. 4 top). Instead, CO2 efflux at the water–air interface was related to pneumatophore abundance (ρ = 0.82) and pneumatophore height (ρ = 0.32; Fig. 4 bottom). Because normality’s assumption was not satisfied for our data, Spearman’s correlations were more convenient.
We performed a Canonical Correlation Analysis (CCA; Fig. 5) to explore the contribution of physicochemical and biological variables to the CO2 effluxes in the three mangrove ecotypes evaluated. As categories, we used mangrove ecotype and as objects the variables measured, including the CO2 efflux. Mangrove ecotypes were distinguished by salinity, temperature, and flood height. Surface water salinity (F2 contribution 47.36%), porewater salinity (F2 contribution 13.00%), and sediment temperature (F1 contribution 14.41%) separated scrub mangrove; flooding conditions (F1 contribution 36.90%) segregated basin mangrove; and CO2 effluxes (F2 contribution 11.37%) separated fringe mangrove.
Temperature and salinity effects
The CO2 effluxes were inversely correlated with sediment or water temperature (Fig. 6A) and with porewater or water salinities at the sediment-air and water–air interface, respectively (Fig. 6B). Sediment temperature was positively correlated with porewater salinity (ρ = 0.38; Fig. 4 top) which ultimately differentiated the mangrove ecotypes (Fig. 5). Higher temperatures were found in saline sites exposed to higher solar radiation (scrub mangrove) and lower temperatures in sites with medium to low salinities below the canopy (fringe and basin mangroves; Fig. 2B,C). Likewise, magnitudes of sediment-air CO2 effluxes in scrub mangrove did not differ from those from the water–air interface (Fig. 3C). This mangrove ecotype was also the only one where flooding was less variable during measurements (Fig. 2A). Indeed, temperature and salinity decreased the CO2 effluxes from pneumatophores in the three mangrove types studied.
Figure 6 shows the relationship between CO2 efflux with salinity and temperature for each exchange interface. Both sediment and surface water temperatures decreased CO2 efflux from sediment-air and water–air interfaces (Fig. 6A,B). A similar pattern is observed for porewater and surface water salinity (Figs. 6C,D, S1). Indeed, CO2 effluxes decreased from fringe to scrub mangroves, similar to the groups formed when porewater salinity and both sediment and surface water temperatures were plotted (Fig. S2).
Interface and seasonal effects on CO2 effluxes
Although no differences were found among mean pneumatophore abundance and mangrove ecotypes and seasons, mean CO2 efflux varied with mangrove ecotype, interface, and season (Fig. 7). Mean pneumatophore abundance was 255.65 ± 145.37 pneu m−2 for basin, 254.32 ± 162.79 pneu m−2 for fringe and 218.41 ± 172.40 pneu m−2 for scrub during all measurements (Table 1). Differences of mean CO2 efflux among mangrove ecotypes were given by differences in mean porewater salinity (dry season for sediment-air interface, H = 218.46; P < 0.001; rainy season for water–air interface, H = 251.41; P < 0.001). However, mean CO2 efflux decreased either from dry to rainy season or from sediment-air to water–air due to increasing flooding levels (Figs. 2A, 7A,B). For the sediment-air interface, the highest CO2 efflux was for the fringe mangrove (7.19 ± 0.74 µmol m−2 s−1), and the lowest was for the scrub mangrove (1.28 ± 0.74 µmol m−2 s−1), both during the dry season (Fig. 7A). For the water–air interface, the highest CO2 efflux was for the basin mangrove (2.14 ± 0.24 µmol m−2 s−1) and the lowest CO2 efflux was for the scrub mangrove during both the dry (0.28 ± 0.83 µmol m−2 s−1) and the rainy (0.59 ± 0.80 µmol m−2 s−1) seasons (Fig. 7B). There was a slight increase of CO2 efflux in the scrub mangrove from the dry to the rainy seasons in both sediment-air and water–air interfaces (Fig. 7A,B).
Median CO2 effluxes from three different mangrove ecotypes during the dry (open boxes) and rainy (filled boxes) seasons at the sediment-air (A) and water–air (B) interfaces. Boxes correspond from first to third quartiles; letters denote significant differences among mangrove ecotypes, capital letters for sediment-air and lowercase for water–air interfaces. Lines and asterisks denote differences between seasons for the same mangrove ecotype (A) or between mangrove ecotypes within seasons (B; P < 0.01).
