Abstract
The largest rivers in developed countries have usually been turned into waterways by straightening them and removing large bedforms hampering navigation. For river restoration and their sustainable management it is important to know how large bedforms support biodiversity, whether they could be protected and what potential conflicts in river management they can pose. We have addressed these questions by studying the role of large bedforms in supporting populations of two inland tern species Sternula albifrons and Sterna hirundo. We spatially analysed the behaviour of these two species with reference to the bedform structure mapped over a long semi-natural reach of the River Wisła (Vistula) (S. Poland). The results show that radiotagged terns breed on islands within the aggradation reaches, foraging in the adjacent shallows inhabited by populations of small fish. For Little Terns, the more complex the water line of emergent forms, the greater their foraging intensity. The islands do not pose any flood risk to human settlements. The whole geofeature forms an integral habitat for fish and birds; it is maintained by its geographic settings and so is stable over long periods of time (over 200 years). Protection of such habitats is thus feasible.
Similar content being viewed by others
Introduction
Geodiversity underpins biodiversity; this relationship should be taken into consideration in nature conservation planning and management1,2. Usually a large-scale phenomenon, it is comparable with the global biodiversity hot-spots in the Caucasus or Mediterranean Basin3. Nevertheless, nature conservation and restoration at such a large scale has to be based on smaller geofeatures, which need to be identified and protected or at least properly managed. This becomes especially important in view of the forthcoming Nature Restoration Law, introduced in the EU, which stipulates that 30% of the EU area should be rewilded or restored by 2030, including 25 000 km of rivers4.
In recent times, freshwater habitats have undergone dramatic changes: river channels have been straightened and narrowed, fragmented by dams, weirs and reservoirs, and isolated from their floodplains by longitudinal obstacles. As a result, freshwater habitats and their associated biodiversity have been assessed as being the globally most seriously threatened5, chiefly as a result of chemical and biological pollution, but also of alterations in their hydrology and physical structure. Biodiversity decline as a result of river training works applies to all biota inhabiting running waters, although some taxonomic groups like birds are better documented6.
The emerging problem for river ecology is to address issues about a river’s physical structure and the hydrological mechanisms shaping the forms of river channels crucial for maintaining river biodiversity. River beds are obviously not flat7; they consist of a variety of forms, the importance of which for hydraulic management and human safety is crucial for proper river management (see8 for a review). Dune bedforms in rivers can be important sources of flow resistance, frequently impeding sediment transport. As such, their dynamics are important in river engineering and management9. Few studies have been published on the biological importance of dune bedforms in large rivers (50 000 km2 of catchment10); they have been hampered by the large spatial scale of the phenomenon and the consequent methodological difficulties. The main problem, however, lies in the fact that large bedforms, being obstacles to navigation, have been removed in most developed countries, where large rivers are still being dredged, as they are regularly used for the water transport of goods. Such simplified channels with almost no biodiversity are of little value for studies of river biodiversity11. In consequence, the biota associated with natural bedforms of large rivers are almost entirely overlooked. In this context, the best example pertains to inland nesting birds, like colonial gulls and terns, the conservation of which focuses on marine shore environments. Their possible abundance due to the presence of large river bedforms, once widespread and still occurring in some parts of the world (e.g.12,13), is completely ignored. This entails the risk of more such habitats being lost, as a result of plans to develop waterways in CEE countries13,14,15 or their being omitted in large-scale restoration plans. Moreover, the standard conservation measures for Laridae target nesting success (e.g.16) and disregard transdisciplinary approaches to the broader habitat context, especially hydromorphological factors. This is precisely what we would like to focus on in this paper.
Intensive sediment transport often results in the emergence of bedforms above the water surface, where sediment aggradations become functional islands17. This type of bedform is isolated from the mainland and so should, as a rule, be safe from terrestrial predators18. For this reason, such sites are valuable for birds as safe nesting grounds, and this way of thinking about the conservation of such sandy islands in rivers predominates in conservation practice. However, a bedform emerging above the water surface is usually only a small part of a larger bedform. A low-angle dune19 creates a lot of shallow areas, with a diversified water line20. The considerable spatial complexity of the island’s inshore zone provides a variety of microhabitats that form nursery habitats required by fish during their early ontogenic development21. The small fish that are present in the shallows during the daytime (22 and the references therein) facilitates the occurrence and reproduction of rare riverine bird species associated with sandbars23.
The combination of such bedforms would create a habitat unit in the river continuity favourable for terns. This is not an obvious feature of river channels, because most large bedforms created by sediment transport in large rivers hardly rise above the water level and are usually flat and unstable. Not only do their boundaries change but so do their locations, depending on the dynamics of sediment transport within the channel24. In our opinion, the main question arising for biodiversity-friendly river management relates to the stability of this habitat, the possibility of its conservation depending on the predictability of its occurrence and permanence. The other question relates to the possibility of restoring river birds’ habitats by launching hydrological processes leading to geofeatures favouring the breeding of these birds.
