Abstract
The impact of ustekinumab (UST) on mucosal- and fistula healing and extraintestinal manifestations (EIM) in Crohn’s disease (CD) were not fully elucidated in the registration trials. In this prospective, multicenter study (EudraCT number: 2017-005151-83) we evaluated the German label real-world-effectiveness of UST to achieve the primary endpoint of combined clinical and endoscopic response at week 52 and several secondary endpoints. Of 79 screened we enrolled 52 patients (female n = 28, bionaïve n = 13, biologic n = 39). At week 52 (per protocol analysis), 52% (n = 13/25) of patients achieved the primary endpoint [50% (n = 3/6) in the bionaïve, 45.5% (n = 5/11) biologic, 62.5% (n = 5/8 ) multiple biologics cohorts, respectively with age as independent predictor [OR 95% CI 0.933 (0.873, 0.998) p = 0.043], 60% (n = 15/25) achieved endoscopic response [50% (n = 3/6) in the bionaïve, 54.5% (n = 6/11) biologic, 75% (n = 6/8) multiple biologics cohorts, respectively], 36% (n = 9/25) achieved endoscopic remission [50% (n = 3/6) in the bionaïve, 27.3% (n = 3/11) biologic, 37.5% (n = 3/8) multiple biologics cohorts, respectively], 48% (n = 12/25) achieved mucosal healing [50% (n = 3/6) in the bionaïve, 36.4% (n = 4/11) biologic, 62.5% (n = 5/8) multiple biologics cohorts, respectively]. All achieved a fistula response and 33.3% (n = 1/3) in the multiple biologics group fistula remission at week 52. EIM decreased (week 0 28.2% vs. week 52 8%). CRP, FCP, PRO-2, EQ-5D-5L improved throughout. 36 patients (69.2%) experienced ≥ 1 treatment emergent adverse event, in 8 (15.4%) cases rated as severe and in 5 (9.6%) leading to UST discontinuation, but no very severe events or deaths. The effectiveness of UST was better than in the registration trials.
Similar content being viewed by others
Introduction
Crohn’s disease (CD) is an idiopathic inflammatory bowel disease (IBD) that cannot be cured and challenges patients and healthcare systems worldwide1. CD results from an inappropriate immune response towards the commensal microbiota in genetically susceptible individuals2, exacerbated and promoted by environmental factors such as Western lifestyle, diet and industrialization3. CD requires lifelong medical therapy and progresses with repeated flare-ups to possible complete digestive failure4. The chronic inflammation is associated with an early, increased risk of various digestive and other cancers5.
Due to its increasing incidence and early age peaks in the 2nd and 3rd decade, CD impacts on patients’ personal and professional lives due to impaired activity, lost work-productivity, fatigue, as well as early disability. The complete economic burden has been estimated at and 2.1 to 16.7 billion € for CD annually in Europe6,7,8. Recent research from our group comparing actual costs and revenues indicates, that inpatient care expenditures are almost double compared with the average population and not fully recovered by German diagnosis related group (G-DRG) proceeds9. Biologic medications10 contribute the most to the deficit. Thus, evidence driven patient and treatment stratification are paramount.
The perceived quality of and satisfaction with CD care delivered varies substantially between patients11 and providers12. Patient reported outcomes (PRO)13 and patient relevant endpoints14 are not systematically collected15 in Germany, despite their demanded implementation by major regulatory bodies14,16 and their immediate impact on the approval and reimbursement of novel compounds such as ustekinumab. More than one third of all IBD patients in Germany are not in remission17 underscoring the need to implement and study the real-world application of new therapies.
Ustekinumab was compared to placebo in the UNITI-1 trial in CD patients who had failed anti-TNFα and in the UNITI-2 trial, carried out in CD patients who had failed conventional therapy as well as in a maintenance study with re-randomized responders (IM-UNITI)18,19. In endoscopic and histologic substudies of patients who participated in UNITI-1, UNITI-2 and IM-UNITI, ustekinumab induced a greater reduction in the Simplified Endoscopic Activity Score for Crohn’s Disease (SES-CD) and global histology activity score reduction at week 8 than placebo20,21. Ustekinumab was approved for CD based on UNITI and IM-UNITI (and later also for ulcerative colitis results based on the UNIFI prospective randomized controlled trial)22.