Discussion
Although CO2 efflux from soils and pneumatophores of mangrove forests dominated by trees of the genus Avicennia has been documented11,12,13,14, only one reports both pneumatophore abundance and mangrove ecotype14. These studies reported lower CO2 effluxes than those in the present study. We found mean CO2 effluxes rates of 1.61–7.19 μmol m−2 s−1 (with a mean pneumatophore abundance from 224.31 to 256.01 pneu m−2) at the sediment-air interface, which is comparable to the mean CO2 effluxes reported globally from mangrove soils throughout the world reported by Akhand et al.10 (− 0.37 to 8.73 μmol m−2 s−1). Data from creeks and estuaries surrounding mangrove forests10,27,35 show a large range of data, from − 0.01 to 7.28 μmol m−2 s−1, but our data from water–air interfaces, in the presence of pneumatophores, has a small range (0.51–2.05 μmol m−2 s−1). However, the use of different methods for CO2 measurements, as well as the large variation between mangrove ecotypes and climate conditions, do not allow us to make direct comparisons.
Even though we did not carry out measurements of sediment or water only, from the regression intercepts of Fig. 3 we can obtain basal respirations of 6.04 μmol m−2 s−1 for fringe, 3.45 μmol m−2 s−1 for basin, and 0.49 μmol m−2 s−1 for scrub mangrove ecotypes at the sediment-air interface; and 0.92, 1.43 and − 0.56 μmol m−2 s−1 from fringe, basin, and scrub mangroves, respectively, at the water–air interface. Then, we can consider that the proportion of aerial autotrophic respiration can be comparable to those CO2 effluxes from sediments or water with pneumatophores minus that basal efflux. Autotrophic respiration is supposed to represent 48.60% of the total soil respiration in forests36. In our study, this proportion would be of 15.62–69.69% and 30.15–209.13% for sediment-air and water–air, respectively, and taking into consideration the CO2 release driven by pneumatophores only. Yet, although Avicennia is one of the most widely distributed mangrove genera and can have more than 10,000 pneumatophores per tree37,38, pneumatophore CO2 effluxes are not being reported as a distinct form of carbon loss in mangroves39.
As predicted, CO2 effluxes were greater at high pneumatophore abundance, which is related to a high plant biomass. When soils are flooded, pneumatophore respiration is supposed to be the main source of CO2 effluxes, because gas diffusion in the water column is limited. Additionally, the individual contribution of the pneumatophores is represented by the slope value of the relationships between CO2 efflux and pneumatophore abundance, allowing comparisons between mangrove ecotypes or tree ages. Pneumatophore CO2 effluxes were greater at the sediment-air than at the water–air interface exchange for the fringe and basin mangrove ecotypes. For the scrub mangrove, such regressions showed data points overlapping from both interfaces (Fig. 3C), but mean CO2 effluxes were higher at the sediment-air than at the water–air interface for both seasons (Fig. 7A,B; Fig. S3). Hydrodynamics in mangrove forests depends on the duration, level, and frequency of flooding, and it is known that CO2 increases during ebb and spring tides and decreases during flow and neap tides27. In our study, we only tested the presence (level) or absence of the water column and found that passing from sediment-air to water–air conditions, the CO2 efflux rates decreased 76.77%, 49.75% and 68.32% for fringe, basin, and scrub mangrove ecotypes. These results can help adjust our current CO2 models in mangrove forests, considering their hydrology.
Under sunlight, flooding can also reduce CO2 fixation by photosynthetic layers of pneumatophores in Avicennia marina24. In preliminary studies we found that 35.36 ± 28.22% of the respired CO2 is offset by pneumatophore photosynthesis (unpublished data), like the 49.58 ± 13.35% observed in A. officinalis and Sonneratia alba40. Thus, pneumatophore photosynthesis can represent a source of oxygen and carbon when leaf stomata are closed at high vapor pressure deficit especially for the scrub mangrove ecotype, allowing survival during the dry season.