Last but not least is the social aspect of large dunes as emergent bedforms. Being visible on the surface, they are, in the case of a catastrophic flood, immediately held responsible for blocking the flow and thus for causing flood damage. This can lead to serious social conflict25, such as the one which provided the motive for this study. Such situations threaten the existence of channel reaches of high natural value, as the people affected call for rivers to be trained, i.e. straightened, with the construction of levees and groynes.
This research aimed to identify the hydrological mechanisms creating and maintaining habitats suitable for rare, threatened, riverine, colonial, fish-eating bird species like Little Tern Sternula albifrons and Common Tern Sterna hirundo (both species are on the IUCN Red List in the “least concern” category26,27). These two species were studied because both used to inhabit river channels, foraging within the channel and nesting on the river dunes13,28, but because the majority of large rivers have been regulated, such habitats have practically ceased to exist. Using radiotelemetry and detailed hydrographic methodologies (GPS RTK and ADCP) applied along a 52 km-long stretch of a large, natural river valley, we aimed to define habitats not only suitable for the nesting of birds but also suitable for fish, thus providing foraging grounds for the breeding birds. The crucial questions with regard to the conservation of such a habitat are the long-term stability of such features of the channel, thus making their potential conservation rational, and whether emergent bedforms constitute a flood threat to adjacent areas inhabited by humans.
Materials and methods
Study area
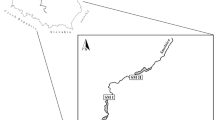
The River Wisła (Vistula), 1047 km long and 300–1000 m wide in its middle and lower reaches, is the largest river in Poland, draining a catchment area of 168.6 thousand km2. In its upper reaches, the Wisła receives water from the Carpathians and the Świętokrzyskie Mts. Near the confluence with the River San, the Wisła valley is 10–25 km wide. Various sections of this stretch of the Wisła were regulated between 1903 and 1980, but since then, the river has in a natural way partly destroyed most of the old regulation structures and nowadays flows more freely downstream of Sandomierz. Near the end of the upper reach, the Wisła valley narrows to 3–4 km, forming a “gorge” through the limestone hills near Annopol. This study was conducted on the 52-km-long stretch from Tarnobrzeg (50°34′9′′N, 21°40′38′′E) to Słupia Nadbrzeżna (50°57′6′′N, 21°48′19′′E; with some additional observations of Little Terns foraging on Kłudzie Island 51°9′43′′N, 21°46′55′′E), broadly encompassing the “Wisła pod Zawichostem” nature reserve.
Hydrological surveys
The measurements were made on the 52-km-long stretch from Tarnobrzeg (50°34′9′′N, 21°40′38′′E) to Słupia Nadbrzeżna (50°57′6′′N, 21°48′19′′E) using Global Positioning System Real Time Kinematic (GPS RTK), with an Acoustic Doppler Current Profiler (ADCP) for measuring the water depth. The water surface elevation, as measured by GPS RTK, formed the basis for the water surface elevation profile and, together with the ADCP depth, yielded the river bed elevation profile. Any object above the water surface, like a sandy island, was measured directly by GPS RTK. These data were then used to generate 3D spatial Digital Elevation Models (DEMs) by interpolation with open source QGIS 2.18 plugins. The sub-5 cm vertical accuracy of this survey reveals local steep sections of the river profile that represent an important habitat scale unrecognizable in publicly accessed DEMs obtained by Airborne Laser Scanning (ALS). DEMs are valuable in the short term because of the rapidly changing condition of river bedforms. River bedforms such as islands that are covered only by high waters had to be monitored as the official survey database contains no contemporary data.
Flood simulation
Flow hydraulic parameters (Q—water flow [m3/s], water table [m]) were modelled numerically using HEC-RAS 1D on the 52 km-long stretch from Tarnobrzeg (50°34′9′′N, 21°40′38′′E) to Słupia Nadbrzeżna (50°57′6′′N, 21°48′19′′E) and calibrated with water surface measurements (GPS RTK). The model is based on the main channel and the floodplain.
Fish distribution
Fish sampling locations were determined by the presence of suitable habitats: complex shallows with unstructured sand bottoms for beach seining and adjacent deep runs and pools for electrofishing. Within each type of habitat, the precise locations of the sampling transects were chosen in a random stratified manner in order to ensure that all fish assemblages were represented and all possible microhabitats covered30. Fish assemblages were sampled using two approaches: (i) deep and otherwise structured habitats, e.g. consisting of large woody debris etc., were electrofished with a boat-mounted generator-powered unit (generator rating 3500 W, output 350 V pulse-DC, 60 Hz, 8–10 A); the fish were collected with a hand-held ring anode and an additional dip net as the boat was moving downstream; (ii) in shallow (< 1.3 m, usually 0.3–0.8 m deep) and unstructured habitats, e.g. sandbars, fish were sampled with a 15-m-long seine net with a bar mesh size of 8 mm. All fish were identified to species, measured for total length (± 1 mm) and released back into the water. Instantaneous mortality was negligible. The efficient electrofishing area covered approximately 2000 m2 at each site. Sampling shallow habitats involved 2–6 individual hauls of the seine net. The pooled seining area at each site was approx. 500 m2. Fish were sampled in August and September 2014–15 at 5 sites, shown by the black triangles in Fig. 1a.