Since clinical trials rarely represent the real world patient population23 and ustekinumab’s impact on induction and maintenance of mucosal healing, fistula healing and extraintestinal manifestations in CD are largely unknown long-term remission patients reported outcomes and quality of life data are incomplete we aim to close these knowledge gaps.
Materials and methods
Study drug ustekinumab
Ustekinumab is a fully human immunoglobulin G1 kappa (IgG1k) monoclonal antibody to human IL-12/23p40 that binds with high affinity to the p40 subunit of human IL-12 and IL-2310. By inhibiting interaction with the cell surface IL-12Rβ1 receptor protein, ustekinumab effectively neutralizes all IL-12 (Th1) and IL-23 (Th17) mediated cellular responses. Abnormal regulation of IL-12 and IL-23 has been associated with multiple immune-mediated diseases including CD.
Study design
In this prospective, open label, nationwide, multicenter, phase IV study we investigated ustekinumab use in adult subjects with active, moderate to severe, ileal and/or colonic CD in a real world setting in 8 (originally planned 10) German centers representing all care levels—private practice, community hospitals and academic institutions. The drug was used in accordance with its German label24,25,26.
An initial target of 100 adult male and female subjects with endoscopic evidence of active disease were planned to be enrolled, but later adjusted to 50–60 due to pandemic related issues. Patients enrolled were either naïve to biologic treatment or previously had an inadequate response, loss of response, been intolerant to, or had medical contraindications to either 1, or 2 or more biologic(s) approved for the treatment of CD. Eligibility of subjects was evaluated locally, and centrally read endoscopic assessments at screening was used for baseline evaluation. At week 0, all eligible subjects initiated IV induction treatment with ustekinumab, in line with the German label, on a weight-tiered basis at a dose of approximately 6 mg/kg IV. At week 8, all subjects received a 90 mg SC injection of ustekinumab. To ensure a balanced, unbiased real world cohort subjects were be stratified 1:1:1 at baseline according to being naïve to biologics (bionaïve), prior exposure to 1 (biologic) or 2 or more biologics (multiple biologic) for treatment of Crohn’s disease. All subjects were to be followed for 52 weeks. Consideration was given to discontinuing treatment in patients who show no evidence of therapeutic benefit by week 16 or 16 weeks after switching to the 8-weekly dose. In accordance with the German label, subjects who lost response during 12-weekly dosing may benefit from an adjustment to 8-weekly maintenance treatment. Subjects were subsequently dosed every 8 weeks or every 12 weeks according to clinical judgment. In contrast, subjects already receiving 8-weekly treatment were not able to adjust the ustekinumab dose following disease flare and left the study (early termination visit).
Study endpoints and assessments
Assessments of vital signs, concomitant medications, procedures (i.e. surgeries and other non-medical interventions) hospitalizations, extraintestinal manifestations, clinical and biochemical disease activity (Harvey Bradshaw Index [HBI]27, complete blood count [CBC], C-reactive protein [CRP], albumin and fecal calprotectin [FCP]) were performed throughout the study at the times indicated in the Time and Events Schedule. For subjects with fistulizing disease, perianal disease activity index (PDAI)28 was assessed. Patient-reported outcomes29 included assessments of PRO-230 and non-disease specific health related quality of life (EQ-5D-5L)31. Adverse event data and information on concomitant therapies according to Medical Dictionary for Regulatory Activities (MedDRA)32 and Common Terminology for Adverse Events (CTCAE)33 were collected throughout the study. Immunogenicity assessments included an inspection of the infusion and injection sites for reactions and monitoring of vital signs for systemic allergic or other immunological reactions. An ileocolonoscopic assessment of endoscopic disease activity with the Simple Endoscopic Score for Crohn’s Disease (SES-CD)34 and mucosal healing documentation via central reading was performed at the Week 0, voluntarily at Week 26 and mandatory at the Week 52 study visits. For patients receiving corticosteroids at baseline tapering was mandatory from Week 16 onwards. For detailed information on the definition and timing of efficacy and safety assessments throughout the study refer to Supplementary Web Appendix Part 1: Web Table 1 Time and Event Schedule and Web Table 2 Objectives and Endpoints.
