Abstract
The present work deals with developing a method for revalorizing steel residues to create sunlight-active photocatalysts based on iron oxides. Commercial-grade steel leftovers are oxidized under different combinations of pH and temperature (50–90 °C and 3 ≥ pH ≤ 5) in a low energy-intensive setup. The material with the highest production efficiency (yield > 12%) and magnetic susceptibility (χm = 387 × 10−6 m3/kg) was further explored and modified by diffusion of M2+ (Zn and Co) ions within the structure of the oxide using a hydrothermal method to create ZnFe2O4, CoFe2O4 and combined Co–Zn ferrite. (Co–Zn)Fe2O4 displayed a bandgap of 2.02 eV and can be activated under sunlight irradiation. Electron microscopy studies show that (Co–Zn)Fe2O4 consists of particles with diameters between 400 and 700 nm, homogeneous size, even distribution, and good dispersibility. Application of the developed materials in the sunlight catalysis of black liquors from cellulose extraction resulted in a reduction of the Chemical Oxygen Demand (− 15% on average) and an enhancement in biodegradability (> 0.57 BOD/COD) after 180 min of reaction. Since the presented process employs direct solar light, it opens the possibility to large-scale water treatment and chemical upgrading applications.
Similar content being viewed by others
Introduction
In recent years, there has been a necessity for new materials and manufacturing methods due to overexploitation, depletion, and speculation over traditional commodities. New bottom-up materials are being explored daily, but there is also a trend for repurposing resources by chemical upgrading1,2. Waste material’s composition and properties can be modified to reach new economic value in a process commonly known as revalorization3,4. For example, iron is a significant player in the metal mechanic industry and is the most used metal globally, either as freshly extracted ores or through recycling. Worldwide, iron is massively produced and discarded by industries and households. As it rapidly oxidizes, it loses its original mechanical and chemical properties, triggering a growing recycling industry that indirectly contributes to a circular economy approach5.
During corrosion of metallic iron, Fe0 transforms to Fe2+O that undergoes further oxidation to Fe3+2O3 in the presence of air, whereas in aqueous systems, multiple and combined Fe2+/Fe3+ hydroxides are formed6,7,8. These compounds usually have low economic value yet have many applications. They are easy to separate and purify and are a cheap source of precursors for specialty-grade steel and iron derivates. Even though some iron oxides can be formed naturally, some sophisticated phases require specific pH, temperatures, and precursor ratios. However, new green chemistry approaches can reach these conditions without utilizing hazardous materials and at a low energy cost9,10.
Among all industrially relevant iron oxides, ferrites are commonly used in electronics and machinery, with an expected market value of 1,865 million USD by 2028 and an annual growth of 1.6%. These materials are oxides composed of Fe2+ and Fe3+ ions with an M2+M3+2O4 formula from an equimolar mixture of FeO and Fe2O3. Ferrites regularly exist in cubic and hexagonal crystal structures, and in the case of cubic ferrites, there are two sub-structures: garnet and spinel. In contrast, hexagonal ferrites have a wider set of structures in which metal and oxygen stoichiometry vary. The principal backbone of ferrites is a close-packed structure of oxygen anions, whereas the metallic cations fill the interstices of the oxygen lattice. They possess moderate values of magnetization, moderate to high permittivity, and low losses at frequencies in submillimeter wavelengths11.
Due to the simplicity and flexibility of cubic spinel, ferrites have gained much more relevance for industrial applications, including magnetic recording, drug delivery systems, and chemical catalysts12,13,14. The spinel structure is expressed as AB2O4 where A is divalent cations occupying four tetrahedral lattice sites, and B has six cubical sites12. Derivative compounds can be formed by substituting Fe2+ and Fe3+ with other transition divalent or trivalent ions to increase their physical, magnetic, or catalytic properties. Ferrites also have a semiconductor nature that allows them to be used as photocatalysts activated by visible light since their bandgaps range from 1.9 to 2.9 eV depending on the composition, particle size, and crystallinity15.
The most common semiconductors are transition metal oxides16 and light-sensitive organic or organometallic compounds. However, a robust, efficient, and non-expensive semiconductor is needed to treat large volumes and highly concentrated effluents. From this point of view, mixtures of iron oxides are an alternative available elsewhere with low toxicity and cost17. Novel degradation processes for pollutants are explored, including nanoengineered photocatalysts. Ti, Cr, Mn, Fe, Co, Ni, Cu, and Zn oxides have been used to treat pollutants because they react under the irradiation of photons within the visible region of the electromagnetic spectrum18. In this way, the M2+Fe3+2O4 composite is an opportunity to research the behavior of new particles from steel waste.
In water treatment technology, high concentration, low biodegradability, and slow kinetics represent challenges to be addressed. Among the most concerning effluents in industry today are the Black Liquors (BLq), produced during paper fabrication and cellulose recovery, which have gained attention as a model recalcitrant pollutant.
BLq is typically composed of water (80–85%), organic (5–10%), and inorganic solids (10–15% w/w). The organic fraction is mainly lignin (20–40%), hemicellulose (50–70%), and multiple secondary metabolites (< 10% w/w)19. Due to its complex composition and high concentration (COD: 6000–12,000 mg L−1), BLq is a dangerous waste and an environmental and health risk. In addition, using hydroxides to treat vegetal tissues during cellulose extraction results in highly alkaline environments20 (pH > 12).
BLq cannot be discarded in municipal sewage and has a very low biodegradability (BOD5/COD < 0.4), limiting biological treatment21,22. Hence, different solutions are being explored to treat these effluents. Chemical oxidation is possible to a certain extent using strong oxidizers such as chlorine dioxide (ClO2) and permanganate (KMnO4), but efficiency is low, and the reagents may be harmful23,24,25. New sustainable approaches in BLq treatment seek to eliminate water to recover bioactive compounds26. However, energy duties are prohibitive if traditional heat transfers are utilized, and in the case of solar thermal energy, extended evaporation time and specialized equipment are needed27. These processes seem promising, but the treatment capacity is limited, and BLq streams in a typical Kraft process car reach 7 tons of BLq (15% solids) per ton of wood pulp28,29. As an alternative, biological treatments have been proposed, but first, it is necessary to increase its biodegradability through preparative chemical oxidations. Advanced Oxidation Processes (AOPs) have been applied to these effluents, the Fenton reaction and UV-powered TiO2 catalysis being the most explored methods30,31. In the Fenton reaction, Fe(II) reacts with hydrogen peroxide in an exothermic reaction that produces highly-oxidant hydroxyl radical (·OH, E0 = 2.80 V)32. Recent AOPs can produce a set of additional radical oxidants such as ·OH, O2·− and ·OOH; these molecules are produced by the decay of semi-stable precursors as peroxides if light, heat, or electrochemical requirements are met (Eqs. 1–3)33,34:
AOPs based on semiconductors rely on the formation of excitons (e−/h+ pair) at the material surface upon excitation of ground state electrons (e−*) in the Valence band (eVB−) by the addition of external energy such as photons (Eq. 4). Moreover, the formation of excitons in aqueous environments can produce either reducing or oxidizing radicals (Eqs. 5 and 6) that accelerate the degradation of organic molecules through catalytic oxidation and reduction processes35.
