Abstract
This study assessed survival for lung cancer patients meeting criteria for the National Lung Cancer Screening Program in Korea launched in 2019 and updated guideline reported by the US Preventive Service Task Force (USPSTF). We assessed all-cause mortality based on the Korean Lung Cancer Registry (KLCR), including lung cancer patients diagnosed in 2014–2016. We compared survival among lung cancer patients eligible for extended USPSTF criteria (age 50–80 years and ≥ 20 pack-years) and those meeting current criteria (age 54–74 years and ≥ 30 pack-years, current or within the past 15 years). The nearest neighbour propensity-score matching was performed to generate a matched set. Kaplan–Meier curves were generated to compare survival among groups; differences in survival were analyzed using the stratified log-rank test. The mortality risk was estimated based on a Cox proportional hazards regression model and the robust standard error was calculated. Of 8110 patients, 37.4% and 24.3% met the extended USPSTF eligibility criteria and National Lung Cancer Screening Program (NLCSP) criteria, respectively. Overall mortality risk was not significantly different between the extended younger age group and the NLCSP group (hazard ratio [HR] [95% confidence interval (CI)]: 0.78 [0.59–1.02]). The extended older age group had a significantly higher mortality risk (HR [95% CI]: 1.41 [1.26–1.58]). Mortality risk was not significantly different between patients who smoked 20–29 pack-years and those who smoked ≥ 30 pack-years (HR [95% CI]: 0.90 [0.79–1.03]). Lung cancer patients aged 50–53 years and those with a 20–29 pack-years smoking history exhibited similar mortality risk to individuals meeting current criteria, while patients aged 75–80 years were at a higher risk of death. Although we verified similar or higher mortality risks in extended subgroups, a careful assessment of the benefits and harms of the screening tests is necessary when contemplating the extension of criteria.
Similar content being viewed by others
Introduction
Lung cancer is the second most common cancer and the leading cause of death worldwide1,2. The GLOBOCAN 2020 recorded 2,206,771 lung cancer cases in 2020, which accounted for 11.4% of all incident cancers1, and 1,796,144 lung cancer-related deaths, which comprised 18.0% of new mortalities due to all cancer types1. In South Korea, lung cancer is the second most common cancer (crude incidence rate: 58.4 per 100,000 persons) and the 5-year relative survival rate (34.7%) was the third lowest among the 10 most common cancers in 2015–20193. Furthermore, lung cancer was the leading cause of death (36.3 per 100,000 persons) among all cancers in 20204. The burden of lung cancer and public health interventions, such as the national screening program and tobacco control, have been discussed previously5,6. The lung cancer screening program involves low-dose computed tomography (LDCT)-based screening of high-risk groups and has been nationally and regionally implemented in some countries7,8,9,10. This program has efficiently reduced the burden of lung cancer and asserted the need for expanding the target population at risk of lung cancer11,12.
The National Lung Cancer Screening Program (NLCSP), launched in Korea in 2019, targets individuals at high risk of lung cancer who are: (1) aged 54–74 years and (2) either current smokers with a smoking history of ≥ 30 pack-years or former smokers who quit smoking within the past 15 years and had smoked ≥ 30 pack-years13. The eligibility criteria were developed based on evidence from the National Lung Screening Trial, which was a randomized clinical trial (RCT) that showed mortality benefits in the United States14. The feasibility of NLCSP implementation in Korea was established as a result of the Korean Lung Cancer Screening Project—a population-based nationwide prospective trial7,15.
The United States Preventive Services Task Force (USPSTF) recently expanded the eligibility criteria for the NLCSP to include individuals aged 50–80 years with a ≥ 20 pack-years smoking history and who currently smoke or have quit within the past 15 years16. Luo et al. reported that two-thirds of newly diagnosed patients with lung cancer did not meet the previous eligibility criteria and recommended expanding USPSTF criteria to include individuals 5 years younger than the original age cutoff and those who quit smoking more than 15 years ago17. The new criteria were implemented, based on the findings of an investigation of the risk–benefit of LDCT, for those with a high risk for lung cancer and were aimed at determining the optimal cutoff points for the age range and the number of smoking pack-years8. Accordingly, the current eligibility criteria for lung cancer screening tests in Korea should be evaluated to determine whether they appropriately include the population at high risk for lung cancer. However, to our knowledge, no previous study has directly compared overall survival according to the various eligibility criteria for lung cancer screening in Korea.