Statistical considerations
All subjects who received at least one dose of ustekinumab were included in the safety analysis set. All subjects who received at least one dose of ustekinumab and have at least one post-stratification efficacy assessment were included in the intent-to-treat (ITT) analysis set. In addition, all subjects who continued to the maintenance phase and received treatment were included in the maintenance ITT population. All ITT subjects who completed all aspects of the trial and did not have any major deviations from protocol were included in the per-protocol (PP) analysis set. PP analyses were performed on the primary efficacy variable.
In line with the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE)35,36 consensus, a combined endpoint of clinical response plus endoscopic disease activity was set as the primary endpoint of this study. This was not an RCT and hence had no placebo comparator. Assuming a combined clinical and endoscopic response rate of 40%, the anticipated asymptotic 95% confidence interval (CI) about the remission rate will be ± 10% with 60 subjects; to account for attrition, 68 subjects were planned to be enrolled. Enrolled patients who stopped treatment before reaching week 52 due to any reason, or patients without endoscopic data at week 52 were analyzed as non-responders. The number and percentage of responders were summarized by stratification groups and compared using a generalized linear model with a logit link. Baseline history of CD related surgeries, baseline mono versus combination therapy status, and baseline fistulizing versus non-fistulizing CD status were included as covariates. Least squares means odds ratios (OR) and 95% confidence intervals (CI) were provided for each stratification group.
All available efficacy and safety data collected for the study were included in data listings and/or summary tables. Categorical endpoints that were assessed at end of the study imputed missing values using non-responder imputation. All other endpoints were assessed using mixed models and not imputed.
Ethical statement
The study was approved by central ethics committee of the state government of Berlin Germany (Landesamt für Gesundheit und Soziales, Ethik Kommission des Landes Berlin 18/0263-EK13). All methods were carried out in accordance with relevant guidelines and regulations. informed consent was obtained from all subjects and/or their legal guardian(s).
Study registration
This non-interventional study was registered with EudraCT (European Union Drug Regulating Authorities Clinical Trials Database) reference number 2017-005151-83. Details can be found here: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2017-005151-83.
Results
Patient disposition
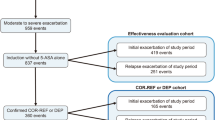
We screened 79 patients, of which 34% (27/79) were screening failures and 66% (52/79) were enrolled in the study. 65 percent of patients (34/52) completed the study, this attrition rate was likely impacted by the unprecedented global pandemic. A greater proportion of patients in the bionaïve group completed the study. The reasons for discontinuation were comparable among the groups. However, the highest number of discontinuations due to non-HBI response was recorded in the biologic group whereas patient discontinuing due loss to follow-up occurred only in the multiple-biologic group. Table 12summarizes treatment compliance. For additional details about treatment compliance and protocol adherence, refer to Supplementary Web Appendix Part 2 Web Tables 14.1.3 and 14.1.7.
Patient baseline characteristics
Sex distribution was for the most part equal. Most patients enrolled were in their 40s and shared the same physical status according to their respective BMI. All patients were of Caucasian descent. Patients in the biologic- and multiple biologics groups had more frequently ileocolonic and fistulizing disease and were more severe as indicated by higher baseline CRP, FCP and SES-CD score values, the most severe group being those previously exposed to multiple biologics. Table 3 For additional details about patient’s prior treatment complications, medical history, medications, concomitant medications please refer to the Supplementary Web Appendix Part 2 Web Tables 14.1.3–14.1.6.
Efficacy
Overall, disease activity decreased throughout the course of the study among all groups. The course of key clinical, endoscopic and laboratory efficacy parameters over the course of the study is depicted in Fig. 1. For additional details please refer to the Supplementary Web Appendix Part 2 Web Tables 14.2.10–14.2.16 and 14.3.5.2.