The main study site and methodology employed. (a) Locations of the studied breeding colonies of terns (red dots) and fish sampling sites (black triangles), shown against the background of the Digital Terrain Model29. (b) Mapping the islands’ shore lines. Photo by D. Kwaśna. (c) A Little Tern with affixed radio transmitter. Photo by D. Kwaśna. (d) Searching for radiotagged terns from a motorboat. Photo by T. Zając.
Bird surveys
The river channel between Sandomierz and Annopol was searched every week at low water in 2014–2016 for breeding Little Terns and Common Terns, usually in May and June. Observers on a motorboat sailed up- and downstream, scanning the islands for nests of both species. The locations and status of nests were recorded using GPS.
Telemetry
The telemetry study was started in 2014 on Chwałowice Island; 4 Little Terns and 1 Common Tern were radiotagged, but because the water level rose rapidly soon after the first birds had been tagged, the study in that year had to be abandoned. In the next two seasons, birds were radiotagged on Opoka Island – 20 Little Terns and 8 Common Terns in 2015, and 5 Little Terns and 16 Common Terns in 2016. The small number of radiotagged Little Terns in 2016 was due to the low overall abundance of this species in that year. All the birds were trapped at the nest using Moudry TR60 spring traps. These were set up only in the mornings, on those nests where hatching was expected in the next few days. The traps were monitored continuously with binoculars. Only one individual from a pair per nest was captured and tagged. Every trapped bird was immediately taken from the nest, fitted with a numbered aluminium ring and a Holohil BD-2 radio transmitter (weight: 1.35 g–2.7% of the weight of the lighter Little Tern; standard lifespan: 8 weeks). The transmitters were affixed to the bird’s back (Fig. 1c) with Superglue. The manipulated birds usually returned to the nests very soon after being released, so that disturbance was minimal. Bird were captured until the first hatchling appeared on the island so as to minimize chick mortality caused by insolation. Both captured and non-captured birds continued to breed normally after capturing had been completed.
The surveys to detect the radiotagged terns were carried out once a week during the nestling feeding period from motorboats (Fig. 1d) travelling between Sandomierz upstream and Jakubowice downstream. Also every week, but on other days, usually 2 teams (equipped with an Australis 26 K and/or a Yaesu FT-817ND receiver with a Yagi antenna) scanned the river channel for radiotagged birds, using fixed check points on the bank, deployed along the channel at distances enabling detection of the BD-2 transmitter within the range of reception, in order to locate the record easily on the map, which was later georeferenced in order to analyse the data using Arc GIS. The whole stretch between Tarnobrzeg and Słupia Nadbrzeżna was also searched from a car fitted with a powerful non-directional whip antenna to detect birds that might have been foraging beyond the channel or a long way from the breeding area.
The density distribution maps of the records of radiotagged Little Terns and Common Terns (Fig. 2) were generated using the ArcGIS 10.1 kernel density method with a 500 m search radius, which corresponds to the approximate width of the Wisła channel near Annopol.
The density of records of radiotagged terns in relation to river bed morphology for the breeding island Opoka. (a) River bed morphology: blue line—water surface elevation profile, black line—river bed elevation profile, red line—river bed aggradation reaches, green line—stabilized or degrading sections of the river bed; the red arrows indicate the locations of the islands studied. (b–e) Density of records of radiotagged terns: (b) Little Terns in 2015, (c) Little Terns in 2016, (d) Common Terns in 2015, (e) Common Terns in 2016.
Foraging
Data on foraging behaviour and its efficiency at a small spatial scale (in the channel section immediately adjacent to the breeding island) were gathered only for the rarer Little Tern during the nestling feeding period in 2017–2019 on Opoka Island. An additional study was conducted in 2018–19 on Kłudzie Island (51°9′43''N, 21°46′55''E), which lay beyond the main surveyed section. Kłudzie Island was included as a control for studying the Little Tern’s foraging behaviour on any island other than Opoka.
GPS was used to generate accurate maps of the islands’ water lines (Figs. 1b, 3a,b); these were then printed and used for mapping the localities of the foraging terns. Four or five permanent observation posts (depending on the visibility in a given season) were established on the channel banks around each island, from which all of the islands’ banks and adjacent areas of the channel could be observed without disturbing foraging individuals, from sunrise until 15:00 h at the latest. Observations were made at each point for one hour, their sequence in consecutive counts being altered at random. The positions of foraging birds were entered into the GIS system (470, 480 and 244 records in 2017, 2018 and 2019, respectively, for Opoka Island; 410 and 117 records in 2018 and 2019, respectively, for Kłudzie Island). 100 buffers with a 50 m radius were delimited at random in that part of the river channel containing the island and for the data from each year (Fig. 3b). The number of tern attacks on fish for each buffer were established during the periods when water levels were low and stable, as well as the length of the water line within the buffer. In order to make allowance for the complexity of the water line, the buffers were used for further analyses if the length of the water line was longer than the diameter of the buffer, which represents a straight line.