In this study, we aimed to ascertain the characteristics of patients who were diagnosed with lung cancer between 2014 and 2016 in relation to the eligibility criteria specified in the lung cancer screening program. We compared the survival of the patients with lung cancer who met the current NLCSP criteria with that of the following subgroups of the extended screening eligibility criteria: extended younger age group (age 50–53 years), extended older age group (age 75–80 years), and extended smoking pack-years group (20–29 pack-years).
Materials and methods
Data source
Data from the Korean Lung Cancer Registry (KLCR) were obtained from the Korea Central Cancer Registry (KCCR), which contains information on more than 95% of all newly diagnosed malignancies in Korea. The KCCR and the Lung Cancer Registration Committee conducted a retrospective sampling survey to create the KLCR database18 that includes 10% of all patients with lung cancer registered in the KCCR. These respondents were randomly selected from 39 general hospitals and 13 regional cancer centers that register more than 75% of patients with lung cancer in Korea; the sample size from each hospital was determined by the probability of selection that was ascertained according to the number of registrations. Patients were stratified by the date of diagnosis, sex, age, and their Surveillance, Epidemiology, and End Results program summary stage. The institutional review board of the National Cancer Center approved the study protocol (NCC 2022-0044) and waived the requirement for informed consent due to the retrospective nature of the study. This study was conducted in compliance with the Declaration of Helsinki.
Study population
The retrospective cohort study was designed to examine the survival of the patients with lung cancer who qualified for the NLCSP criteria and were categorized into subgroups based on the extended USPSTF criteria. We initially included patients with lung cancer who were registered in the KLCR between January 1, 2014, and December 31, 2016. All registered patients with lung cancer in the KLCR were followed up until December 31, 2020. After excluding patients with multiple primary cancers, 2621 patients in 2014, 2660 patients in 2015, and 2829 patients in 2016 were enrolled from the 52 centers. Of these 8110 patients, those with missing values for the diagnosis path (n = 132), clinical stage (n = 208), smoking status (n = 439), and index date (n = 579) were excluded. Patients who met the extended USPSTF criteria were selected and stratified according to the current criteria, extended age (50–53 and 75–80 years), and extended smoking pack-years (20–29 pack-years) groups. The smoking pack-years group consisted of both current smokers and former smokers who had quit within the last 15 years. To focus our analysis on the marginal survival associated with a single criterion and to provide a concise interpretation of the results, we excluded patients who met both the extended age and smoking history criteria (n = 132).
Selection of study variables
Baseline patient variables included age, sex, diagnosis path, smoking pack-years, clinical stage, and morphology of the tumor. We categorized age groups as 50–53, 54–74, and 75–80 years. The number of smoking pack-years was categorized as 20–29 or ≥ 30 years. The tumor stage was determined according to the pathologic types of lung cancer, such as non-small cell lung cancer (stage I–IV) and small cell lung cancer (limited and extensive stage), based on the American Joint Committee on Cancer 7th edition. Other variables, such as diagnosis path (with and without symptoms) and morphology (squamous cell carcinoma, adenocarcinoma, small cell carcinoma, non-small cell carcinoma, and others) were selected.
Study outcome
The primary outcome was overall survival which is defined as the time from the date of diagnosis to the date of death from any cause. Patients who were alive were defined as censored. The lung cancer caused death was defined as the secondary outcome of this study, and any other cause of death was regarded as a competing event.