Primary endpoint—combined clinical and endoscopic response
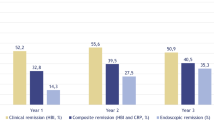
In the ITT population, 23.1%, 22.7% and 29.4% of patients in the bionaïve, biologic, and multiple biologics population achieved the primary endpoint, respectively. In the PP population, 50%, 45.5% and 62.5% of patients in the naïve, biologic, and multiple biologic population achieved the primary endpoint, respectively. There was no statistically significant difference between the ORs of all groups in both the ITT and PP analyses, respectively. Figure 2 top Web Appendix Part 2 Web Table 14.2.1.1.
Endoscopic response, remission and mucosal healing
In the ITT population, 23.1%, 27.3% and 35.3% of patients in the bionaïve, biologic, and multiple biologics groups achieved an endoscopic response, respectively. In the PP population, 50%, 54.5% and 75% of patients in the naïve, biologic, and multiple biologic population achieved an endoscopic response at week 52, respectively. Figure 3 Web Appendix Part 2 Web Tables 14.2.3.1 and 14.2.3.2.
In ITT population, 23.1%, 13.6% and 17.6% of patients in the bionaïve, biologic, and multiple biologics population achieved an endoscopic remission, respectively. In the PP population, 50%, 27.3% and 37.5% of patients in the naïve, biologic, and multiple biologic population achieved endoscopic remission at week 52, respectively. Figure 3 Web Appendix Part 2 Web Tables 14.2.4.1 and 14.2.4.2.
In ITT population, 23.1%, 18.2% and 35.3% of patients in the bionaïve, biologic, and multiple biologics population achieved mucosal healing, respectively. In the PP population, 50%, 36.4% and 62.5% of patients in the bionaïve, biologic, and multiple biologic population achieved mucosal healing at week 52, respectively. (Fig. 2 bottom) Web Appendix Part 2 Web Tables 14.2.5.1 and 14.2.5.2.
There was no statistically significant difference between the ORs of all groups regarding endoscopic response, endoscopic remission and mucosal healing in both the ITT and the PP analyses, respectively.
The SES-CD score decreased throughout the study. There were no statistically significant differences among the SES-CD change score LS means of all groups. Figure 1 Web Appendix Part 2 Web Table 14.2.11.
Clinical response, clinical remission, steroid free clinical remission and steroid sparing
In the ITT population, 61.5%, 40.9% and 47.1% of patients in the bionaïve, biologic, and multiple biologics populations achieved a clinical response at week 52. There was no statistically significant difference between the OR of all groups, at week 52, respectively. Figure 4 Web Appendix Part 2 Web Table 14.2.2.
In the ITT population, 53.8%, 45.5% and 41.2% of patients in the bionaïve, biologic, and multiple biologics groups achieved a clinical remission at week 52. There was no statistically significant difference between the ORs of all groups, at all timepoints, respectively. Figure 4 Web Appendix Part 2 Web Table 14.2.6.
In the ITT population, 33.8%, 40% and 22.2% of patients in the bionaïve, biologic, and multiple biologics populations achieved steroid free clinical remission at week 52. At week 16 significantly more patients in the bionaïve vs. biologic or multiple biologic groups (p < 0.001) achieved steroid free clinical remission, but there was no statistically significant difference between the ORs of all groups, at all other timepoints, respectively. Overall, the steroid dose was successively reduced throughout the study. Figure 4 Web Appendix Part 2 Web Tables 14.2.7 and 14.3.1.11.
The HBI score decreased throughout the study. There were no statistically significant differences between HBI change score LS means of all groups. Figure 1 Web Appendix Part 2 Web Table 14.2.10.
Fistula response, remission and PDAI Score
There were no patients with fistulizing CD in the bionaïve group. In the ITT population, all patients in the biologic and multiple biologic populations achieved a fistula response at week 52. There was no statistically significant difference between the ORs of all groups, at week 52 and all timepoints, respectively.
In the ITT population, no patients in the biologic and 33.3% patients in the multiple biologics groups achieved fistula remission at week 52. A statistically significant difference (p < 0.001) between the ORs of the biologic vs. multiple biologic groups was noted at week 8, 16, and 52, respectively.