Few studies have reported CO2 effluxes as a function of salinity variability within the same mangrove ecotype10,13. It is well known that salinity decreases photosynthesis and respiration in plants33,34,41,42,43, accordingly, the site with the highest salinity (scrub ecotype) had the lowest CO2 effluxes despite its high sediment and surface water temperatures. This was unexpected, as typically high temperatures increase respiration34. In this study, the scrub ecotype showed lower CO2 efflux during both dry and rainy seasons (Fig. 7), suggesting that high salinity also reduces soil respiration44. In fact, in our study, the CO2 efflux was the result of the effect of salinity, temperature, and flooding that differed between mangrove ecotypes (Figs. 2, 6; Fig. S2). Although the basin mangrove had the lowest porewater salinities, it showed low CO2 effluxes because it had the highest flooding level; however, during the dry season in non-flooded places, CO2 effluxes were high (Table S1, Fig. 7). Also, during the dry season, flooding level was inversely correlated with sediment temperature and pneumatophore temperature, which reduced CO2 efflux. In studies conducted in soils of different forests, a positive relationship between CO2 effluxes and soil temperature has been found7,8, because high temperatures favor microbial activity in the soil33. However, in our study, an interaction between high temperatures and high salinities led to a lower CO2 efflux in the scrub mangrove forest for both seasons and both exchange interfaces.
Mangrove soils that are rich in carbon, organic matter, and nutrient availability (e.g., N, P and Fe) favor CO2 efflux9,45. In some mangrove flooded soils, the enzyme that regulates phosphorus (P) cycling (alkaline phosphatase) is strongly related to CO2 effluxes, but no relationship between the enzyme that regulates nitrogen (N) cycling (β-N-acetylglucosaminidase) and CO2 efflux was found46. Lower CO2 effluxes in the scrub mangrove forest could then indicate that this site is P-limited, because previous reports assign the stature of this mangrove forests to P deficiency in soils47,48,49, although this is still controversial50. In our study, the CO2 effluxes in flooded soils of scrub mangroves were like those in non-flooded soils and, in some cases, these were very low or even negative values (Figs. 2C, 7). Further research on salinity and P availability would also be needed in arid regions, where scrub mangroves are dominated by Avicennia germinans51.
During the rainy season, we found the tallest pneumatophores in all mangrove forests because of a higher level and frequency of flooding events29 and confirmed a positive correlation between pneumatophore height and flooding level (ρ = 0.48; Fig. 3). Pneumatophore height has also been positively correlated to flooding level in several studies52,53,54, and both pneumatophore height and density have been reported as indicators of soil and hydrodynamics conditions in mangroves37,54. Pneumatophores of Avicennia spp. are supposed to be up to 30 cm in height38, but, in our study, pneumatophores were up to 48 cm in height in the scrub mangrove ecotype. This pneumatophore's extreme length can be related to the increase in water level due to the heavy rain caused by four tropical storms in 2020 in the Yucatan Peninsula55.
The average CO2 effluxes from A. germinans pneumatophores in our study sites, at the water–air and sediment-air interfaces, were comparable to those reported in several studies11,44,56,57. The highest CO2 effluxes in this study were even higher than those from other forest ecosystems around the world 58,59,60,61,62, reflecting the high productivity of the mangrove ecosystems. Our findings question the current understanding of the carbon budget in mangrove forests and highlight the importance of considering integrating physiology and anatomy within carbon-based studies. Indeed, we propose that further studies on CO2 (or other greenhouse gases) effluxes should also consider other mangrove tissues, such as stilts, stems, and even leaves, to evaluate their contribution to ecosystem respiration under different global change scenarios.
Materials and methods
Study sites and field measurements
Three mangrove ecotypes, dominated by Avicennia germinans (L.) L. were chosen in the northwestern coast of Yucatan: fringe and basin mangroves in the Ria Celestun Biosphere Reserve (20° 51′ 27.4″ N, − 90° 22′ 33.9″ W; 20° 51′ 03.6″ N, − 90° 17′ 42.5″ W, respectively; Ramsar Site 1333; Fig. 1) and scrub mangrove in the State Reserve Cienagas y Manglares de la Costa Norte de Yucatán (21° 13′ 17.7″ N, − 89° 49′ 49.4″ W; Ramsar Site 2468; Fig. 1). Field measurements were made in May 2021 and in September 2021 for the dry and rainy seasons, respectively. Sediment-air and/or water–air interfaces were considered depending on the inundation state of each mangrove ecotype on the day of measurements. When possible, both interfaces were measured. Sediment-air interface was measured for all mangrove ecotypes during the dry season and only for scrub mangrove forests during the rainy season. The water–air interface was evaluated for all mangrove ecotypes during the rainy season, and for basin and scrub mangroves during the dry season.