Statistical analyses
The baseline characteristics were descriptively presented according to the measured variables. Continuous and categorical variables were expressed as the mean ± standard deviation and the frequency with percentage, respectively. The two-sample t-test and the chi-square test were employed to compare the distributions of continuous and categorical variables between groups, respectively. To assess survival difference between lung cancer patients eligible to the current criteria and those meeting the extended USPSTF criteria, we created three separate matched sets. Nearest neighbor propensity-score matching was performed to generate a matched set with a 1:1 ratio and caliper width of 0.1. For matching groups, we considered continuously measured variables such as smoking pack-years and age, as well as categorical variables including sex, tumor stage, and morphology. Meier curves were generated to compare survival among groups according to a matched subset of age and smoking pack-years. The difference in survival between groups in the matched subset was investigated based on the stratified log-rank test. The mortality risk was estimated based on a Cox proportional hazards regression model that included clustering subgroups (which comprised matched pairs), and the robust standard error was calculated. To verify the consistency of our findings, we applied a multivariable Cox proportional hazard model to estimate the risk of death while adjusting for the variables involved in the matching process. The level of statistical significance was set at p < 0.05. All statistical analyses and visualization procedures were performed using R software 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 8110 patients, we identified 3034 who met the extended USPSTF criteria (Fig. 1). These patients were categorized into the following three groups: patients who met the current criteria (n = 1978), extended age group (n = 586), and extended smoking pack-years group (n = 338). Patients who met both the extended age (50–53 and 75–80 years) and smoking history (20–29 pack-years) criteria (n = 132) were excluded from the study. Patients in these three groups were followed up until December 31, 2020, and their cause of death was investigated. The median follow-up duration was 1.17 (interquartile range: 0.42–4.08) years, and the median overall survival was 1.17 years (95% confidence interval [CI]: 1.08–1.25).
Table 1 shows the baseline characteristics of the study groups. The characteristics of patients in the current criteria group were compared to those of the extended age and extended smoking groups. The mean age and number of smoking pack-years among patients in the current criteria group were 65.7 years and 49.8 pack-years, respectively. The patients in the extended-age group were more likely to have symptomatic and advanced lung cancer than those who met the current screening criteria. Female were more prevalent in the extended pack-years group and more likely to have squamous histology. The results of the survival analysis for risk of all-cause death and lung cancer death using the Cox hazard regression model are presented in Supplementary Tables S4 and S5.
To balance the distribution of the characteristics, nearest neighbor propensity-score matching was performed that yielded 581 patient pairs for the age extension group (97 pairs for patients aged 50–53 years and 484 pairs for those aged 75–80 years) and 333 patient pairs for the extended pack-years group. Supplementary tables S1, S2, and S3 present well-balanced characteristics between matched pairs. Figure 2 shows the Kaplan–Meier curves and risk tables for comparing survival between the current and extended criteria groups after matching for each. Overall survival did not differ significantly between those in the younger age group and the current screening criteria groups (stratified log-rank test p = 0.1). However, patients in the older age group showed poorer survival compared with patients in the current screening criteria group (stratified log-rank test p < 0.001). In the set of matched pairs for the smoking groups, the survival of patients who smoked 20–29 pack years did not differ significantly from those who smoked ≥ 30 pack-years (stratified log-rank test p = 0.7).
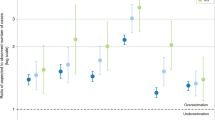
Figure 3 presents the forest plot for the median survival and the risk of death for groups in each matched set. In the matched set, the median survival time of patients in the extended younger age group (median survival time [95% CI]: 1.50 [1.17–2.25] years) was not significantly different from that in the current criteria group (median survival time [95% CI]: 1.17 [0.91–1.58] years); both groups had similar risks of death (hazard ratio [HR] [95% CI]: 0.78 [0.59–1.02]). In another matched set for the older age group, the median survival of the extended older age group (median survival time [95% CI]: 0.67 [0.58–0.75] years) was lower than that of the current criteria group (median survival time [95% CI]: 1.08 [0.92–1.33] years). The risk of death in the extended older age group (HR [95% CI]: 1.41 [1.26–1.58]) was significantly higher than that in the current criteria group. In the matched set for smoking pack-years, patients with 20–29 pack-years (median survival time [95% CI]: 1.41 [1.08–1.92] years) and those with ≥ 30 pack-years (median survival time [95% CI]: 1.25 [1.08–1.58] years) had similar median survival and risk of death (HR [95% CI]: 0.90 [0.79–1.03]). Additional comparison for the risk of both all-cause death and lung cancer death between the current criteria group and extended groups, analyzed using the multivariable cox proportional hazard regression model, showed consistent results (see Table 2 and Supplementary Table S6).