The PDAI score decreased throughout the study, especially in the multiple biologic group that included most of the fistulizing CD patients at baseline. There were no statistically significant differences between PDAI change score LS means of groups. Figure 5 Web Appendix Part 2 Web Tables 14.2.8.1, 14.2.8.2, and 14.2.9.
Extraintestinal manifestations
Of the tracked extraintestinal manifestations (Supplementary Web Appendix Part 1: Web Table 2 Objectives and Endpoints) only arthralgia/arthritis, iritis/uveitis and erythema nodosum were present at baseline, with arthralgia/arthritis being the most common one across all strata, and no additional ones developed throughout the study. In the ITT population, 38.5%, 22.7% and 23.5% of patients in the bionaïve, biologic, and multiple biologics populations at baseline, which decreased to 7.7%, 4.5% and 11.8%, respectively at week 52. Figure 6 Web Appendix Part 2 Web Table 14.3.1.13.
Patient reported outcome (PRO-2)
The PRO-2 score decreased throughout the study. Overall, fewer patients reported severe disease determined by PRO-2 activity at week 52 compared with baseline across all groups. There were no statistically significant differences between PRO-2 change score LS means of all groups. Figure 7 Web Appendix Part 2 Web Table 14.2.16.
Health related quality of life (EQ-5D-5L)
The EQ-5D-5L score increased throughout the study. There were no statistically significant differences between HBI change score LS means among of all groups. Figure 8 Web Appendix Part 2 Web Table 14.2.15.
Crohn’s disease related surgeries or hospitalizations
While 9.1% of patients in biologic group were hospitalized for CD [85.5 ± 40.3 (mean ± SD) days], none were admitted in the bionaïve or multiple biologics groups. 7.7%, 9.1% and 0% of patients in the bionaïve, biologic, and multiple biologic groups required CD related surgery after 374 ± 0 vs. 207.5 ± 212.84 (mean ± SD) days, respectively. Web Appendix Part 2 Tables 14.3.1.14, 14.3.1.15. There were no statistically significant differences between groups in the Kaplan Meier analysis. Web Appendix Part 2 Tables 14.4.1 and 14.4.2.
Other secondary and exploratory efficacy endpoints
Additional data on CBC, hemoglobin and albumin can be found in the Web Appendix Part 2. Table 14.3.5.2.
Only patient age remained statistically significantly [OR 95% CI 0.933 (0.873, 0.998) p = 0.043] associated with the primary outcome in the ITT population, while prior Crohn’s treatment strata, sex, age at diagnosis, disease phenotype, extraintestinal manifestations, CRP, FCP, albumin, EuroQoL-5D health questionnaire scores had no impact. Web Appendix Part 2. Table 14.4.3. No independent predictors for secondary outcomes could be identified (data not shown).
Safety
Adverse events
Thirty-six patients (69.2%) across all groups (bionaïve 84.6%, biologic, 63.3%, multiple biologics 64.7%) experienced ≥ 1 treatment emergent adverse event (TEAE), in 8 (15.4%) cases rated as severe and in 5 (9.6%) across all groups (bionaïve 15.4%. biologic 9.1%, multiple biologics 5.9%) leading to discontinuation of ustekinumab, but no very severe events or deaths (Table 4).
The TEAEs SOC and PT summary shows that gastrointestinal AEs (i.e., uncontrolled, or complicated Crohn’s disease) occurred more commonly in the bionaïve group. No very severe or deadly AEs occurred. Most TEAEs were judged as non-related. Patients in the multiple-biologic group—as expected—experienced more possibly related and more moderate TEAEs. Apart from the gastrointestinal disorders, infections were the second most frequently occurring TEAEs. Web Appendix Part 2 Tables 14.3.1.2–14.3.1.4, 14.3.1.6. Overall, we did not notice any new safety signals.
Infusion reactions
No infusion reaction TEAEs occurred. Web Appendix Part 2 Table 14.3.1.8.
Injection site reactions
Eight patients (15.5%) across all groups (bionaïve 15.4%, biologic 13.6%, multiple biologics 17.6%) experienced ≥ 1 injection site TEAE, most commonly edema followed by pyrexia and erythema. Web Appendix Part 2 Table 14.3.1.9.