Physicochemical measurements
Air temperature and relative humidity were recorded with a 12-bit Temp/RH Smart Sensor (S-THB-M002, Onset Computer Corporation, Bourne, MA) every 10 s, and 10-min averages were stored with a data acquisition system (HOBO U30-NRC Weather Station, Onset) at each mangrove ecotype during the fieldwork in both seasons. Porewater samples were taken at 30 cm soil depth adjacent to each CO2 efflux tube, then porewater salinity was measured. When sediment was flooded, surface water salinity, surface water temperature and flooding level were taken. Both porewater and surface water parameters were obtained with a portable conductivity meter (YSI, Model Pro2030, Yellow Springs, OH). Porewater salinity was measured in all cases for both sediment-air and water–air CO2 efflux measurements. Sediment temperature (Type “T” Omega Soil Temperature Probe, Omega Engineering Inc., Stamford, CT) and volumetric water content (Theta Probe ML2x, The Macaulay Land Use Research Institute and Delta T Devices, Cambridge, UK) were recorded at 0.1 m depth of the sediment next to each PVC tube at the same time as CO2 efflux was being measured for non-flooded conditions, while surface water temperature was measured for flooded conditions. During the dry season, we also measured pneumatophore temperature (Table 1).
Pneumatophores characterization
At each mangrove ecotype and season, 32–37 plots (0.5 m2 each; divided into 4 subplots) were randomly chosen for pneumatophores counting to estimate the mean pneumatophore abundance per unit area (pneu m−2; Table 1). Within each plot a polyvinyl chloride (PVC) tube (0.2 m diameter, and 0.30 ± 0.08 m height) was inserted 0.03 m into the sediment. PVC tubes were placed at each site before CO2 efflux measurements were taken. To represent different pneumatophore abundances, PVC tubes were placed enclosing from 3 to 72 pneumatophores each, which cover pneumatophore abundances ranging from 95 to 2292 pneu m−2 (Fig. 3). Additionally, the distance from the PVC tube to the nearest tree was also taken. After CO2 efflux measurements, five pneumatophores per PVC tube were characterized in the field measuring total height, basal diameter, top diameter, and distance between the top diameter and apex of the pneumatophores. Three pneumatophores per PVC tube were taken to the laboratory to obtain pneumatophore-dried biomass (g). Then, pneumatophore basal area (cm2), lateral area (cm2), total volume (cm3), and density (g cm-3) were calculated.
CO2 efflux measurements
CO2 efflux rates from pneumatophores or sediment-air and water–air were quantified using a dynamic-closed chamber system (0.2 m in diameter: 8200–103 Smart Chamber, LI-COR Biosciences; Lincoln, NE) connected to an infrared gas analyzer (LI-8100A, LI-COR). For each sampling point, chambers were placed on PVC tubes previously installed (one week before), and then CO2 efflux was recorded for 7 min. At each season, CO2 efflux measurements were made from 8:30 to 12:30 h. The CO2 efflux data were first analyzed using the SoilFlux Pro-4.2.1 software (LI-COR Biosciences) to recognize possible leaks or disturbances inside the chamber during measurements. Then, effluxes were re-computerized using asymptotic fits, previously employed for soils, plants, and waters to reduce underestimations63,64,65. Only fits with an R2 ≥ 0.95 were chosen (n = 171).
Data analysis and representation
Normality (Shapiro–Wilk) and homoscedasticity (Levene) were tested for all data before parametric analysis. Then, simple regressions were run to elucidate the relationship between pneumatophores abundance and CO2 effluxes by mangrove ecotype, season, interface, and its interactions (Fig. 3; see Supplementary Table S1). Kruskal–Wallis (or one-way ANOVA on ranks) analyses were performed to find differences among mangrove ecotypes within each interface or season, then reported as H-values (Table 1; Figs. 2, 7). Also, Mann–Whitney-Wilcoxon were performed to test two groups’ differences for ecotype or season, then reported as W-values (Fig. 7). For pairwise multiple comparisons either Dunn’s or Tukey’s procedures with or without Bonferroni or Holm adjustments were used. Analysis for Figs. 3, 4 and Table S1 were made from raw field data (n = 171). To elucidate the role of biophysical variables, a multi-comparison matrix using Spearman’s correlation was performed. To account for the contribution of physicochemical and biological variables to the CO2 efflux and the differentiation of mangrove ecotypes, a Canonical Correspondence Analysis (CCA) was used. Models from Table S1 and Fig. 3 were applied to the pneumatophore abundance dataset (from characterization), and then it was unified with the CO2 efflux dataset containing only CO2 effluxes from the mean pneumatophore abundance observed at each mangrove ecotype, to exclude outliers. This new dataset from observed and predicted data was used for Fig. 7 (totaling n = 850). All statistical analyses were performed using R language version 2023.03.1.44666. Results are usually presented in terms of the mean ± standard error, unless specified.