Discussion
This study investigated the characteristics of patients with lung cancer registered during 2014–2016 based on the eligibility criteria for the lung cancer screening program. The mortality risk among patients who met the current criteria was compared to that of patients included in the extended criteria group, in which the lower and upper age boundaries were extended to 50 and 80 years, respectively, and the lower limit of smoking pack-years was decreased to 20 pack-years. The study demonstrated that the patients in the extended younger age group (patients aged 50–53 years) and the extended smoking pack-year group (patients who smoked 20–29 pack-years) had a similar mortality risk as compared to those who met the current criteria. However, the extended older age group, which included patients aged 75–80 years, had a higher mortality risk than those in the current criteria group.
Previous studies have shown a reduction in lung cancer mortality due to the implementation of LDCT screening, which has been widely recommended for patients at high risk for lung cancer19. An RCT from the US showed that LDCT reduced the mortality rate by 20% due to the follow-up of the target population for 5 years20. The Dutch-Belgian Lung Cancer Screening trial confirmed that the implementation of the screening program reduced the mortality rate by 25% for individuals who were followed up for 10 years21. Furthermore, the United Kingdom Lung Cancer Screening trial revealed that the LDCT might reduce mortality by 16%22. A study in China reported that the implementation of the screening program was associated with reductions in lung cancer and all-cause mortality of 31.0% and 32.0%, respectively10.
Improved survival rates, observed in association with the implementation of the NLCSP, may be attributable to the shift from diagnosing late-stage to early-stage lung cancer. The USPSTF 2013 recommendation and the announcement of the Centers for Medicare & Medicaid Service in 2015 were associated with the increase of the proportion of stage I and the reduction in stage III and IV among non-small cell lung cancer patients in the US23,24. According to a study from Taiwan, the initiation of LDCT screening in 2015 accelerated the diagnostic shift of the lung cancer stage following health policies including smoking cessation and precision medicine25. The study also found that the reduction of mortality and survival improvement was prominent after the implementation of the health policy regarding LDCT25.
In Korea, following the announcement of the integration of LDCT into the lung cancer screening guidelines, pilot studies were conducted across multiple centers to evaluate the effectiveness and feasibility of implementing the NLCSP program7,15. The NLCSP in Korea increased the detection rates of early-stage lung cancer, while yielding a low false-positive rate, in compliance with the guidelines of the Lung Imaging Reporting and Data System26. Cost-effectiveness studies have shown benefits for individuals aged 54–74 who smoked ≥ 30 pack-years, while the impact of smoking cessation was not assessable27. Additionally, a short-term evaluation of the NLCSP implementation in Korea demonstrated an improvement in one-year survival rates28. Therefore, the initiation of the NLCSP may successfully benefit lung cancer patients by increasing the detection of curable-stage cases and reducing medical expenditures, while long-term evaluation of the program is still required.
However, the extension of eligibility criteria for screening should be considered to appropriately increase the number of potential beneficiaries. The current criteria included only 24.3% of patients with lung cancer from the KLCR, which comprised approximately 10% of patients with lung cancer registered in the KCCR during 2014–2016. In the US, one of the reasons for the expansion of the USPSTF criteria was that the initial criteria included only one-third of patients with lung cancer29. We confirmed that the extension of the criteria would increase the proportion of eligible patients with lung cancer for the screening test to 37.4%. As the KLCR includes systematically sampled lung cancer patients, and the distribution of characteristics between the KCCR and KLCR has been shown to be similar30, we can generalize the increase in the proportion of patients with lung cancer eligible for extended criteria to the wider population in Korea.