In the present work, we are producing a set of paramagnetic Fe2+ and Fe3+ oxides from residual steel, which have been upgraded with other metals (Co2+ and Zn2+) to create composites that are applied as photocatalysts in the treatment black liquor process. M2+Fe3+2O4 is expected to degrade complex structures by breaking down long organic strings, making the final product more biodegradable.
Materials and methods
Reagents
All reagents were purchased from Sigma-Aldrich and used as received. Co(NO3)2·6H2O, Ni(NO3)2·6H2O and Zn(NO3)2·6H2O, Na2SO4 and isopropanol were used as reagent grade. The HCl and NaOH utilized were provided by JT Baker, and ethanol and distilled water were used as local distributors supplied solvents.
Instruments
UV–Vis/DRS absorption studies were performed in a HACH DR6000 spectrophotometer. Dynamic Light Scattering (DLS) was completed in a Malvern Zetasizer ZSU3100, and Mass magnetic susceptibility was determined in an MSB MK1 Sherwood balance. Transmission Electron Microscopy was performed in a JEOL 2010 (200 kV) using C-coated copper grids. A JEOL JSM 5900 Scanning Electron Microscopy (20 kV) equipped with an Oxford AZtec Energy Dispersive X-Ray Spectrometry was utilized to study morphology. The X-ray diffraction studies were obtained using a Bruker D8 Advance equipped with a Cu Ka = 0.154 nm radiation source. COD was measured through the dichromate modification of the EPA 410.4 method with HI94754C-25 kits for high ranges (0 to 15,000 mg/L). BOD5 was determined using the Standard 5210B method (APHA) with a HACH BOD Trak II kit.
Steel residues obtention
Steel residues were obtained from the Department of Mechanical Engineering at Tecnológico de Monterrey Campus Puebla. The residues came from various metal-industry processes and were mainly composed of steel alloys. Metal precursors were obtained by sieving and recovering shavings (d < 3.0 cm); a subsequent washing with Extran detergent (1.0% v/v) removed oil and dust, and the obtained fragments were dried at room conditions.
Iron oxides preparation
Different treatments were performed to remove iron oxides (FexOy) from recovered steel in full aqueous environments and under different pH and temperatures (Table 1). The method is based on the controlled oxidation of metallic iron previously reported by Hsu36. All reactions utilized NaOH or HCl for pH adjustments, and the process took place under standard STP conditions. In a standard experiment, a certain amount of iron scraps was dispersed in water (1:100 m/v) under constant stirring (600 rpm) for 20 h; at the end of the reaction, magnetic material was recovered with a 180 N electromagnet. The obtained dark magnetic powders were washed with water, dried in an oven (36 °C), and stored in dry and dark conditions.
Mixed iron oxides preparation
The modification of selected iron oxides with divalent metallic ions was achieved by adding different M2+ (Co and Zn) nitrate salts to an aqueous dispersion of prepared iron oxides, followed by a hydrothermal treatment. In 50 mL of distilled water, 1.0 mmol (231 mg) of FeOE with theoretical composition Fe3O4 (FeO + Fe2O3) was dispersed, and the amount of metal nitrate was adjusted according to Eqs. 7–9:
This mixture added 5 mL of ethylene glycol while pH adjusted to 6 and heated for 20 h at 180 °C in a Teflon-lined 80 mL autoclave. Samples were labeled as ZnFe2O4 or CoFe2O4, depending on the modifying agent. After the reaction, the supernatant was discarded, and a 100 T magnet was used to sort the recovered magnetic materials and washed with ethanol. The obtained powders were finally dried overnight at 36 °C in an oven and stored in dry, dark condition.
Black liquor treatment studies
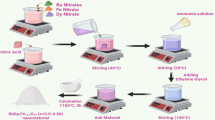
Black liquor (BLq) from alkaline hydrolysis of Ananas comosus leaves for cellulose production was employed as a model pollutant. The physical–chemical characterization of raw BLq is presented in Table S1. We studied the effect of different photocatalytic systems in the degradation of BLq by measuring Chemical Oxygen Demand (COD) and 5-day Biological Oxygen Demand (BOD). The biodegradability index (BI) was also determined based on the BOD/COD ratio, where it is typically established that values above 0.5 correspond to degradable substrates that can be treated by established biological methods37. In a typical experiment, 200 mL of a reaction mixture consisting of a 1:2 BLq/H2O volumetric ratio was prepared in a 250 mL round bottom flask, and a certain amount of the selected catalysts was added to this blend. Samples were stirred for 30 min without light to quantify the adsorption of the catalyst surface. The reaction mixture was then placed at the focal point of a sunlight Cylindrical-Parabolic Collector (CPC) positioned in accordance with the geographical location (19.017669510342138, − 98.24227379261022). 24 V peristaltic pumps were used to recirculate the mixture to ensure constant mixing and air diffusion, including the entire setup, was powered by a 4 Panel 270W Photovoltaic array (Fig. 1). Samples (2.0 mL) were taken at regular time intervals, centrifuged (3000 rpm, 5 min), and analyzed for COD and BOD quantification. Total reaction time was 360 min and, in all cases, reactions were only reported and considered valid if sunlight irradiation was above 120 kLux during at least 80% of the reaction time.
Photocatalyst effect over BLq treatment
As a starting point, changes in the composition of BLq were monitored using the Fenton (Fe2+/H2O2) reaction as a reference, including H2O2, Fe2+, and pure sunlight irradiation controls. Hydrogen peroxide concentration was set to 0.5 mg/L, and Fe2+ (as FeSO4) loading was 0.75 mg/L, within ranges typically utilized in organic pollutants degradations and previously reported38. The amount of iron oxide catalysts varied from 0.25 to 0.75 and to 1.5 mg/L in order to study the effect of catalyst loadings (Table S3).
Role of e− and h+ in photocatalytic treatment of BLq
To understand the semiconductor properties of prepared catalysts, the photocatalytic process was repeated in the presence of isopropanol as an h+ scavenger and AgNO3 as e− scavenger. These reagents have been reported previously in the study of the catalytic mechanism of ferrites39,40. In a typical experiment, studied catalysts (0.75 mg/L) were dispersed in a BLq solution containing 1500 ppm of the selected scavenger (Table S4).
Magnetic recovery and reusability of catalysts
The recoverability and reusability of the developed mixed iron catalysts were assessed by measuring the efficiency in treating BLq upon successive catalytic cycles. Initially, Mass magnetic susceptibility for fresh catalysts and the recoverability of the catalysts (0.75 mg/L) was determined in distilled water (200 mL) as a control. A typical photocatalytic experiment was carried out in the following stage using 0.75 mg/L of the catalysts in 200 mL of BLq solution. After 360 min of reaction, catalysts were magnetically recovered and dried in an oven (80 °C, 12 h). The mass of recovered catalysts was determined, and a new photocatalytic cycle was carried out, adjusting the volume of the solution to maintain catalyst concentration to 0.75 mg/L without altering BLq concentration. The whole process was repeated three times and the efficiency of the treatment and recovered catalyst was recorded.