Relationship between water line complexity and foraging. (a) Monthly changes in the water line during the 2015 breeding season on Opoka Island, shown by coloured lines on the orthophotomap29. (b) 50 m random buffers (orange circles) used for counting the number of foraging attempts of Little Terns (yellow dots) and for measuring the water line length, shown on the orthophotomap29).
Foraging and aggradation areas
500 points were randomly selected within the Wisła river channel and the adjacent area (150 m wide) along the 26 km-long stretch of the river from the village of Kamień Łukawski (50°41′23''N, 21°47′10''E) to the town of Annopol (50°53′7''N, 21°51′25′′). A buffer of 200 m radius was generated for each point. In each buffer that coincided with the river bed elevation profile, the standardized river bed slope was calculated using S = (Hs-He)/l∙100%, where Hs is the bed elevation at the buffer start, He is the bed elevation at the buffer end, and l is the distance between Hs and He. Negative values of the parameter S indicate the areas with an aggrading river bottom, a 0 value of S indicates the areas with a flat bottom, and a positive value of S indicates the areas with a degrading river bottom. The number of radiotagged Little Terns and Common Terns was attributed to each buffer.
History
The stability of the aggradation reaches was analysed by comparison with the historical maps and aerial photographs listed in Table 1, dated since 1975.
Regulations
The research was conducted in full compliance with the ethical codes and legislation of Poland, according to the following permits from the nature conservation authorities: DZP-WG.6401.00.5.2014.km, DZP-WG.6401.03.119.2016.dł, WPN-I.6401.309.2018.PK, WPN.6401.78.2019.KC.
Statistical analyses
Differences in density and the total length of fish between the deep and shallow habitats at the sampling sites were investigated using a model-based approach, i.e. Generalized Linear Mixed-effects Models (GLMM) with either normal (for measurements) or Poisson error distribution (for count data). We used raw (untransformed) data. In each model, the “habitat type” variable was set as a fixed, categorical factor, and the “site” variable was set as a random effect. The models were compared to null (intercept-only) models. The best fitted models were selected using the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC).
The influence of habitat features on the foraging intensity was analysed using a Generalized Linear Model (GLZ; Poisson, log; dependent variable: number of attacks; categorical factors: season, island; continuous variables: water line length, water area; interactions between variables: season*island, season*water line length, island*water line length, season*water area, island*water area, water line length*water area, season*water line length*water area, island*water line length*water area).
The differences in bed channel morphology and in flight distance between Little Terns and Common Terns were Median-tested.
The influence of the river bottom slope on the presence/absence of Little Terns and Common Terns was analysed using Generalized Linear Models (GLZ; logit, binomial; dependent variable: species present/absent; categorical variable: year; continuous predictor: bottom slope). The analyses were performed for each species separately.
The influence of the river bottom slope on the numbers of terns of both species observed was analysed using Generalized Linear Models (GLZ; log, Poisson; dependent variable: number of observations; categorical variable: year, continuous predictor: bottom slope). The analyses were performed for each species separately.
Results
Only three breeding colonies of terns were identified along the studied reach: (shown by red dots in Fig. 1a): (1) Sandomierz Groyne (50°40′6''N, 21°44′11''E) with 5 Little Tern nests and 4 Common Tern nests found only during 2015, all destroyed by a predator, (2) Chwałowice Island (50°45′41''N, 21°50′35''E) was functional during two years: 2014 (25 Little Tern nests, 13 Common Tern nests) and 2015 (25 Little Tern nests, 16 Common Tern nests), abandoned when the island became joined to the right-hand river bank in 2016 and (3) Opoka Island (50°51′45''N, 21°50′53''E) which was functional for 3 years: 2014 (Little Tern 10, Common Tern 63), 2015 (Little Tern 23, Common Tern 55) and 2016 (Little Tern 8, Common Tern 77); this island was stable during the entire study period.
Distribution of the tern colonies in relation to channel morphology
Channel bottoms measured from Sandomierz to Annopol consisted of interchanging degrading, balanced and aggrading sections (Fig. 2a). Chwałowice and Opoka Islands comprised two aggrading sections, whereas the Sandomierz colony occupied part of a destroyed groyne in the regulated part of the channel. Analysis of the historical maps shows that the sites inhabited by breeding colonies have always been areas of sand aggradation ever since the nineteenth century (Table 1) and, except for a few years, sandy islands have always existed in these areas.
Foraging flight and aggradation areas
In relation to the channel bed morphology, the data differed significantly between the two species (Median test, Md = 897 m, Chi sq = 20.8, df = 1, p < 0.0001; Fig. 2): Little Terns flew shorter distances (Md = 465 m, max 11 km; Fig. 2b,c) than Common Terns (Md = 1207 m, max 15 km; Fig. 2d,e). The data were similar in all the years of the telemetry study. The river bed slope was not significantly related to either the probability of the presence or the number of observed Common Terns (Table 2). Nevertheless, the lower the river bottom slope (i.e. upgrading), the higher the probability of observing Little Terns and the higher the numbers of this species (Table 2, Fig. 4).