Our study showed that the patients who met the extended criteria for age and smoking pack-years had a similar or higher mortality risk as those who met the current criteria. Among those who smoked ≥ 30 pack-years, patients aged 50–53 years were likely to have a similar mortality risk as those aged 54–74 years. This is consistent with the finding that the mortality risk in the younger age group in the US did not differ from the mortality risk of those aged 54–74 years17. In addition, among those aged 54–74 years, patients who had ever smoked 20–29 pack-years had the same mortality risk as those who had ever smoked ≥ 30 pack-years. Previous studies have not only shown the positive correlation between the amount of smoking pack years and lung cancer death31, but also indicated that those who currently smoke 20–29 pack-years are at a similar risk of developing lung cancer as those smoking more32. Meanwhile, the distribution of tumor stages was similar between these groups, indicating that identifying lung cancer patients at a curable stage through screening could improve the survival of lung cancer patients in Korea. Therefore, it may be worth looking into reducing the cutoff point for smoking pack-years, as studies have reported that screening of patients with a ≥ 20 pack-year smoking history reduced the mortality risk10,24. However, further research is needed to comprehensively analyze the cost-effectiveness of lung cancer screening for individuals eligible under the extended criteria for younger age and smoke 20–29 pack-years, to determine whether expanding eligibility can provide significant clinical benefits while remaining economically sustainable17.
Lung cancer screening for older individuals at high risk of lung cancer remains a complex decision. Our study showed that the extended age group (75–80 years) had a higher mortality risk than the current eligible age population (54–74 years). However, older individuals typically have multiple risk behaviors and comorbidities that can affect their life expectancy, which naturally tends to be shorter than that of younger individuals33,34. Screening in the elderly should prioritize ensuring they live as long as expected without being harmed by unnecessary interventions such as LDCT. Therefore, the extension of the age criterion requires additional evidence to balance the benefits and harms of LDCT in terms of overdiagnosis risk and cost-effectiveness35. Future studies should evaluate the benefit of LDCT for those aged between 75 and 80 years before the upper age limit is extended for the national screening lung cancer test in Korea.
While smoking and older age are well-established and the most important risk factors for lung cancer8, our study revealed that female were largely excluded from the eligible screening criteria due to their smoking status and age. Recent studies have reported that the lung cancer incidence rate in Korean female has steadily increased for a decade36,37. In our study, 29.2% of all patients with lung cancer in the KLCR were female. However, less than 8% met either the current or extended criteria. This could be a result of a low prevalence of smoking and its intensity in female compared to male20,38, and a sex difference in susceptibilities to lung cancer in terms of exposure to smoking39. The prevalence of smoking in female with lung cancer is less than 20% in Asian regions40,41, whereas 70–85% of female with lung cancer in Western populations, including North America, northern Europe, and Australia/New Zealand, were reported to be smokers42. In our data, we also confirmed that the 87% of female lung cancer patients had never smoked and were thus largely excluded from the NSCLP eligibility criteria. Similar to the USPSTF 2013 guidelines, but current NSCLP eligibility criteria were even more likely to include male than female43, highlighting a potential sex disparity in the benefit from the NSCLP that should be addressed when considering the extension of eligibility criteria.
The screening criteria for lung cancer should be considered from multiple perspectives, including tumor characteristics and potential overdiagnosis, to balance the benefit between males and females. Choi et al. reported that 74.8% of female with lung cancer were more likely to develop primary adenocarcinoma, compared to 38.0% of male with primary adenocarcinoma, and that EGFR mutation was more frequently detected among female with stage IV lung cancer30. It is well-known that the prevalence of epidermal growth factor receptor (EGFR) mutation is higher in Asian females than in Western populations44,45. Since EGFR mutation is well-established oncogenic driver in NSCLC, investigating family history along with the detection of the EGFR mutation for both males and females at high risk of lung cancer would not only contribute to the early diagnosis of lung cancer but also determining the effective treatment for these patients46. Meanwhile, Goo et al. recently discussed the potential overdiagnosis of CT screening among female with lung cancer patients at early stage in that the incidence of lung cancer for female at early stage has been increasing while the incidence in female at late stage and male at both early and late stage remained similar since 200637. Therefore, further research is needed to appropriately extend the criteria for lung cancer screening, balancing the risk of lung cancer among male and female, while comprehensively exploring risk factors related to lung cancer development in female beyond age and smoking status. Additionally, such research should carefully consider overdiagnosis issues, particularly in female with early-stage lung cancer, to ensure that expanded screening criteria do not lead to unnecessary diagnosis and treatment.