Results and discussion
Metal residues characterization
The average composition of residual steel (Fig. S1) utilized as a precursor was monitored for 60 with a total average flow of 2.9 ± 0.6 kg/day. Composition by weight was AISI 1045 (3% ± 0.2), AISI 1018(5% ± 1.2), AISI D2 (2% ± 0.1) and AISI A36 (90% ± 6.0).
Metal oxide formation
The applied chemical treatments yield different products, and the magnetic recovery was utilized as the key parameter to determine production efficiency (denoted as magnetic yield, ym), which was determined according to Eq. 10:
mm corresponds to magnetically recovered dry mass, and mr corresponds to the system's initial steel mass. FeOA and FeOE had the higher production; in the case of FeOA (pH = 3; T = 60 °C), a dark brown powder with ym11.25% and χm = 468 × 10−6 m3/kg was obtained (Fig. 2a). Under this strong acidic environment, freshly formed Fe2+ ions are stabilized, and the oxidation rate decreases, allowing the formation of a stable mixture between Fe2+ and Fe3+ oxides that could be related to magnetite (Fig. 2b). This hypothesis is further confirmed by the high χm values similar to those obtained for Fe3O441.
The other reactions at 60 °C, pH = 5 (FeOB), had a negligible yield (ym = 0.22%), with no noticeable presence of any other residue or dispersion (Fig. S2). For pH = 7 (FeOC), a diamagnetic yellowish suspension and an orange precipitate were formed. Given the conditions, its composition is associated with different iron (II) and (III) hydroxides42,43. There was also the presence of magnetic oxides (ym = 6.27%); however, its magnetic susceptibility was relatively small (χm = 6 × 10−6 m3/kg), suggesting hydroxides adsorbed on the surface of maghemite.
For FeOE (pH = 5, T = 90 °C), a dark brown compound was obtained with ym = 12.56% and a magnetic susceptibility (χm = 387 × 10−6 m3/kg) similar to that of maghemite (γ-Fe2O3, χm = 400 × 10−6 m3/kg)44. No other precipitates were observed in this system, implying an ordered oxidation process in which all formed oxides are magnetically recovered. For the other systems at 90 °C, pH = 3 (FeOD) resulted in a mixture between a black diamagnetic precipitate and a dark brown paramagnetic powder (ym = 8.25, χm = 228 × 10−6 m3/kg) identified as a mixture of Fe3O4 and γ-Fe2O3. For pH 7, ym decreased to 6.49% with a magnetic susceptibility of 243 × 10−6 m3/kg.
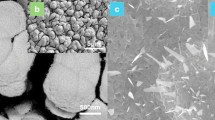
Based on the potential reusability and higher yields, FeOA and FeOE were further characterized by Scanning Electron Microscope (Fig. 3). Analyses reveal the presence of small particles (d < 100 nm) grouped in micrometric agglomerates. Even though agglomerations are easily dispersed in most aqueous solvents, when dried, the catalysts agglomerate, given its observed ferromagnetism and remanent magnetization45,46.
Energy Dispersive X-Ray diffraction of FeOA and FeOE show structural similarities between both materials with a peak pattern similar to that of cubic spinels, particularly magnetite (JCPDS 11-614) and major signals at 30.25° [220]; 35.61° [311], 43.29° [400], 53.91° [422], 57.32° [511] and 63.00° [440] (Fig. 4a). From the Bragg equation, the crystallite size was determined to be 27.24 nm for FeOA and 24.21 nm for FeOE. In the case of FeOA, an additional signal at 46.2° is identified as [110] austenite (JCPDS 31-0619), and its presence can be explained by steel leftovers within the ferrite structure47. Chemical composition from EDS reveals the presence of metallic impurities derived from the different types of steel utilized (Fig. 4b). The main components of FeOA and FeOE were Fe (66.6 and 71.21%) and O (32.12 and 28.83%), which agree in metal–oxygen ratio with theoretical values of Fe3O4 (Fe = 72.35% and O = 27.64%) and γ-Fe2O3 (Fe = 69.94% and O = 30.06%)48,49. As the utilized precursor was a mixture of different steel residues, metallic leftovers were the main impurities. Al was the major secondary component (2.32% and 2.54% for FeOA and FeOE), followed by Mg (1.04 and 1.52%). Other minor components were Si (1.25 and 0.2%), presumably from glassware and silicates present in water; Na, K, and Ca were also found in small amounts (less than 1.0%), and their presence is associated with salts naturally present in distilled water.
DLS characterization of selected materials showed good dispersibility with an average diameter of 485 nm for FeOA and 570 nm for FeOE (Fig. S3a); the materials have a uniform distribution, and no secondary peaks were observed, discarding micrometric agglomerations, suggesting uniformity in the size of material flocs. The charge of the particles is positive as they were prepared under acidic conditions (FeOA zmax = 28.8 mV, whereas for FeOE, zmax = 1.87 mV), and it has been reported to be a direct relationship between Z potential and pH for magnetite nanoparticles, being this value shifted towards negative values as pH decreases (Fig. S3b)50,51. Nevertheless, this trend is not absolute as different steel revalorizing methods could yield iron oxides with different stabilities, as has been reported by Borth et al.52 where controlled oxidation of steel followed by an alkaline washing and a subsequent thermal method yield a z potential near 40 mV at pH 7. Visible light excitation exists in both materials, and the energy-direct band gap was 2.10 eV for FeOA and 1.873 eV for FeOE (Fig. S3c). This corresponds to a region between 590 and 660 nm in the transition region between Vis and near-infrared, which would allow thermal excitation. The light response characterization shows that prepared iron oxides can effectively harvest visible light, and their values are closer to those of standard magnetite hydrothermally produced53. Moreover, similar values (2.2 eV) have been reported for iron oxides recovered by steel waste52.
Co and Zn modified metal oxides
FeOE oxides were utilized as precursors for a modification consisting of Co and Zn in addition to iron oxides by metallic ion substitution under hydrothermal conditions. DLS characterization in Fig. 5a shows that ZnFe2O4 has diameters around 550 nm (30%) with a maximum size below 1000 nm. In the case of CoFe2O4, two groups of particles are observed; first, there is a material finely dispersed with an average size of 250 nm (11%) and a narrow distribution with a minimum of 120 and a maximum of 530 nm. There is also the existence of agglomerations with a varied size ranging from 600 nm to < 5 mm with a higher population around 2000 nm (4.8%). The observed size distribution suggests that the agglomeration is formed by individual particles observed below 500 nm. This assumption is supported by the Z potential data, where only one set of charged particles is observed (zmax = -9 mV). In the case of ZnFe2O4, zmax = − 11 mV with a minor set of particles with z = 25 mV. For CoFe2O4 (Fig. 5b), It is evident that the hydrothermal process allows the reconfiguration by recrystallizing iron oxides. In the case of Co oxides, the size of individual particles decreased compared to parent FeOE, and a new set of agglomerations appeared, which would suggest changes in its magnetic properties as Co is a ferromagnetic metal and gives origin to soft magnetic materials as it has been reported for cobalt ferrites54. Also, the z potential of materials shows changes in composition as the parent material had positive values like those reported for maghemite prepared under acidic conditions (zmax > 10 mV)55. After the hydrothermal process in the presence of M2+ ions, values switched to negatively similar to those reported for magnetite, Zn, and Co ferrites under similar synthetic conditions (zmax from − 6 to − 15 mV)56. The activation energy determined through bandgap determination is presented in Fig. 5c. ZnFe2O4 requires 2.73 eV, whereas CoFe2O4 requires 1.85 eV. Comparable to parent iron oxides, these oxides can be activated with visible light (λ > 450 nm). Estrada et al.53 reported a band gap of 2.795 eV for CoFe2O4, and Huerta-Aguilar et al.57 synthesized ZnFe2O4 with a bandgap of 1.9 eV.