Fish prey distribution
A total of 6049 fish were caught: 2072 by electrofishing and 3695 by seining. Fish from 10 to 772 mm TL (mean 80.0 mm) were caught by means of electrofishing, from 15 to 226 mm TL (mean 42.3 mm) by seining. The size distributions in both habitat types differed significantly (ΔAIC = 905, ΔBIC = 899). Fish found in shallow habitats were consistently smaller than in the adjacent deep part of the river channel (Fig. 5a,c). In addition, the density of foraging fish (< 50 mm TL) was significantly higher in shallow than in deep habitats (ΔAIC = 674, ΔBIC = 668; Fig. 5b,c). The shallows were dominated by Alburnus alburnus and Leuciscus leuciscus, which made up 87% of the fish caught. Alburnus alburnus, Squalius cephalus, Gobio gobio and Vimba vimba were most numerous species in deep habitats (81%).
Characteristics of fish caught in tern foraging habitats of different depths. (a) Pooled distribution of the total length of fish in deep vs shallow habitats. (b) Density (ind./m2) of fish shorter than 50 mm TL, i.e. presumably prey items of foraging terns, in deep vs shallow habitats. (c) Mean sizes and numbers for deep and shallow areas.
Tern foraging
The islands’ water lines changed in shape depending on the water level (Fig. 3a). The preliminary study conducted in 2017 only for Opoka Island showed that wherever the water line was more complex, the overall numbers of tern attacks were higher (total number of attacks: rS = 0.73, N = 100, p < 0.0001). This significant relationship was also confirmed for both Opoka and Kłudzie Islands in 2018 and 2019 (Table 3).
Flood security
We simulated the interference to flow caused by Opoka Island by comparing the numerical model results of the channel with and without the island (Fig. 6a). The simulation showed that the island’s existence had no influence on the water level until Q = 537 m3/s. When flows were catastrophically high (Q1, 1% water) and the water overflowed from the channel on to the flood terrace, the islands within the channel could cause the water level in the whole valley to rise by 7.5 cm (Fig. 6b).
Discussion
In our study sites, sediment aggradation has been producing islands and their surrounding shallows for over 200 years. Given its geological origin, this process may have been going on for very much longer, whereas the presence of breeding colonies of terns on the Opoka aggradation area has been documented since the 1960s34. This justifies the formal protection of these islands, because the probability of their disappearance, e.g. as happens with some other islands after a big flood, is very small.
The considerable spatial complexity of the islands’ inshore zones provides a variety of microhabitats forming the nursery habitats required by fish during their early ontogenic development21. The small fish that are present in the shallows during the daytime (see22 and the references therein) sustain the occurrence and reproduction of rare riverine bird species associated with sandbars23, such as Little Terns. The foraging intensity of Little Terns is thus closely related to the complex water lines of the islands. Large islands are created when water levels are high, but when the water level goes down, the hydraulic forces weaken and the bottom shape and water line become diversified at a finer scale24. This mechanism creates a habitat promoting the growth of fry, because predation is less efficient in a more complex spatial system35, although this applies only to predatory fish. A complicated spatial structure does not protect fish fry against avian predators; on the contrary, a complex water line may act as an easily perceived visual signal showing terns where to forage.
The shallows in an aggradation area form a complementary foraging habitat to an emergent breeding island36, optimizing the terns’ feeding effort, and are an integral part of the same geofeature. However, it may also be that the food capacity of an aggradation area is too small for terns to raise young, even though the river section in question does offer perfect conditions for nesting. Terns breeding on groynes are a case in point. Then, only a few, usually solitary pairs will breed there (pers. observ.).
Of course, this study relates to one river, and the objection may be raised that this situation differs from those in other rivers. However, it is reasonable to assume that in any sandy river, terns will forage on small fish which are known to aggregate in shallows (e.g. in the Missouri River23:). Shallows are more frequent in aggradation areas, where dry islands suitable for breeding must appear, even by chance. Hence, this mechanism should apply to all rivers where abiotic factors give rise to sediment aggradation, an aspect that might be usefully applied to nature conservation.
Conservation implications
With regard to agreement and commitment among societies on the necessity of restoring the ecological functions and services of freshwater bodies, and hence their biodiversity, the pressing problem for river ecology is to determine in what way the channel should or should not be managed. Ambitious plans for river restoration are forthcoming (e.g. 25 000 km of rivers in the EU are to be restored (“rewilded”) according to the “European Biodiversity Strategy”4, followed by the Restoration Law, subject to consultation by EU Member States in 202337), which must be based on sound science. The first question to be addressed therefore relates to a river’s physical structure: which channel forms and which bedforms are crucial for maintaining or even enhancing river biodiversity.