Data availability
Data will be made available on request to any of the correspondent authors.
References
Bouillon, S. et al. Mangrove production and carbon sinks: A revision of global budget estimates. Glob. Biogeochem. Cycles 22, GB2013. https://doi.org/10.1029/2007GB003052 (2008).
Donato, D. C. et al. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 4, 293. https://doi.org/10.1038/ngeo1123 (2011).
Friess, D. A., Adame, M. F., Adams, J. B. & Lovelock, C. E. Mangrove forests under climate change in a 2 °C world. WIREs Clim. Change 13(4), e792. https://doi.org/10.1002/wcc.792 (2022).
Leal, M. & Spalding, M. D. (eds.). The State of the World’s Mangroves 2022. Global Mangrove Alliance, 92 p (2022).
de Gerenyu, L. et al. Daily and seasonal dynamics of CO2 fluxes from soils under different stands of monsoon tropical forest. Eurasian Soil Sci. 44(9), 984–990. https://doi.org/10.1134/S1064229311090067 (2011).
Leon, E. et al. Hot spots, hot moments, and spatio-temporal controls on soil CO2 efflux in a water-limited ecosystem. Soil Biol. Biochem. 77, 12–21. https://doi.org/10.1016/j.soilbio.2014.05.029 (2014).
Lovelock, C. E. Soil respiration and belowground carbon allocation in mangrove forests. Ecosystems 11, 342–354. https://doi.org/10.1007/s10021-008-9125-4 (2008).
Poungparn, S. et al. Carbon dioxide emission through soil respiration in a secondary mangrove forest of eastern Thailand. J. Trop. Ecol. 25(4), 393–400 (2009).
Chen, G., Tam, N. & Ye, Y. Spatial and seasonal variations of atmospheric N2O and CO2 fluxes from a subtropical mangrove swamp and their relationships with soil characteristics. Soil Biol. Biochem. 48, 175–181. https://doi.org/10.1016/j.soilbio.2012.01.029 (2012).
Akhand, A., Chanda, A., Das, S., Hazra, S. & Kuwae, T. CO2 fluxes in mangrove ecosystems. In Blue carbon in shallow coastal ecosystems, carbon dynamics, policy, and implementation (eds Kuwae, T. & y Hori, M.) 185–221 (Springer, Singapore, 2019). https://doi.org/10.1007/978-981-13-1295-3_7.
Kristensen, E. et al. Emission of CO2 and CH4 to the atmosphere by sediments and open waters in two Tanzanian mangrove forests. Mar. Ecol. Prog. Ser. 370, 53–67. https://doi.org/10.3354/meps07642 (2008).
Penha-Lopes, G. et al. The role of biogenic structures on the biogeochemical functioning of mangrove constructed wetlands sediments—A mesocosm approach. Mar. Pollut. Bull. 60(4), 560–572. https://doi.org/10.1016/j.marpolbul.2009.11.008 (2010).
Troxler, T. G. et al. Component-specific dynamics of riverine mangrove CO2 efflux in the Florida coastal Everglades. Agric. For. Meteorol. 213, 273–282. https://doi.org/10.1016/j.agrformet.2014.12.012 (2015).
Kristensen, E. et al. Pneumatophores and crab burrows increase CO2 and CH4 emission from sediments in two Brazilian fringe mangrove forests. Mar. Ecol. Prog. Ser. 698, 29–39. https://doi.org/10.3354/meps14153 (2022).
Nie, S. et al. Sediment CO2 flux from a mangrove in southern China: Is it controlled by spatiotemporal, biotic or physical factors?. Forests 14, 782. https://doi.org/10.3390/f14040782 (2023).
Purjava, R., Ramesh, R. & Frenzel, P. Plant-mediated methane emission from an Indian mangrove. Glob. Change Biol. 10, 1825–1834. https://doi.org/10.1111/j.1365-2486.2004.00834.x (2004).
Krithika, K., Purjava, R. & Ramesh, R. Fluxes of methane and nitrous oxide from an Indian mangrove. Curr. Sci. 94(2), 218–224 (2008).