The mix of Co and Zn ion used to create (Co–Zn)Fe2O4 composite was also characterized by DLS and DRS (Fig. S4). The size of (Co–Zn)Fe2O4 is larger than CoFe2O4 and ZnFe2O4 with a maximum at 660 nm (~ 30%) and some agglomeration reaching 1 μm. Z potential of this material was -25 mV on average. This value is typically observed in iron oxides formed under pH ranges above 6.0 and would suggest the absence of cations on the surface58,59, confirming the adequate substitution of metallic ions rather than a superficial deposition. Band gap determination showed an activation energy (ΔE = 2.02 eV) between the previously prepared Co and Zn materials.
TEM characterization was carried out for CoFe2O4, ZnFe2O4, and (Co–Zn)Fe2O4 mixed oxides to determine morphology and crystalline configuration. In the case of CoFe2O4, there is the presence of big agglomerations (d > 2000 nm) composed of individual particles with rounded shapes and sizes between 150 and 600 nm (Fig. 6a), agreeing with DLS determinations. Single Area Electron Diffraction (SAED) shows a polycrystalline material, and indexing the main rings allowed us to identify a cubic structure with dimensions similar to ferrites (Fig. 6b). The main rings are measured and associated with their respective hkl Miller Indexes: 4.93 [111]; 2.51 [311]; 2.47 [222]; 2.16 [400]; 1.51 [440] and 1.05 Å [731]. All these values are in close accordance with Fe3O4 (JCPDS 11-614), and an additional diffraction signal was observed at 1.63 Å, which would correspond to the [711] index of cobalt ferrite (Table S2). In the case of ZnFe2O4, small groups of particles with an average of 500 nm exist, whereas large agglomerations are less noticeable. The surface morphology of individual particles reveals sharp edges and angular vertices that suggest an ordered epitaxial growth (Fig. 6c)60. For this material, SAED shows a high crystallinity with a reticular pattern and main indexes found at 2.59 [311] and 2.16 Å [400]. These values are near to those reported for magnetite (2.53 Å [311] and 2.09 Å [400] and zinc ferrite (2.54 Å [311] and 2.10 Å [400])61. The increase in interplanar spacing could be explained by a distorted structure created upon forced diffusion of Zn at high pressures and temperatures62,63. A third diffraction signal was identified at 1.96 Å and identified with [331] facet that is usually observed in ZnFe2O4 but not Fe3O4. This data made it possible to locate the ZnFe2O4 sample as a zinc-iron hybrid ferrite with a cubic spinel structure (Fig. 6d)64,65.
Transmission Electron Microscope analysis of modified iron oxides: (a) CoFe2O4 8000x; (b) CoFe2O4 SAED pattern; (c) ZnFe2O4 8000x; (d) (Co–Zn)Fe2O4 SAED pattern. (e) (Co-Zn)Fe2O4of mixed iron oxide 30000x; (f) SAED pattern of the selected area (f(Co–Zn)Fe2O4); (g) (Co–Zn)Fe2O4 30000x; (h) SAED pattern of the selected area (red).
For the mixed (Co–Zn)Fe2O4 sample, it was possible to observe two types of particles: small round particles with sizes between 100 and 150 nm and quadrangular prism-shaped particles with sizes ranging from 100 to 300 nm. The rounded bodies in Fig. 6e can be associated with CoFe2O4 modified ferrites as the SAED pattern of a selected area in Fig. 6f (e(Co–Zn) Fe2O4) exhibits a polycrystalline material with close resemblance to cobalt ferrite. [311], [222], [400], and [440] hkl Miller indexes of magnetite are identified, and also, the characteristic [711] plane is identified from reported cobalt ferrite (CoFe2O4, JCPDS 22-1086). The prism-shaped particles observed were in different sizes, but their geometry had a consistent quadrangular outline (Fig. 6g). High crystallinity is often observed in zinc ferrites where consistent geometries can be obtained under hydrothermal conditions66,67. This assumption is confirmed in the SAED pattern of the selected area (g(Co–Zn)Fe2O4), where a highly crystalline material is obtained with a well-defined epitaxial growth (Fig. 6h). [311] and [400] were associated with magnetite, whereas [331] from zinc ferrite was identified.
Generally, studied catalysts consist of a homogeneous mixture of cubic ferrites; moreover, all materials are magnetically active and active under visible light. In mixed (Co–Zn)Fe2O4, the two sets of ferrites are evenly distributed despite differences in geometry. In all materials, particles were not typically isolated, which gives a clue about their magnetic nature66,68. They suggested a self-aggregating nature given by the magnetic inductance of the materials present. SAED patterns in all analyzed samples coincide with the most common Miller indexes, and their values are in very close agreement with standard JCPDS values previously reported for cubic Fe, Co, and Zn ferrites (Figure S5).
Application of mixed oxides in the photocatalytic degradation of black liquor
Crude Black liquor directly produced at cellulose mill, had a COD = 12,568 mg/L, BOD = 4602 mg/L with a Biological Index of 0.37 BOD/COD with an alkaline pH > 10. This material was thus diluted in a 1:2 ratio (COD = 6284 mg/L; BOD = 2,301 mg/L; Biological Index = 0.37 BOD/COD; pH > 9.6) with distilled water and utilized as a substrate to all photocatalytic reactions unless noted.