Quantifying foraging flight distances using telemetry demonstrates not only that the distribution of foraging flights tallies with the spatial distribution of aggradation reaches, but also that a quantitative approach can be applied to decision making. The “effective” area of the terns’ occurrence can be identified using very expensive and time-consuming telemetric methods, but this is also possible by means of basic geomorphological surveys (ascertaining the presence of aggradation features like islands and shallows) and modelling38.
In the wake of the catastrophic flood on the Wisła in 2010, riparian communities were very wary about the aggradation reaches. Local people were convinced that aggradation sites had blocked the water flow and caused the levees to collapse. On the other side of the conflict, conservation activists blocked any activities within or near the river channel on very long reaches, simply on the basis that terns had been observed there during the breeding period, without having checked whether these birds had actually been breeding. This generated a lot of unnecessary tension between local people and conservationists25 and no solution was found. By taking a quantitative approach to the terns’ distribution as regards both breeding and foraging, determined using a reliable methodology (telemetry), one can delimit the area of habitat effectively used by the terns. This, in turn, makes for easier decisions regarding flood control works. As an additional argument, one can demonstrate that structures like an in-channel island pose no threat to surrounding areas because its resistance to the water flow is negligible when the water level is high.
It is well known that migrating sand dunes within river channels are frequently the result of human-induced changes to river hydrology39; such dunes can migrate, be flattened and/or completely disappear with time40. However, analysis of historical maps indicates that at these particular sites, aggradation of bottom sediments leading to island formation has been taking place for a very long time. The geomorphic and hydrological conditions here have always favoured aggradation: the valley narrows where the river passes by the limestone ridge and where a large, highly dynamic mountain river (the San) enters the Wisła channel, carrying large amounts of sediment into it. Analysis of geomorphic settings should be the first step taken by decision makers when planning river restoration. It is also worth mentioning that blocking the channel with sediment is an easy method of restoring rivers; in many locations, it is sufficient for river managers not to combat bottom aggradation.
It is important that islands as breeding sites comprise an integral habitat with complementary habitats (shallows). This offers a guide to the construction and location of artificial breeding habitats for terns, which is the broadly adopted method for their active conservation. In such measures, sand or gravel is left exposed for the birds on moored river barges or as islands in gravel pits; sandbars within the channel could also be constructed from dredged material41,42). If none of these structures is associated with shallows, they may be colonized by Common Terns, which forage over long distances, and hence within a broader spectrum of supplementary habitats. In contrast, the artificial breeding habitats of Little Terns must be closely associated with shallows as a complementary foraging habitat. These conditions have always been favourable for small/young fish and thus also Little Terns, and should be taken into account in every river restoration scheme. It should be noted that this study provides quantitative information about the resources (fish size and quantity) available to terns in natural sites – information which could be useful in restoration projects in order to determine which fish population parameters must be attained in a given habitat during river restoration.
In contemporary rivers, most bedforms are too flat to emerge above the water surface to form breeding islands, but the shallows could offer perfect foraging areas for these birds. If breeding sites are provided on barges moored on rivers, locating them in place of an island on the aggradation areas will be an effective way of conserving terns, as recently confirmed by Martinović et al.43.
Conclusion
Areas of sediment aggradation in the riverbed create easily identifiable areas of favourable breeding and foraging habitats for terns, which are stable in the long term and can thus be protected. They are made suitable for terns by the presence of a complex geofeature: sediment aggradation forming an emergent island and adjacent shallows and, in the case of the Little Tern, the complex water line of emergent forms. The emergent islands responsible for the occurrence of both species pose no threat of flooding, because they do not significantly increase the water level during a spate. These geofeatures are easily identifiable by remote sensing and can be used in planning the protection and restoration of river valleys.
Data availability
The dataset used and analysed during the current study (including GPS data not presented in the paper, such as the positions of foraging terns and hydromorphological measurements) is available from the corresponding author on reasonable request.
References
Hjort, J., Heikkinen, R. K. & Luoto, M. Inclusion of explicit measures of geodiversity improve biodiversity models in a boreal landscape. Biodivers. Conserv. 21, 3487–3506. https://doi.org/10.1007/s10531-012-0376-1 (2012).
Matthews, T. J. Integrating geoconservation and biodiversity conservation: Theoretical foundations and conservation recommendations in a European Union context. Geoheritage 6, 57–70. https://doi.org/10.1007/s12371-013-0092-6 (2014).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858. https://doi.org/10.1038/35002501 (2000).
European Commission. EU Biodiversity Strategy for 2030 COM(2020) 380 final. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0380. Accessed 22 Feb 2023
Dudgeon, D. et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182. https://doi.org/10.1017/S1464793105006950 (2006).
Buckton, S. T. & Ormerod, S. J. Global patterns of diversity among the specialist birds of riverine landscapes. Freshw. Biol. 47, 695–709. https://doi.org/10.1046/j.1365-2427.2002.00891.x (2002).
Kennedy, J. F. The formation of sediment ripples, dunes, and antidunes. Annu. Rev. Fluid Mech. 1, 147–168. https://doi.org/10.1146/annurev.fl.01.010169.001051 (1969).