Our study had some limitations. First, this study compared survival in patients with lung cancer based on age and smoking history, in accordance with the extended guideline for lung cancer screening, and there is a limit to examining the benefits and harms of screening. Nevertheless, our findings indicated a specific high-risk subgroup, which provides evidence to facilitate decisions for extending the lung cancer screening eligibility criteria. Second, as this was an observational study, we could not collect information on some confounders that could have affected patient survival, such as family history of cancer including lung cancer, details of treatment, other comorbidities, molecular characteristics, environment exposure, and secondhand smoking37. Third, the scarcity of females in our study population and the heterogeneity in prognosis and treatment between NSCLC and SCLC may have potentially affected the stability of our regression models. To address this imbalance, we evaluated the balance of propensity scores between groups and confirmed that the standardized mean difference among variables was within 0.1. Fourth, our study did not account for the lung cancer treatments received during follow-up due to dataset limitations. While we focused on controlling for baseline characteristics to compare groups, future studies should investigate the impact of time-varying treatments on survival, stratifying by tumor characteristics such as SCLC and NSCLC. Fifth, the follow-up period was not consistent for all patients. We included lung cancer patients diagnosed from 2014 to 2016 and followed them until 2020; consequently, patients diagnosed in 2016 had a follow-up duration of less than five years, contributing to a shorter median follow-up time. Lastly, our findings did not provide sufficient evidence to support the extension of the eligibility criteria. The decision for expanding the criteria would need to be based not only on a high mortality rate but also on other indicators such as the number of patients that are needed to be screened to prevent one death due to lung cancer and gain one life-year, as well as the number of patients that are needed to be treated to prevent one death due to lung cancer. Changing risk factor distribution in lung cancer patient also should be considered in depth. Nevertheless, our study had several strengths in that we used representative national lung cancer sampling data from the KCCR, and we could confirm all-cause mortality of lung cancer patients from death certificates provided by Statistics Korea.
The current NLSCP eligibility criteria in Korea only encompass a quarter of lung cancer patients. Our findings suggest that patients with lung cancer who have a smoking history of over 20 pack years or are 4 years younger than the age cutoff in the NLCSP exhibit similar risk of death to those meeting the current NLCSP criteria. Expanding the NLCSP criteria to include these subgroups could facilitate earlier detection of lung cancer and potentially improve outcomes for these individuals. While this study also reported a significant increase in the risk of death among the 75–80-year-old population, cost-effectiveness considerations should be warranted when deciding whether to include this age group in the target population for lung cancer screening. Careful consideration of optimal screening criteria is crucial to guide decision-making. The current eligibility criteria are unlikely to capture the high-risk individuals who would benefit most from screening. In the future, more sophisticated screening programs integrating low-dose CT and biomarkers could be developed to better identify high-risk individuals who would derive the greatest benefit from screening.
Data availability
The dataset supporting the conclusions of this article is available in the Korea Central Cancer Registry. (https://kccrsurvey.cancer.go.kr/index.do).
Abbreviations
- LDCT:
-
Low-dose computed tomography
- NLCSP:
-
National Lung Cancer Screening Program
- RCT:
-
Randomized clinical trial
- USPSTF:
-
United States Preventive Services Task Force
- KLCR:
-
Korean Lung Cancer Registry
- KCCR:
-
Korea Central Cancer Registry
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Fitzmaurice, C. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 2006 to 2016: A systematic analysis for the Global Burden of Disease study. J. Clin. Oncol. 36, 1553–1568 (2018).
Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2019 [accessed Sep 5, 2022]. http://ncc.re.kr/main.ncc?uri=english/sub04_Statistics.
Korean Statistical Information Service. Frequency and rate of mortality by cause of death (236 factors), sex, and age; 2022 [accessed Mar 11, 2022]. https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B34E07&conn_path=I2.