Two removal stages were identified: first, adsorption, and second, photocatalysis once irradiation started (Fig. 7a). For all tested heterogeneous catalysts, average adsorption yields a removal of 2.9% of the original COD concentration. (Co–Zn)Fe2O4 had the highest adsorption at 4.14%, and the lowest was for CoFe2O4 (1.89%). In the controls, H2O2 showed a decrease of 5.29% in COD; for Fe2+ , the change was 5.53%. In the case of H2O2, its oxidant capacity and alkaline media would activate radicals that allow the oxidation of some organic matter69 while in the case of Fe2+ alone, the changes were attributed to the formation of Fe2+ complexes that would precipitate and indirectly decrease COD of the samples70. For the photocatalytic stage, utilization of the sunlight parabolic collector allowed the system to reach 70 °C after 40 min with a max light intensity of 26 kLux measured at the focal point of the CPC. In addition to residual ultraviolet sunlight, it has been reported that these conditions could cause hydrolysis of dissolved saccharides even in the absence of catalysts71. Because of this, there was a marginal removal of 1.62% COD in control and 3.22% in H2O2. The highest degradation was observed in the Fenton reaction (Fe2+/H2O2). This setup removed 27.84% of the total COD and was followed by ZnFe2O4 (18.13%), (Co–Zn)Fe2O4 (14.99%), FeOE (10.12%) and CoFe2O4 (8.82%). Even though total COD removal was less than 20% in all cases, BOD increased, and the BOD/COD ratio changes showed interesting results (Fig. S6). From initial 0.37 BOD/COD, biodegradability was increased to 0.64 in the case of the Fenton reaction, and for heterogeneous catalysts, these values went up to 0.68 ((Co–Zn)Fe2O4), 0.65 (ZnFe2O4) and 0.52 (CoFe2O4 and FeOE). These changes suggest that complete degradation of BLq is hard to achieve with these methods. Still, partial oxidation occurs, breaking down complex structures such as lignin and residual cellulose, thereby increasing its biodegradability and subjecting the treated stream to conventional biological oxidation processes72. When the loading of catalysts was modified, so did changes in BLq composition (Fig. 7b); in the case of low catalyst dosage (0.25 mg/L), the adsorption diminished during the dark stage and the total COD removal upon photocatalysis. For example, the average adsorption was 1.08%, with a maximum for (Co–Zn)Fe2O4 (2.86%) and a minimum for ZnFe2O4 with only 0.3% removal. In the case of photocatalysis, the maximum COD removal was for ZnFe2O4 (13.34%) and the minimal for (Co–Zn)Fe2O4 (5.88%). With this loading, the increase in biodegradability was still observed but at a lower degree, being only 0.55 BOD/COD for ZnFe2O4, and for FeOE, the change was minimal, reaching only 0.42 BOD/COD. When a large dosage was utilized, there was an increase in COD removal by adsorption and photocatalysis and an enhancement in BI. In this case, COD change by adsorption was 6.08%, with a maximum in the case of FeOE at 7.16%. Photocatalysis maximum degradation was for ZnFe2O4 (19.71%) and minimum for FeOE (5.77%), which may suggest a saturation of active sites upon adsorption causing low photocatalytic oxidation (Fig. S7). For BI, the highest BOD/COD ratio was for ZnFe2O4 (0.71), closely followed by (Co–Zn)Fe2O4 (0.70), and the minimal was FeOE (0.55). These trends show FeOE as a weak catalyst that can be easily deactivated by adsorbed organic matter, and the addition of Zn2+ and Co2+ within ferrite structure creates defects that could increase heterojunctions and active sites, creating robust and efficient sunlight photocatalysts. These observations are supported by previous reports, where zinc and cobalt ferrites show enhanced activity compared to bare iron ferrites73,74.
Biodegradability properties of treated BLq with (Co-Zn)Fe2O4 under sunlight. (a) Changes in composition upon different oxidation treatments; (b) Changes in composition upon sunlight photocatalysis; (c) Effect of electron (AgNO3) and hole (isopropanol) scavengers; (d) Reusability tests for iron-based photocatalysts.
Degradation tests were performed in the presence of radical scavengers to understand the semiconductor nature of catalysts (Fig. 7c). Hole scavengers such as isopropanol reduce the h+ density at valence bands by donating electrons and annihilating these quasi-particles75. Holes at the semiconductor surface typically react with water to form ·OH radicals, but by inhibiting them, e−*CB density increases, average lifetime extends, and thus, formed ROS are mainly O2·−. In the presence of isopropanol, there was a slight increase in COD removal in all catalysts (1.0% ± 0.2), and in the case of ZnFe2O4, it reached 1.78%. The changes in BI were also small (+ 0.1 BOD/COD on average) compared to the standard reaction with no scavenger.
On the other hand, the presence of e-scavengers yields an important increase in COD removal and enhancement in biodegradability (Fig. S8). Silver nitrate electron scavenger accepts the freshly excited e−*CB, minimizing its role in the production of ROS. As electron scavengers limit the exciton recombination and increase h+ lifetime, oxidized species such as ·OH are the main radicals39,76. For COD, the average increase was 3.57%, with a maximum in ZnFe2O4 (22.31%) followed by mixed (Co–Zn)Fe2O4 (18.58%). The biodegradability index also showed important changes, reaching 0.8 BOD/COD in ZnFe2O4 and (Co–Zn)Fe2O4. From these results, we can conclude that oxidation of BLq by ·OH is the driving mechanism behind photocatalysis, and the presence of electron-donating species may hinder photocatalysis by coupling h+VB77.
Magnetic recovery and reusability of catalysts
The mass magnetic susceptibility for the mixed iron oxides was determined to be 397.0 × 10−6 m3/kg for FeOE, 443.50 × 10−6 m3/kg for CoFe2O4, 357.10 × 10−6 m3/kg for ZnFe2O4 and 410.0 × 10−6 m3/kg for (Co–Zn)Fe2O4. Co2+ addition to ferrites yields a higher magnetic response78, being in between χm of maghemite and magnetite, and the lower was for ZnFe2O4 (Fig. S9a). Differences in final morphology and recovery of the final oxides could arise because of the inherent magnetic properties of Co3+ and Zn2+ ions, as zinc diminishes magnetic activity79,80. In the case of Co, paramagnetic properties given by the unpaired electrons compete with Fe2+ ions in ferrites81. On the other hand, Zn2+ ions with diamagnetic tendency have less magnetic hindrance than Fe2+ ions, allowing a well-ordered crystallization as was inferred from prismatic crystals observed in TEM and associated with ZnFe2O4. In recoverability tests, in aqueous systems and upon 360 min stirring, FeOE had a ym = 93.8% recovery, and for mixed iron oxides, this value increased to 94.3% in CoFe2O4 and 93.1% for (Co–Zn)Fe2O4. However, ym decreased to 89.1% ZnFe2O4; it was observed that this trend follows the magnetic susceptibility values, which allow us to relate recoverability with χm and discard loss of mass because of decomposition or solvation of ions during short periods. In photocatalytic tests with BLq, there was a gradual decrease in recovered catalysts after successive cycles. FeOE ym was 92.1% in Cycle 1, 81.2% in Cycle 2, and 73% in Cycle 3. In the case of CoFe2O4, ym recoverability was less impacted and went from 92.4% in Cycle 1 to 91.3 and 89.4% in the following cycles. For ZnFe2O4, changes were also important, going from ym = 84–76.7% after 3 cycles, and in mixed (Co–Zn)Fe2O4, they went from 87.3 to 85.4%. It can be observed that bare iron oxides in FeOE lose more than 25% in mass after three cycles. This behavior could be explained based on the natural tendency to oxidize to soluble Fe3+ ion and the formation of hydroxides in alkaline aqueous systems. Like recoverability, the photocatalytic performance of the studied catalysts tends to decrease as oxidation cycles take place (Fig. S10) and the most affected material was FeOE, where COD removal went to 10.12% in the first cycle to 5.10% and 3.18% in the second and third cycles. On the other hand, ZnFe2O4 still showed an important decrease in COD of 12.58 even after 3 cycles. This behavior highlights the catalytic activity of zinc ferrites despite their poor magnetic susceptibility and recoverability. In the case of (Co–Zn)Fe2O4 mixed oxides, its catalytic performance changed from 14.99 to 12.58% in terms of COD removal, showing an intermediate behavior compared to Co and Zn ferrites (Fig. 7d). In terms of the Biodegradability Index the BOD/COD in all catalysts dropped to less than 0.5 BOD/COD in the third catalytic cycle. From these results, we can conclude that even though photocatalysis still takes place after multiple cycles, the changes in biodegradability are not enough to consider these effluents biodegradable. Therefore, a catalyst regeneration would be suggested between treatment cycles.