Gilvear, D. J. Fluvial geomorphology and river engineering: Future roles utilizing a fluvial hydrosystems framework. Geomorphology 31, 229–245. https://doi.org/10.1016/S0169-555X(99)00086-0 (1999).
Bradley, R. W. & Venditti, J. G. The growth of dunes in rivers. J. Geophys. Res. Earth Surf. 124, 548–566. https://doi.org/10.1029/2018JF004835 (2019).
INSPIRE 2024. http://inspire.ec.europa.eu/glossary/LargeRiver:1. Accessed 8 Jul 2024.
Dynesius, M. & Nilson, C. Fragmentation and flow regulation of river systems in the Northern Third of the World. Science 266, 753–762. https://doi.org/10.1126/science.266.5186.753 (1994).
Bukaciński, D., Bukacińska, M. & Buczyński, A. Threats and the active protection of birds in a riverbed: Postulates for the strategy of the preservation of the middle Vistula River avifauna. Stud. Ecol. Bioethicae 16, 5–23. https://doi.org/10.21697/seb.2018.16.4.01 (2018).
Shurulinkov, P., Daskalova, G., Michov, S. & Koev, V. The distribution, numbers, and breeding of terns and waders on the sand islands along the Bulgarian-Romanian section of the Danube. North West. J. Zool. 12, 65–77 (2016).
Jarzębińska, T. The role of inland ports in integration of polish waterways with the European network. Pol. Marit. Res. 14, 30–33 (2007).
Doubrovsky, M. Ukrainian and Russian waterways and the development of European transport corridors. Eur. Transport Trasp. Eur. 30, 14–36 (2005).
Wilson, L. J., Rendell-Read, S., Lock, L., Drewitt, A. L. & Bolton, M. Effectiveness of a five-year project of intensive, regional-scale, coordinated management for little terns Sternula albifrons across the major UK colonies. J. Nat. Conserv. 53, 125779. https://doi.org/10.1016/j.jnc.2019.125779 (2020).
Leli, I. T., Stevaux, J. C. & Assine, M. L. Origin, evolution, and sedimentary records of islands in large anabranching tropical rivers: The case of the Upper Paraná River, Brazil. Geomorphology 358, 107118. https://doi.org/10.1016/j.geomorph.2020.107118 (2020).
Schlesselmann, A. K. V., O’Donnell, C. F., Monks, J. M. & Robertson, B. C. Clearing islands as refugia for black-fronted tern (Chlidonias albostriatus) breeding colonies in braided rivers. N. Z. J. Ecol. 42, 137–148. https://doi.org/10.20417/nzjecol.42.23 (2018).
Kostaschuk, R. A. & Venditti, J. G. Why do large, deep rivers have low-angle dune beds?. Geology 47, 919–922. https://doi.org/10.1130/G46460.1 (2019).
Kocurek, G., Ewing, R. C. & Mohrig, D. How do bedform patterns arise? New views on the role of bedform interactions within a set of boundary conditions. Earth Surf. Process. Landf. 35, 51–63. https://doi.org/10.1002/esp.1913 (2010).
Schiemer, F., Keckeis, H. & Kamler, E. The early life history stages of riverine fish: Ecophysiological and environmental bottlenecks. Comp. Biochem. Physiol. A 133, 439–449. https://doi.org/10.1016/S1095-6433(02)00246-5 (2002).
Nowak, M. et al. Diel changeover of fish assemblages in shallow sandy habitats of lowland rivers of different sizes. Knowl. Manag. Aquat. Ecosyst. 420, 41. https://doi.org/10.1051/kmae/2019037 (2019).
Stucker, J. H., Buhl, D. A. & Sherfy, M. H. Emergent sandbar construction for Least Terns on the Missouri River: Effects on forage fishes in shallow-water habitats. River Res. Appl. 28, 1254–1265. https://doi.org/10.1002/rra.1525 (2012).
Reesink, A. J. H. et al. The adaptation of dunes to changes in river flow. Earth Sci. Rev. 185, 1065–1087. https://doi.org/10.1016/j.earscirev.2018.09.002 (2018).
Echodnia.eu. Rezerwat zagraża mieszkańcom, ekolodzy protestują [The nature reserve threatens local community, ecologists protest], echodnia.eu, posted 18 Feb 2011. Accessed 8 Jul 2024.
BirdLife International. 2019a. Sternula albifrons (amended version of 2018 assessment). The IUCN Red List of Threatened Species 2019: e.T22694656A155476219. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22694656A155476219.en. Accessed 8 Jul 2024.
BirdLife International. 2019b. Sterna hirundo (amended version of 2018 assessment). The IUCN Red List of Threatened Species 2019: e.T22694623A155537726. https://doi.org/10.2305/IUCN.UK.2019-3.RLTS.T22694623A155537726.en. Accessed 8 Jul 2024.
Cramp, S., Bourne, W. R. P. & Saunders, D. The Seabirds of Britain and Ireland (Collins, 1974).