Deng, Y. et al. Epidemiological trends of tracheal, bronchus, and lung cancer at the global, regional, and national levels: A population-based study. J. Hematol. Oncol. 13, 98 (2020).
Park, J. & Look, K. A. Health care expenditure burden of cancer care in the United States. Inquiry 56, 0046958019880696 (2019).
Lee, J. et al. Development of protocol for Korean Lung Cancer Screening Project (K-LUCAS) to evaluate effectiveness and feasibility to implement national cancer screening program. Cancer Res. Treat. 51, 1285–1294 (2019).
Krist, A. H. et al. Screening for lung cancer: US preventive services task force recommendation statement. JAMA 325, 962–970 (2021).
Yang, D., Liu, Y., Bai, C., Wang, X. & Powell, C. A. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett. 468, 82–87 (2020).
Li, N. et al. One-off low-dose CT for lung cancer screening in China: A multicentre, population-based, prospective cohort study. Lancet Respir. Med. 10, 378–391 (2022).
Tanoue, L. T., Tanner, N. T., Gould, M. K. & Silvestri, G. A. Lung cancer screening. Am. J. Respir. Crit. Care Med. 191, 19–33 (2015).
Pastorino, U. et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann. Oncol. 30, 1162–1169 (2019).
Kim, H. Y. National lung cancer screening in Korea: introduction and imaging quality control. J. Korean Soc. Radiol. 80, 826–836 (2019).
Jang, S. H. et al. The Korean guideline for lung cancer screening. J. Korean Med. Assoc. 58, 291–301 (2015).
Lee, J. et al. Feasibility of implementing a national lung cancer screening program: interim results from the Korean Lung Cancer Screening Project (K-LUCAS). Transl. Lung. Cancer Res. 10, 723–736 (2021).
Potter, A. L., Bajaj, S. S. & Yang, C. J. The 2021 USPSTF lung cancer screening guidelines: A new frontier. Lancet Respir. Med. 9, 689–691 (2021).
Luo, Y. H. et al. 5-year overall survival in patients with lung cancer eligible or ineligible for screening according to US Preventive Services Task Force criteria: A prospective, observational cohort study. Lancet Oncol. 20, 1098–1108 (2019).
Lim, J. U. et al. Characteristics of female lung cancer in Korea: Analysis of Korean National Lung Cancer Registry. J. Thorac. Dis. 12, 4612–4622 (2020).
Detterbeck, F. C., Mazzone, P. J., Naidich, D. P. & Bach, P. B. Screening for lung cancer: diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143, e78S-92S (2013).
The National Lung Screening Trial Research Team, Aberle, D. R., Adams, A. M., Berg, C. D., Black, W. C., Clapp, J.D. et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 365: 395–409 (2011).
van Iersel, C. A. et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (Nelson): Risk-based selection from the general population in a lung cancer screening trial. Int. J. Cancer 120, 868–874 (2007).
Field, J. K. et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg. Health Eur. 10, 100179 (2021).
Potter, A. L. et al. Association of computed tomography screening with lung cancer stage shift and survival in the United States: quasi-experimental study. BMJ 367, e069008 (2022).
Khouzam, M. S. et al. Impact of federal lung cancer screening policy on the incidence of early-stage lung cancer. Ann. Thorac. Surg. 115, 827–833 (2023).
Yang, C. et al. Stage shift improves lung cancer survival: Real-world evidence. J. Thorac. Oncol. 18, 47–56 (2023).
Kim, Y. Evidence of national lung cancer screening program in Korea. Korean J. Health Promot. 19, 161–165 (2019).
Kim, J. et al. Cost utility analysis of a pilot study for the Korean lung cancer screening project. Cancer Res. Treat. 54, 728–736 (2022).
Kim, W., Lee, S. C., Lee, W. R. & Chun, S. The effect of the introduction of the national lung cancer screening program on short-term mortality in Korea. Lung Cancer. 186, 107412 (2023).
Yang, P. et al. Trends in subpopulations at high risk for lung cancer. J. Thorac. Oncol. 11, 194–202 (2016).