Conclusions
A controlled oxidation process has been developed to form ferrite catalysts from residual steel in aqueous system. Several reaction conditions have been explored, and results show that at pH = 5 and 90 °C, steel can be converted to magnetic iron oxides, mostly Fe3O4 and γFe2O3. The experimental setup for obtaining iron catalysts is reliable and scalable up to the gram scale. Obtained materials comprise submicron conglomerates, homogeneous in size with ferrite-like crystal structure and a semiconductor nature active under visible light (ΔE < 2.0 eV). Catalytic properties have been enhanced upon modification by hydrothermal diffusion of Zn2+ and Co2+ ions. When applied to the degradation of black liquor under sunlight irradiation, there was an increase in BLq biodegradability from 0.36 to a maximum of 0.71 BOD/COD and with a decrease in total COD of more than 25% when utilizing ZnFe2O4 [1.5 mg/L]. CoFe2O4 was the best option in terms of recoverability, with 92.4% mass recovery after a single treatment but with poor degradation capabilities. Finally, the production of mixed (Co–Zn)Fe2O4 yields a robust catalyst with balanced recoverability (88%) and decent COD removal (< 15%). The presented materials and production methods represent a feasible option for producing sunlight-active catalysts that can be applied to treat non-biodegradable effluents.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AISI:
-
American Iron and Steel Institute
- AOPs:
-
Advanced oxidation processes
- BLq:
-
Black liquor
- BOD:
-
Biological oxygen demand
- BOD5 :
-
5-Day biological oxygen demand
- COD:
-
Chemical oxygen demand
- CPC:
-
Cylindrical parabolic collector
- DLS:
-
Dynamic light scattering
- JCPDS:
-
Joint Committee on Powder Diffraction Standards
- SAED:
-
Single area electron diffraction
- TEM:
-
Transmission electron microscope
- UV:
-
Ultraviolet light
- Vis:
-
Visible light
References
Zhang, F. et al. From trash to treasure: Chemical recycling and upcycling of commodity plastic waste to fuels, high-valued chemicals and advanced materials. J. Energy Chem. 69, 369–388 (2022).
Yuan, X. et al. Review on upgrading organic waste to value-added carbon materials for energy and environmental applications. J. Environ. Manage. 296, 113128 (2021).
Rial, J. B. & Ferreira, M. L. Potential applications of spent adsorbents and catalysts: Re-valorization of waste. Sci. Total Environ. 823, 153370 (2022).
Dupont, D. & Binnemans, K. Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3: Eu3+ from lamp phosphor waste. Green Chem. 17, 856–868 (2015).
Survey, U. S. G. Mineral Commodity Summaries. https://doi.org/10.3133/MCS2022.
Misawa, T., Hashimoto, K. & Shimodaira, S. The mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room temperature. Corros. Sci. 14, 131–149 (1974).
Morcillo, M., Díaz, I., Cano, H., Chico, B. & de la Fuente, D. Atmospheric corrosion of weathering steels. Overview for engineers. Part I: Basic concepts. Constr. Build. Mater. 213, 723–737 (2019).
Dillmann, P., Mazaudier, F. & Hœrlé, S. Advances in understanding atmospheric corrosion of iron. I. Rust characterisation of ancient ferrous artefacts exposed to indoor atmospheric corrosion. Corros. Sci. 46, 1401–1429 (2004).
Sharma, P., Holliger, N., Pfromm, P. H., Liu, B. & Chikan, V. Size-controlled synthesis of iron and iron oxide nanoparticles by the rapid inductive heating method. ACS Omega 5, 19853–19860 (2020).
Zhu, J., Li, L., Xiong, Z., Hu, Y. & Jiang, J. Evolution of useless iron rust into uniform α-Fe2O3 nanospheres: A smart way to make sustainable anodes for hybrid Ni–Fe cell devices. ACS Sustain. Chem. Eng. 5, 269–276 (2017).
GonzálezOchea, R. A. & Encina, E. R. Light harvesting in magnetite-coated plasmonic metal nanospheres. J. Phys. Chem. C 126, 885–891 (2022).
Amiri, M., Salavati-Niasari, M. & Akbari, A. Magnetic nanocarriers: Evolution of spinel ferrites for medical application. Adv. Colloid Interface Sci. 265, 29–44 (2019).
Berman, D. et al. 6.7 Gb/in2 recording areal density on barium ferrite tape. IEEE Trans. Magn. 43, 3502–3508 (2007).
Narang, S. B. & Pubby, K. Nickel spinel ferrites: A review. J. Magn. Magn. Mater. 519, 167163 (2021).
Ismael, M. Ferrites as solar photocatalytic materials and their activities in solar energy conversion and environmental protection: A review. Solar Energy Mater. Solar Cells 219, 110786 (2021).
Kusmierek, E. A CeO2 semiconductor as a photocatalytic and photoelectrocatalytic material for the remediation of pollutants in industrial wastewater: A review. Catalysts 10, 1435 (2020).
Yoshikawa, T. et al. Production of phenols from lignin-derived slurry liquid using iron oxide catalyst. Appl. Catal. B 146, 289–297 (2014).
Casbeer, E., Sharma, V. K. & Li, X. Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 87, 1–14 (2012).
Cardoso, M., de Oliveira, É. D. & Passos, M. L. Chemical composition and physical properties of black liquors and their effects on liquor recovery operation in Brazilian pulp mills. Fuel 88, 756–763 (2009).
Ding, Z. et al. Green synthesis of chemical converted graphene sheets derived from pulping black liquor. Carbon N Y 158, 690–697 (2020).
Edwiges, T. et al. Comparison of various pretreatment techniques to enhance biodegradability of lignocellulosic biomass for methane production. J. Environ. Chem. Eng. 7, 103495 (2019).
Owens, J. W. Environmental Fate and Effects of Pulp and Paper Mill Effluents, 661–671 (2020).
Hobbs, M. S., Grippo, R. S., Farris, J. L., Griffin, B. R. & Harding, L. L. Comparative acute toxicity of potassium permanganate to nontarget aquatic organisms. Environ. Toxicol. Chem. 25, 3046–3052 (2006).