GUGiK, Head Office of Geodesy and Cartography in Poland. https://www.gov.pl/web/gugik/dane-pzgik4, https://www.geoportal.gov.pl/. Accessed 1 Feb 2023.
Copp, G. H. & Peňáz, M. Ecology of fish spawning and nursery zones in the flood plain, using a new sampling approach. Hydrobiologia 169, 209–224. https://doi.org/10.1007/BF00007312 (1988).
Mapster “Old Maps of Poland and Central Europe”. http://igrek.amzp.pl/. Accessed 1 Feb 2023.
Arcanum “Mapire. The Historical Map Portal”. https://mapire.eu/en/. Accessed 1 Feb 2023.
IMGW-PIB, Institute of Meteorology and Water Management – National Research Institute. https://danepubliczne.imgw.pl/. Accessed 1 Feb 2023.
Luniak, M. Ptaki środkowego biegu Wisły. Acta Ornithol. 13, 17–113 (1971).
Huffaker, C. Experimental studies on predation: Dispersion factors and predator-prey oscillations. Hilgardia 27, 343–383. https://doi.org/10.3733/hilg.v27n14p343 (1958).
Dunning, J. B., Danielson, B. J. & Pulliam, H. R. Ecological processes that affect populations in complex landscapes. Oikos https://doi.org/10.2307/3544901 (1992).
European Commission. Proposal for a Nature Restoration Law. https://environment.ec.europa.eu/topics/nature-and-biodiversity/nature-restoration-law_en. Accessed 22 Feb 2023.
Tracy-Smith, E., Galat, D. L. & Jacobson, R. B. Effects of flow dynamics on the aquatic-terrestrial transition zone (ATTZ) of Lower Missouri River sandbars with implications for selected biota. River Res. Appl. 28, 793–813. https://doi.org/10.1002/rra.1492 (2012).
Wu, S., Xu, Y. J., Cheng, H. & Wang, B. Difference in riverbed micromorphology of two world large lowland rivers: Implication of natural and human effects. Estuar. Coast. Shelf Sci. 276, 108001. https://doi.org/10.1016/j.ecss.2022.108001 (2022).
Ten Brinke, W. B. M., Wilbers, A. W. E. & Wesseling, C. Dune growth, decay and migration rates during a large-magnitude flood at a sand and mixed sand–gravel bed in the Dutch Rhine River System. Fluv. Sedimentol. VI https://doi.org/10.1002/9781444304213.ch2 (1999).
Sherfy, M. H., Stucker, J. H. & Buhl, D. A. Selection of nest-site habitat by interior Least Terns in relation to sandbar construction. J. Wildl. Manag. 76, 363–371. https://doi.org/10.1002/jwmg.301 (2012).
Stucker, J. H., Buhl, D. A. & Sherfy, M. H. Consequences of Least Tern (Sternula antillarum) microhabitat nest-site selection on natural and mechanically constructed sandbars in the Missouri River. Auk 130, 753–763. https://doi.org/10.1525/auk.2013.13048 (2013).
Martinović, M., Plantak, M., Jurinović, L. & Kralj, J. Importance of shallow river topography for inland breeding Common Terns. J. Ornithol. https://doi.org/10.1007/s10336-023-02060-0 (2023).
Acknowledgements
We would like to express our sincere gratitude to Marek Chruściel and the Sandomierz municipal authorities, who provided invaluable help in organizing a base for our activities and motorboat transport. We also thank Marcin Oberc for his help with the fieldwork. Special thanks are due to A. Klaczak, P. Szczerbik and W. Popek (University of Agriculture in Kraków) for the extensive field work during the fish sampling. The study was supported by project EOG PL02 2009-2012 (“Zarządzanie kryzysowe obszarem Natura 2000 w warunkach powodzi na przykładzie Małopolskiego Przełomu Wisły (km 254 + 000—307 + 000)”) [Crisis management of Natura 2000 area during flood on the example of Małopolski Przełom Wisły (km 254 + 000—307 + 000)], by the Kraków University of Agriculture, by the Institute of Nature Conservation, Polish Academy of Sciences, Kraków, and by INC PAS grants for young scientists and PhD students (to Dorota Kwaśna).
Author information
Authors and Affiliations
Contributions
T.A.Z. conceived the idea. T.A.Z., J.F., D.K. and M.N. designed the study. D.K., J.F., M.N., T.A.Z., A.M.Ć., P.A., W.B., L.K., and M.W. collected the data. D.K., A.M.Ć., J.F., M.N., P.A., W.B., L.K., M.W. and T.A.Z. analysed and interpreted the data. D.K., A.M.Ć., J.F. and M.N. visualised the data. D.K. and T.A.Z. wrote the main text of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kwaśna, D., Ćmiel, A.M., Florek, J. et al. Radiotelemetry reveals the dependence of inland tern breeding and foraging habitats on ADCP-identified sediment aggradation reaches in lowland rivers. Sci Rep 14, 18735 (2024). https://doi.org/10.1038/s41598-024-69723-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-69723-7