Lin, C. W., Kao, Y. C., Lin, W. J., Ho, C. W. & Lin, H. J. Effect of pneumatophore density on methane emissions in mangroves. Forests 12, 314. https://doi.org/10.3390/f12030314 (2021).
Sheng, N., Wu, F., Liao, B. & Xin, K. Methane and carbon dioxide emissions from cultivated and native mangrove species in Dongzhai Harbor, Hainan. Ecol. Eng. https://doi.org/10.1016/j.ecoleng.2021.106285 (2021).
Zhang, C. et al. Massive methane emission from tree stems and pneumatophores in a subtropical mangrove wetland. Plant Soil 473, 489–505. https://doi.org/10.1007/s11104-022-05300-z (2022).
Purnobasuki, H. & Suzuki, M. Aerenchyma tissue development and gas-pathway structure in root of Avicennia marina (Forsk.) Vierh. J. Plant Res. 118(4), 285–294. https://doi.org/10.1007/s10265-005-0221-7 (2005).
Purnobasuki, H., Purnama, P. R. & Kobayashi, K. Morphology of four root types and anatomy of root-root junction in relation gas pathway of Avicennia marina (Forsk) Vierh roots. Vegetos https://doi.org/10.5958/2229-4473.2017.00143.4 (2017).
Yánez-Espinosa, L. & Ángeles, G. Does mangrove stem bark have an internal pathway for gas flow?. Trees 36, 361–377. https://doi.org/10.1007/s00468-02102210-y (2022).
Aiga, I., Nakano, Y., Ohki, S., Kitaya, Y. & Yabuki, K. Photosynthetic CO2 fixation in pneumatophores of gray mangrove. Avicennia marina. Environ. Control Biol. 33(2), 97. https://doi.org/10.2525/ecb1963.33.97 (1995).
Kitaya, Y. et al. Gas Exchange and oxygen concentration in pneumatophores and prop roots of four mangrove species. Trees 16, 155–158. https://doi.org/10.1007/s00468-002-0167-5 (2002).
Lin, C. W. et al. Methane emissions from subtropical and tropical mangrove ecosystems in Taiwan. Forests 11, 470. https://doi.org/10.3390/f11040470 (2020).
Jacotot, A., Marchand, C. & Allenbach, M. Tidal variability of CO2 and CH4 emissions from the water column within a Rhizophora mangrove forest (New Caledonia). Sci. Total Environ. 631–632, 334–340. https://doi.org/10.1016/j.scitotenv.2018.03.006 (2018).
Lugo, A. E. & Snedaker, S. C. The ecology of mangroves. Annu. Rev. Ecol. Syst. 5, 39–64. https://doi.org/10.1146/annurev.es.05.110174.000351 (1974).
Zaldívar-Jiménez, et al. Conceptual framework for mangrove restoration in the Yucatán Peninsula. Ecol. Restor. 28, 3. https://doi.org/10.3368/er.28.3.333 (2010).
Adame, M. F. et al. Root biomass and production of mangroves surrounding a karstic oligotrophic coastal lagoon. Wetlands 34, 479488. https://doi.org/10.1007/s13157-014-0514-5 (2014).
Herrera-Silveira, J. A. et al. Blue carbon of Mexico, carbon stocks and fluxes: A systematic review. PeerJ 8, e8790. https://doi.org/10.7717/peerj.8790 (2020).
Velázquez-Salazar et al. Manglares de México. Actualización y análisis de los datos 2020. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. México CDMX, 168 (2021).
Luo, Y. & Zhou, X. Soil Respiration and the Environment (Elsevier, Burlington, 2006). https://doi.org/10.1016/B978-0-12-088782-8.X5000-1.
Lambers, H. & Oliveira, R. S. Plant Physiological Ecology 3rd edn, 736 (Springer, Cham, 2019).
Call, M. et al. Spatial and temporal variability of carbon dioxide and methane fluxes over semi-diurnal and spring–neap–spring timescales in a mangrove creek. Geochim. Cosmochim. Acta 150, 211–225. https://doi.org/10.1016/j.gca.2014.11.023 (2015).
Hanson, P. J., Edwards, N. T., Garten, C. T. & Andrews, J. A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 48, 115–146. https://doi.org/10.1023/A:1006244819642 (2000).