Chen, J., Qu, R., Pan, X. & Wang, Z. Oxidative degradation of triclosan by potassium permanganate: Kinetics, degradation products, reaction mechanism, and toxicity evaluation. Water Res. 103, 215–223 (2016).
Svecevičius, G., Šyvokiene, J., Stasiunaite, P. & Mickeniene, L. Acute and chronic toxicity of chlorine dioxide (ClO2) and chlorite (ClO2) to rainbow trout (Oncorhynchus mykiss)(4 pp). Environ. Sci. Pollut. Res. 12, 302–305 (2005).
Speight, J. G. Heavy Oil Recovery and Upgrading 1–839 (Gulf Professional Publishing, 2019).
Karlsson, E., Gourdon, M., Olausson, L. & Vamling, L. Heat transfer for falling film evaporation of black liquor up to very high Prandtl numbers. Int. J. Heat Mass Transf. 65, 907–918 (2013).
Algehed, J. & Berntsson, T. Evaporation of black liquor and wastewater using medium-pressure steam: Simulation and economic evaluation of novel designs. Appl. Therm. Eng. 23, 481–495 (2003).
Bajpai, P. Biermann’s Handbook of Pulp and Paper 295–351 (Elsevier, 2018).
Arutanti, O. et al. Advanced degradation of lignin from palm oil mill effluent (POME) by a combination of photocatalytic-fenton treatment and TiO2 nanoparticle as the catalyst. Water Air Soil Pollut. 231, 1–10 (2020).
Crites, C. O. L. et al. Exploiting the photocatalytic activity of TiO2 towards the depolymerization of Kraft lignin. New J. Chem. 45, 15371–15377 (2021).
Ghernaout, D. Advanced oxidation phenomena in electrocoagulation process: A myth or a reality?. Desalin. Water Treat. 51, 7536–7554 (2013).
Asghar, A., Raman, A. A. A. & Daud, W. M. A. W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 87, 826–838 (2015).
Wang, J. L. & Xu, L. J. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. Crit. Rev. Environ. Sci. Technol. 42, 251–325 (2012).
Wang, H. et al. An excitonic perspective on low-dimensional semiconductors for photocatalysis. J. Am. Chem. Soc. 142, 14007–14022 (2020).
Hsu, P. H. & Wang, M. K. Crystallization of goethite and hematite at 70 °C. Soil Sci. Soc. Am. J. 44, 143–149 (1980).
Saravanathamizhan, R. & Perarasu, V. T. Wastewater Treatment: Cutting-Edge Molecular Tools, Techniques and Applied Aspects, 103–136 (2021).
Wibowo, A., Sihombing, A. R. S. A., Putra Parmita, A. W. Y., Triadhi, U. & Ardy, H. The influence of hydrogen peroxide concentration on catalytic activity of fenton catalyst@bacterial cellulose. IOP Conf. Ser. Mater. Sci. Eng. 509, 012020 (2019).
FakhrulRidhwanSamsudin, M. et al. Exploring the role of electron-hole scavengers on optimizing the photocatalytic performance of BiVO4. Mater. Today Proc. 5, 21703–21709 (2018).
Sharma, R., Bansal, S. & Singhal, S. Tailoring the photo-Fenton activity of spinel ferrites (MFe2O4) by incorporating different cations (M= Cu, Zn, Ni and Co) in the structure. RSC Adv. 5, 6006–6018 (2014).
Gomes, S. et al. Characterization of magnetite in silico-aluminous fly ash by SEM, TEM, XRD, magnetic susceptibility, and Mössbauer spectroscopy. Cem. Concr. Res. 29, 1705–1711 (1999).
Song, S., Lu, S. & Lopez-Valdivieso, A. Magnetic separation of hematite and limonite fines as hydrophobic flocs from iron ores. Miner. Eng. 15, 415–422 (2002).
Zhang, K. et al. Phase transition and magnetic properties of low-grade limonite during reductive roasting. Vacuum 167, 163–174 (2019).
Liu, X. M. et al. Analysis on variety and characteristics of maghemite. Sci. China Earth Sci. 53, 1153–1162 (2010).
Song, Q. & John Zhang, Z. Correlation between spin− orbital coupling and the superparamagnetic properties in magnetite and cobalt ferrite spinel nanocrystals. J. Phys. Chem. B 110, 11205–11209 (2006).
Nguyen, M. D., Tran, H. V., Xu, S. & Lee, T. R. Fe3O4 nanoparticles: structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl. Sci. 11, 11301 (2021).
Martínez-Mera, I., Gutiérrez-Wing, C., Argánis-Juárez, C. & Vilchis-Nestor, A. R. Reduction of maghemite to magnetite over 304SS, in the presence of silver nanoparticles. Surf. Coat. Technol. 324, 338–344 (2017).
Nadoll, P., Angerer, T., Mauk, J. L., French, D. & Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 61, 1–32 (2014).
Carta, D. et al. EDS, HRTEM/STEM, and X-ray absorption spectroscopy studies of co-substituted maghemite nanoparticles. J. Phys. Chem. C 117, 9496–9506 (2013).
Lee, J. M., Lim, D. S., Jeon, S. H. & Hur, D. H. Zeta potentials of magnetite particles and alloy 690 surfaces in alkaline solutions. Materials 13, 3999 (2020).
Favela-Camacho, S. E., Samaniego-Benítez, E. J., Godínez-García, A., Avilés-Arellano, L. M. & Pérez-Robles, J. F. How to decrease the agglomeration of magnetite nanoparticles and increase their stability using surface properties. Colloids Surf. A Physicochem. Eng. Asp. 574, 29–35 (2019).
Borth, K. W., Galdino, C. W., de Teixeira, V. C. & Anaissi, F. J. Iron oxide nanoparticles obtained from steel waste recycling as a green alternative for Congo red dye fast adsorption. Appl. Surf. Sci https://doi.org/10.1016/j.apsusc.2021.149126 (2021).
Estrada, S. O. et al. uning of the magnetic response in cobalt ferrite CoxFe3-xO4 by varying the Fe2+ to Co2+ molar ratios: Rietveld refinement and DFT structural analysis. J. Alloys Compd. 695, 2706–2716 (2017).
Ratkovski, G. P. et al. Spinel cobalt ferrite nanoparticles for sensing phosphate ions in aqueous media and biological samples. Langmuir 36, 2920–2929 (2020).
Allwin Mabes Raj, A. F. P. et al. Removal of Pb2+, CrT, and Hg2+ ions from aqueous solutions using amino-functionalized magnetic nanoparticles. Int. J. Mol. Sci. 23, 16186 (2022).
Ucbas, Y., Bozkurt, V., Bilir, K. & Ipek, H. Concentration of chromite by means of magnetic carrier using sodium oleate and other reagents. Physicochem. Probl. Miner. Process. 50, 767–782 (2014).