Infections
Sixteen patients (31.7%) across all groups (bionaïve 38.5%, biologic 27.3%, multiple biologics 29.4%) experienced ≥ 1 infection TEAE, most commonly nasopharyngitis followed by urinary tract infections and COVID-19 infections. Web Appendix Part 2 Table 14.3.1.10.
Discussion
First and foremost, it speaks to the spirit of our team and our commitment to the patients entrusted to our care, that we successfully finished a nationwide clinical research study in the middle of the COVID-19 pandemic37, where many non-pandemic related research projects were either cancelled or completely failed.
The strengths of our work include the national, multicentric approach, the definition of a combined clinical and endoscopic endpoint, inclusion of patients who failed multiple biologics and/or immunomodulators, recording and central (investigator independent) reading of the endoscopy videos, investigation of fistula healing, capturing the effect on important extraintestinal manifestations and the comprehensive efficacy and safety analyses usually not seen in real world studies.
Up to one third vs. two thirds (ITT vs. PP population), respectively, achieved the combined primary endpoint (combined clinical and endoscopic response) in our cohort. Interestingly, prior exposure to multiple biologics (not just anti-TNFα as in the ustekinumab randomized registration trial program18,19) did not a have negative impact on the primary outcome. These patients did numerically better although this was not statistically significant. This unexpected observation is likely related to sample size disparities among the different strata and does not challenge our general experience that biologic drugs generally work better when given first line.
Our biologic exposed patients achieved slightly higher clinical remission rates than re-randomized responders in the UNITI-1 randomized controlled trial (stratified for primary or secondary loss of response to anti-TNFα cohort) at week 44 (90 mg q8w) in the IM-UNITI study18,19. This is remarkable, since we used a classic treat-through-design, included patients who in some cases failed immunomodulators or biologics or both, compared with the UNITI program where biologic failures were limited to anti-TNFα and strictly separated from conventional treatment failures in UNITI-1 and UNITI-2 cohorts and re-randomization of responders (with the resulting tendency to overestimate the therapeutic effect size) occurred. However, this comparison should still be interpreted with caution, given other trial design differences like assessment timepoints (week 44 vs. 52) or outcome assessment instruments (HBI vs. CDAI).
Roughly one third vs. two thirds (ITT vs. PP population) of our patients achieved an endoscopic response and mucosal healing, respectively. Prior biologic exposure had no statistically significant impact on these outcomes. Both endoscopic outcomes were much better (mucosal healing two times better) than in the ustekinumab development program, where both outcomes were only assessed in pooled subgroup analyses of UNITI-1, UNITI-2 and IM-UNITI in patients with ulcerations at baseline20. To date, no randomized controlled trial with ustekinumab focused on mucosal healing was conducted.
All four perianal fistula patients achieved a fistula response and more than one third in the multiple biologics group achieved fistula remission. Our fistula healing data are similar to the ustekinumab development program, where it was defined as complete resolution from baseline. A pooled subgroup analysis of the CERTIFI, UNITI-1 and IM-UNITI trials in abstract format38 is available where there was only a numerical, but not statistically significant difference against placebo. To date, no placebo controlled randomized controlled trial with ustekinumab focused on fistula healing was conducted.
Ustekinumab also impressively improved the most common extraintestinal manifestations such as arthropathy, uveitis and erythema nodosum. Overall, the fraction of patients with extraintestinal manifestations decreased by more than one third.
Other secondary indicators of inflammation like CRP, FCP, PRO-2 and quality of life like the EQ-5D-5L improved throughout the study in line with the results of the composite primary endpoint and its components underscoring the plausibility and validity of our findings. Moreover, there were no statistically significant differences between groups in the Kaplan Meier analysis of CD related surgeries or hospitalizations among the three different prior biologic exposure strata.
Overall, we did not identify any new safety signals which is comparable to the ustekinumab development program extension study in which ustekinumab responders of the UNITI-1, UNITI-2 and IM-UNITI program were re-randomized and did not detect new safety signals over 96 weeks39.