Tomlinson, P. B. The Botany of Mangroves 2nd edn, 418 (Cambridge University Press, Cambridge, 2016).
Chen, L. Pneumatophores. In Encyclopedia of Estuaries. Encyclopedia of Earth Sciences Series (ed. Kennish, M. J.) (Springer, Dordrecht, 2016). https://doi.org/10.1007/978-94-017-8801-4_282.
Adame, M. F. et al. Deconstructing the mangrove carbon cycle: Gains, transformation, and losses. Ecosphere 15(3), e4806. https://doi.org/10.1002/ecs2.4806 (2024).
Yabuki, K. Gas exchange between the pneumatophores and roots of mangroves by photosynthesis of pneumatophore. in Photosynthetic Rate and Dynamic Environment. https://doi.org/10.1007/978-94-017-2640-5_5 (Springer, Dordrecht, 2004).
Kozlowski, T. Responses of woody plants to flooding and salinity. Tree Physiol. Monogr. https://doi.org/10.1093/treephys/17.7.490 (1997).
Medina, E. Mangrove physiology: the challenge of salt, heat, and light stress under recurrent flooding, pp. 109–126. in Ecosistemas de Manglar en América Tropical (ed. Yáñez-Arancibia, A., Lara-Domínguez, A. L.) 380 (Instituto de Ecología A.C. México, Costa Rica, 1999).
Krauss, K. W. et al. Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 89, 105–127. https://doi.org/10.1016/j.aquabot.2007.12.014 (2008).
Kristensen, E., Bouillon, S., Dittmar, T. & Marchand, C. Organic carbon dynamics in mangrove ecosystems: A review. Aquat. Bot. 89, 201–219. https://doi.org/10.1016/j.aquabot.2007.12.005 (2008).
Chen, G. C., Tam, N. F. Y. & Ye, Y. Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Sci. Total Environ. 408(13), 2761–2767. https://doi.org/10.1016/j.scitotenv.2010.03.007 (2010).
Chambers, L., Guevara, R., Boyer, J. N., Troxler, T. & Davis, S. E. Effects of salinity and inundation on microbial community structure and function in a mangrove peat soil. Wetlands 36, 361–371. https://doi.org/10.1007/s13157-016-0745-8 (2016).
Feller, O. C., McKee, K. L., Whigham, D. F. & O’Neill, J. P. Nitrogen vs. phosphorus limitation across an ecotonal gradient in a mangrove forest. Biogeochemistry 62, 145–175. https://doi.org/10.1023/A:1021166010892 (2003).
Lovelock, C. E., Feller, I. C., McKee, K. L., Engelbrechts, M. L. & Ball, M. C. The effect of nutrient enrichment on growth, photosynthesis and hydraulic conductance of dwarf mangroves in Panama. Funct. Ecol. 18, 25–33. https://doi.org/10.1046/j.0269-8463.2004.00805.x (2004).
Medina, E., Cuevas, E. & Lugo, A. E. Nutrient relations of dwarf Rhizophora mangle L. mangroves on peat in eastern Puerto Rico. Plant Ecol. 207, 13–24. https://doi.org/10.1007/s11258-009-9650-z (2010).
Cisneros de la Cruz, D. J. et al. Short-distance barriers affect genetic variability of Rhizophora mangle L. in the Yucatan Peninsula. Ecol. Evol. 8, 11083–11099. https://doi.org/10.1002/ece3.4575 (2018).
Adame, M. F. et al. Mangroves in arid regions: Ecology, threats, and opportunities. Estuar. Coast. Shelf Sci. https://doi.org/10.1016/j.ecss.2020.106796 (2021).
Toma, T., Nkamura, K., Patanaponpaiboon, P. & Ogino, K. Effect of flooding water level and plant density on growth of pneumatophore of Avicennia marina. Tropics 1, 75–82. https://doi.org/10.3759/tropics.1.75 (1991).
Dahdouh-Guebas, F., Kairo, J. G., De Bondt, R. & Koedam, N. Pneumatophore height and density in relation to microtopography in the grey mangrove Avicennia marina. Belg. J. Bot. 140(2), 213–221 (2007).
Al-Khayat, J. A. & Alatalo, J. M. Relationship between tree size, sediment mud content, oxygen levels, and pneumatophore abundance in the mangrove tree species Avicennia Marina (Forssk) Vierh. J. Mar. Sci. Eng. 9, 100. https://doi.org/10.3390/jmse9010100 (2021).