Huerta-Aguilar, C. A. et al. Crystal phase induced band gap energy enhancing the photo-catalytic properties of Zn–Fe2O4/Au NPs: experimental and theoretical studies. Catal. Sci. Technol. 9, 3066–3080 (2019).
Milanovic, M., Stijepovic, I., Pavlovic, V. & Srdic, V. V. Functionalization of zinc ferrite nanoparticles: Influence of modification procedure on colloidal stability. Process. Appl. Ceram. 10, 287–293 (2016).
Peralta, M. E. et al. Highly efficient removal of heavy metals from waters by magnetic chitosan-based composite. Adsorption 25, 1337–1347 (2019).
Hong, X. et al. A facile chemical solution-based method for epitaxial growth of thick ferrite films. Adv. Electron. Mater. 1, 1500102 (2015).
Branscomb, L. M., Swanson, H. E., Mcmurdie, H. F., Morris, M. C., Evans, E. H., Assisted, B. P., Degroot, J. H., Carmel, S. J. & Washington, D. C. Bur. Stand. (U.S.), Monogr. 25-Section 9, 128 (1971).
Liu, W., Han, J., Yamada, I. & Yagi, S. Effects of zinc ions at tetrahedral sites in spinel oxides on catalytic activity for oxygen evolution reaction. J. Catal. 394, 50–57 (2021).
Makovec, D., Kodre, A., Arčon, I. & Drofenik, M. The structure of compositionally constrained zinc-ferrite spinel nanoparticles. J. Nanoparticle Res. 13, 1781–1790 (2011).
Cao, C., Xia, A., Liu, S. & Tong, L. Synthesis and magnetic properties of hydrothermal magnesium–zinc spinel ferrite powders. J. Mater. Sci.: Mater. Electron. 24, 4901–4905 (2013).
Tsay, C. Y., Chiu, Y. C. & Tseng, Y. K. Investigation on structural, magnetic, and FMR properties for hydrothermally-synthesized magnesium-zinc ferrite nanoparticles. Phys. B Condens. Matter 570, 29–34 (2019).
Zviagin, V., Grundmann, M. & Schmidt-Grund, R. Impact of defects on magnetic properties of spinel zinc ferrite thin films. Phys. Status Solidi (b) 257, 1900630 (2020).
Oliver, S. A., Harris, V. G., Hamdeh, H. H. & Ho, J. C. Large zinc cation occupancy of octahedral sites in mechanically activated zinc ferrite powders. Appl. Phys. Lett. 76, 2761–2763 (2000).
Karvelas, E. G., Lampropoulos, N. K., Benos, L. T., Karakasidis, T. & Sarris, I. E. On the magnetic aggregation of Fe3O4 nanoparticles. Comput. Methods Programs Biomed. 198, 105778 (2021).
Liu, X., Lu, J., Ayele, B. A., Li, D. & Chen, Q. Coupling of alkaline precipitation and alkali-activated hydrogen peroxide oxidation for reuse of cotton pulp black liquor. J. Clean. Prod. 288, 125094 (2021).
Liu, X., Lu, J., Fang, X., Zhou, J. & Chen, Q. Complexation modelling and oxidation mechanism of organic pollutants in cotton pulp black liquor during iron salt precipitation and electrochemical treatment. Chemosphere 308, 136374 (2022).
Yatagai, M. & Zeronian, S. H. Effect of ultraviolet light and heat on the properties of cotton cellulose. Cellulose 1, 205–214 (1994).
Peralta-Zamora, P. et al. Evaluation of ZnO, TiO2 and supported ZnO on the photoassisted remediation of black liquor, cellulose and textile mill effluents. Chemosphere 36, 2119–2133 (1998).
López, J. et al. Magnetic nanostructured based on cobalt–Zinc Ferrites designed for photocatalytic dye degradation. J. Phys. Chem. Solids 150, 109869 (2021).
Raina, O. & Manimekalai, R. Photocatalysis of cobalt zinc ferrite nanorods under solar light. Res. Chem. Intermed. 44, 5941–5951 (2018).
Mureithi, A. W., Sun, Y., Mani, T., Howell, A. R. & Zhao, J. Impact of hole scavengers on photocatalytic reduction of nitrobenzene using cadmium sulfide quantum dots. Cell Rep. Phys. Sci. https://doi.org/10.1016/j.xcrp.2022.100889 (2022).
Tan, H. L., Wen, X., Amal, R. & Ng, Y. H. BiVO4 010 and 110 relative exposure extent: Governing factor of surface charge population and photocatalytic activity. J. Phys. Chem. Lett. 7, 1400–1405 (2016).
Ghaly, M. Y., Jamil, T. S., El-Seesy, I. E., Souaya, E. R. & Nasr, R. A. Treatment of highly polluted paper mill wastewater by solar photocatalytic oxidation with synthesized nano TiO2. Chem. Eng. J. 168, 446–454 (2011).
Imanipour, P. et al. The effect of divalent ions of zinc and strontium substitution on the structural and magnetic properties on the cobalt site in cobalt ferrite. J. Magn. Magn. Mater. 510, 166941 (2020).
da Silva, F. G. et al. Structural and magnetic properties of spinel ferrite nanoparticles. J. Nanosci. Nanotechnol. 19, 4888–4902 (2019).
Omelyanchik, A. et al. Green synthesis of Co–Zn spinel ferrite nanoparticles: magnetic and intrinsic antimicrobial properties. Materials 13, 5014 (2020).
Fairweather, A., Roberts, F. F. & Welch, A. J. E. Ferrites. Rep. Progress Phys. 15, 142 (1952).
Acknowledgements
Zacek Flores-Lopez and Aylin Solis-Diaz acknowledge the Delfin project for scholarship and funding. The authors acknowledge Unidad de Servicios de Apoyo a la Investigacion (USAI) at the Faculty of Chemistry, UNAM, for facilities and analytical instrumentation.
Funding
The research was co-funded by CAPEX: Renewable Energies Lab 22 and OPEX EIC- PHFT070-22ZZ00003 at Tecnologico de Monterrey, Puebla. Co-funded by ITESM UOttawa Seed Grant Program 23-25 No. IJST070-23OT77001-UO-TEC Revalorization of steel residues. The project was also sponsored by Delfin Projects 01809 (A. B. Solis-Diaz) and 04063 (Z. D. Flores-Lopez).
Author information
Authors and Affiliations
Contributions
Zacek David Flores-López, Aylín Belén Solís-Díaz, and Deepak Kumar, Conceptualization, writing-original draft preparation, and validation. Pabel Antonio Cervantes-Aviles, Pandiyan Thangarasu, Harpreet Kaur, Jashanpreet Singh, Prasad Lokande, Carlos Alberto Huerta-Aguilar and Nabisab Mujawar Mubarak Writing- Reviewing and Editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Flores-López, Z.D., Solís-Díaz, A.B., Cervantes-Aviles, P.A. et al. Insight mechanism of magnetic activated catalyst derived from recycled steel residue for black liquor degradation. Sci Rep 14, 19057 (2024). https://doi.org/10.1038/s41598-024-70072-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70072-8