Recently, the real-world effectiveness and safety of ustekinumab in the treatment of Crohn’s Disease was published by the North American SUCCESS40 consortium giving the opportunity to view our findings with a more appropriate cohort.
The SUCCESS cohort included 1,113 patients, almost all of which (90%) were exposed to anti-TNFα prior and achieved cumulative clinical-, steroid free- clinical and endoscopic remission rates of 40%, 32% and 39% as opposed to 45.5% and 41.2% vs. 40% and 22.2%, 13.6% / 27.3% (ITT vs. PP) and 17.6%/37.5% (ITT vs. PPP) in our biologic and multiple biologics groups, at week 52, respectively. Overall, these data confirm our findings. The discrepancy in the endoscopy data sets likely results from the high attrition rate reflected in the discordant ITT vs. PP population results of our study. Like in the SUCCESS consortium biologic-naive patients did achieve higher rates of clinical and endoscopic remission.
Our study has important limitations. The negative impact of the unprecedented COVID global pandemic most likely greatly contributed to the higher than previously experienced attrition rate of the study.
An unprecedented challenge was the conduction of a research study during a global pandemic with all the imposed restrictions and limitations including but not limited to sick study personnel, sick patients, sick patient relatives, limited pharmacy, laboratory in- and outpatient hospital services [clinic access, lab availability, endoscopy availability], mandatory adherence to institutional policies (i.e. shutdown of all research activities) and repeated government curfews. With recruitment severely impaired and an unusually high attrition rate, fewer patients than planned were enrolled and completed the study. Thus, we were unable to complete the originally planned statistical modeling to identify independent predictors of mucosal healing and had to default to mostly basic statistics and reporting.
As a non-interventional RWE study with application of medication according to its national label, it did not include a control group. Drug application according to the national label also precluded any dose escalation or reinductions, that are now known to be effective and common practice to improve outcomes and extend medication use41,42.
In summary, we demonstrated, when prescribed within its German label, ustekinumab effectively and safely induces mucosal- and fistula healing, alleviates many extraintestinal manifestations and improves quality of life in patients with Crohn’s disease. Ustekinumab’s overall effectiveness was better than in the original ustekinumab development program and comparable with the large SUCCESS consortium real world experience.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Baumgart, D. C. & Sandborn, W. J. Crohn’s disease. Lancet 380, 1590–1605. https://doi.org/10.1016/S0140-6736(12)60026-9 (2012).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 42, 1118–1125. https://doi.org/10.1038/ng.717 (2010).
Baumgart, D. C. Crohn's Disease and Ulcerative Colitis From Epidemiology and Immunobiology to a Rational Diagnostic and Therapeutic Approach: https://doi.org/10.1007/978-3-319-33703-6
Pariente, B. et al. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm. Bowel Dis. 17, 1415–1422. https://doi.org/10.1002/ibd.21506 (2011).
Beaugerie, L. & Itzkowitz, S. H. Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 372, 1441–1452. https://doi.org/10.1056/NEJMra1403718 (2015).
Cohen, R. D. et al. Systematic review: The costs of ulcerative colitis in Western countries. Aliment. Pharmacol. Ther. 31, 693–707. https://doi.org/10.1111/j.1365-2036.2010.04234.x (2010).
Yu, A. P., Cabanilla, L. A., Wu, E. Q., Mulani, P. M. & Chao, J. The costs of Crohn’s disease in the United States and other Western countries: A systematic review. Curr. Med. Res. Opin. 24, 319–328. https://doi.org/10.1185/03007990SX260790 (2008).
Baumgart, D. & Le Claire, M. (2015) Die Aufwendungen für die universitäre Behandlung von PatientInnen mit chronisch-entzündlichen Darmerkrankungen im Vergleich zu anderen hochschulmedizinischen Fällen in der Gastroenterologie und Hepatologie sind fast doppelt so hoch und werden nicht vollständig durch DRGs abgebildet. Z Gastroenterol 53, KG022. https://doi.org/10.1055/s-0035-1559048.
Baumgart, D. C. & Le Claire M. The expenditures for academic inpatient care of inflammatory bowel disease patients are almost double compared with average academic gastroenterology and hepatology cases and not fully recovered by diagnosis-related group (DRG) proceeds. PLoS One.. 11(1), e0147364 (2016).