Choi, C. et al. Report of the Korean association of lung cancer registry (KALC-R), 2014. Cancer Res. Treat. 51, 1400–1410 (2019).
Ai, F., Zhao, J., Yang, W. & Wan, X. Dose–response relationship between active smoking and lung cancer mortality/prevalence in the Chinese population: A meta-analysis. BMC Public Health 23, 1–8 (2023).
Pinsky, P. F., Lau, Y. K. & Doubeni, C. A. Potential disparities by sex and race or ethnicity in lung cancer screening eligibility rates. Chest 160, 341–350 (2021).
Fabrikant, M. S., Wisnivesky, J. P., Marron, T., Taioli, E. & Veluswamy, R. R. Benefits and challenges of lung cancer screening in older adults. Clin. Ther. 40, 526–534 (2018).
Howard, D. H., Richards, T. B., Bach, P. B., Kegler, M. C. & Berg, C. J. Comorbidities, smoking status, and life expectancy among individuals eligible for lung cancer screening. Cancer 121, 4341–4347 (2015).
Han, S. S. et al. The impact of overdiagnosis on the selection of efficient lung cancer screening strategies: overdiagnosis and lung cancer screening. Int. J. Cancer 140, 2436–2443 (2017).
Lee, J. G., Kim, H. C. & Choi, C. M. Recent trends of lung cancer in Korea. Tuberc. Respir. Dis. 84, 89–95 (2021).
Goo, J. M., Jung, K. W., Kim, H. Y. & Kim, Y. Potential overdiagnosis with CT lung cancer screening in Taiwanese female: status in South Korea. Korean J. Radiol. 23, 571–573 (2022).
Chang, Y., Kang, H. Y., Lim, D., Cho, H. J. & Khang, Y. H. Long-term trends in smoking prevalence and its socioeconomic inequalities in Korea, 1992–2016. Int. J. Equity Health 18, 148 (2019).
Park, B., Kim, Y., Lee, J., Lee, N. & Jang, S. H. Sex difference and smoking effect of lung cancer incidence in Asian population. Cancers 13, 113 (2020).
Cho, J. et al. Proportion and clinical features of never-smokers with non-small cell lung cancer. Chin. J. Cancer 36(1), 20 (2017).
Zeng, Q. et al. Tobacco smoking and trends in histological subtypes of female lung cancer at the Cancer Hospital of the Chinese Academy of Medical Sciences over 13 years. Thorac. Cancer 10, 1717–1724 (2019).
Parkin, D. M. et al. Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 (2005).
Ritzwoller, D. P. et al. Evaluation of population-level changes associated with the 2021 US Preventive Services Task Force lung cancer screening recommendations in community-based health care systems. JAMA Netw. Open 4, e2128176 (2021).
Shi, Y. et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J. Thorac. Oncol. 9, 154–162 (2014).
Dearden, S. et al. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap). Ann. Oncol. 24, 2371–2376 (2013).
Cheng, P. C. & Cheng, Y. C. Correlation between familial cancer history and epidermal growth factor receptor mutations in Taiwanese never smokers with non-small cell lung cancer: a case-control study. J. Thorac. Dis. 7, 281–287 (2015).
Funding
This study was funded by the Grant-in-Aid for Cancer Research and Control of the National Cancer Center, Republic of Korea [Grant #2210791].
Author information
Authors and Affiliations
Contributions
S.L.: conceptualization, methodology, investigation, data curation, formal analysis, visualization, writing—original draft E.H.P.: conceptualization, methodology, investigation, data curation, writing—original draft B.Y.J.: conceptualization, investigation, data curation, writing—review and editing Y.J.K.: conceptualization, investigation, data curation, writing—review and editing K.W.J.: resources, writing—review and editing; H.S.C.: conceptualization, project administration, writing—review and editing, supervision K.S.C.: conceptualization, methodology, project administration, funding acquisition, writing—review and editing, supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, S., Park, E.H., Jang, B.Y. et al. Survival of lung cancer patients according to screening eligibility using Korean Lung Cancer Registry 2014–2016. Sci Rep 14, 22585 (2024). https://doi.org/10.1038/s41598-024-69994-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69994-0