Romero, D. & León-Cruz, J. F. Spatiotemporal changes in hurricane-force wind risk assessment in the Yucatan Peninsula, Mexico. Nat. Hazards https://doi.org/10.1007/s11069-023-06397-w (2024).
Penha-Lopes, G. et al. Organic carbon dynamics in a constructed mangrove wastewater wetland populated with benthic fauna: a modelling approach. Ecol. Model. https://doi.org/10.1016/j.ecolmodel.2012.02.005 (2012).
Nóbrega, G. N. et al. Edaphic factors controlling summer (rainy season) greenhouse gas emissions (CO2 and CH4) from semiarid mangrove soils (NE-Brazil). Sci. Total Environ. 542, 685–693. https://doi.org/10.1016/j.scitotenv.2015.10.108 (2016).
Litton, C. M., Giardina, C. P., Albano, J. K., Long, M. S. & Asner, G. P. The magnitude and variability of soil-surface CO2 efflux increase with mean annual temperature in Hawaiian tropical montane wet forests. Soil Biol. Biochem. 2011(43), 2315–2323. https://doi.org/10.1016/j.soilbio.2011.08.004 (2011).
Katayama, A. et al. Effect of forest structure on the spatial variation in soil respiration in a Bornean tropical rainforest. Agric. For. Meteorol. 149, 1666–1673. https://doi.org/10.1016/j.agrformet.2009.05.007 (2009).
Krauss, K. W. & Whitbeck, J. L. Soil greenhouse gas fluxes during wetland forest retreat along the lower Savannah River, Gerogia (USA). Wetlands 32, 73–81. https://doi.org/10.1007/s13157-011-0246-8 (2012).
Arellano-Martín, F., Dupuy, J. M., Us-Santamaría, R. & Andrade, J. L. Soil CO2 efflux fluctuates in three different annual seasons in a semideciduous tropical forest in Yucatan, Mexico. Terra Latinoam. 40, e968. https://doi.org/10.28940/terra.v40i0.968 (2022).
Maher, D. T., Cowley, K., Santos, I. R., Macklin, P. & Eyre, B. D. Methane and carbon dioxide dynamics in a subtropical estuary over a diel cycle: Insights from automated in situ radioactive and stable isotope measurements. Mar. Chem. 168, 69–79. https://doi.org/10.1016/j.marchem.2014.10.017 (2015).
Pedersen, A. R., Petersen, S. O. & Schelde, K. A comprehensive approach to soil atmosphere trace-gas flux estimation with static chambers. Eur. J. Soil Sci. 61(6), 888–902. https://doi.org/10.1111/j.1365-2389.2010.01291.x (2010).
Pihlatie, M. K. et al. Comparison of static chambers to measure CH4 emissions from soils. Agric. For. Meteorol. 171–172, 124–136. https://doi.org/10.1016/j.agrformet.2012.11.008 (2013).
Salas-Rabaza, J. A. et al. Impacts of leaks and gas accumulation on closed chamber methods for measuring methane and carbon dioxide fluxes from tree stems. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2023.166358 (2023).
Posit Team. RStudio: Integrated Development Environment for R. Posit Software, PBC, Boston. http://www.posit.co/ (2023).
Acknowledgements
We thank Gisela Mayora, Karina González, Jesús Garrido and Juan Andrés for fieldwork support. We are grateful to Frédéric Thalasso for his comments on the earliest version of the manuscript. We also thank the authorities of the Comisión Nacional de Áreas Naturales Protegidas (CONANP) for help to do field work in Celestún and Chuburná.
Funding
This research was partially supported by the International Tropical Timber Organization (ITTO) (Fellowship's number 050/20A to J.A.S.R.). J.A.S.R. and G.C.A. received a fellowship from Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT).
Author information
Authors and Affiliations
Contributions
J.A.S.R. and J.L.A. conceived the study; wrote the manuscript. L.Y.E., E.C., and J.L.A. conceptualization, methodology, supervision. J.A.S.R, G.C.A., and R.U.S. organized and conducted field sampling. J.A.S.R. and J.L.A. analyzed the data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Salas-Rabaza, J.A., Yáñez-Espinosa, L., Cejudo, E. et al. Pneumatophore CO2 effluxes decrease with increased salinity in mangrove forests of Yucatan, Mexico. Sci Rep 14, 18449 (2024). https://doi.org/10.1038/s41598-024-68822-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68822-9