Baumgart, D. C. & Le Berre, C. Newer biologic and small-molecule therapies for inflammatory bowel disease. N. Engl. J. Med. 385, 1302–1315. https://doi.org/10.1056/NEJMra1907607 (2021).
Peyrin-Biroulet, L. What is the patient’s perspective: How important are patient-reported outcomes, quality of life and disability?. Dig. Dis. 28, 463–471. https://doi.org/10.1159/000320403 (2010).
Altschuler, A. et al. Gastroenterologists’ attitudes and self-reported practices regarding inflammatory bowel disease. Inflamm. Bowel Dis. 14, 992–999. https://doi.org/10.1002/ibd.20416 (2008).
Spiegel, B. M. et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am. J. Gastroenterol. 109, 1804–1814. https://doi.org/10.1038/ajg.2014.237 (2014).
Williet, N., Sandborn, W. J. & Peyrin-Biroulet, L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 12, 1246-1256.e1246. https://doi.org/10.1016/j.cgh.2014.02.016 (2014).
Stallmach, A., Dennler, U., Marschall, U. & Schmidt, C. Patient-relevant endpoints in inflammatory bowel diseases—Have changes occurred in Germany over the past twelve years?. J. Crohns. Colitis. https://doi.org/10.1093/ecco-jcc/jjv041 (2015).
Sawicki, P. T. Patient-relevant end points: Present state of the discussion at the Institute for Quality and Cost-Effectiveness in Public Health. Dtsch. Med. Wochenschr. 131, S16–S20 https://doi.org/10.1055/s-2006-941735 (2006).
Bokemeyer, B. et al. Clinical status, psychosocial impairments, medical treatment and health care costs for patients with inflammatory bowel disease (IBD) in Germany: An online IBD registry. J. Crohns. Colitis. 7, 355–368. https://doi.org/10.1016/j.crohns.2012.02.014 (2013).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn’s Disease. N. Engl. J. Med. 375, 1946–1960. https://doi.org/10.1056/NEJMoa1602773 (2016).
Sandborn, W. J. et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N. Engl. J. Med. 367, 1519–1528. https://doi.org/10.1056/NEJMoa1203572 (2012).
Rutgeerts, P. et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s Disease. Gastroenterology 155, 1045–1058. https://doi.org/10.1053/j.gastro.2018.06.035 (2018).
Li, K. et al. Effects of ustekinumab on histologic disease activity in patients with Crohn’s Disease. Gastroenterology 157, 1019-1031.e1017. https://doi.org/10.1053/j.gastro.2019.06.037 (2019).
Sands, B. E. et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 381, 1201–1214. https://doi.org/10.1056/NEJMoa1900750 (2019).
Ha, C., Ullman, T. A., Siegel, C. A. & Kornbluth, A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin. Gastroenterol. Hepatol. 10, 1002–1007. https://doi.org/10.1016/j.cgh.2012.02.004 (2012).
Fachinformation für STELARA® 130 mg Konzentrat zur Herstellung einer Infusionslösung. (2024). https://www.fachinfo.de/suche/fi/021296/STELARA%C2%AE%20130%20mg%20Konzentrat%20zur%20Herstellung%20einer%20Infusionsl%C3%B6sung.
Fachinformation für STELARA® 45 mg/-90 mg Injektionslösung in einem Fertigpen. (2024). https://www.fachinfo.de/suche/fi/024130/STELARA%C2%AE%2045%20mg%2F-90%20mg%20Injektionsl%C3%B6sung%20in%20einem%20Fertigpen.
Fachinformation für STELARA® 45 mg Injektionslösung STELARA® 45 mg/-90 mg Injektionslösung in einer Fertigspritze. (2024). https://www.fachinfo.de/suche/fi/020726/STELARA%C2%AE%2045%20mg%20Injektionsl%C3%B6sung%20STELARA%C2%AE%2045%20mg%2F-90%20mg%20Injektionsl%C3%B6sung%20in%20einer%20Fertigspritze.
Harvey, R. F. & Bradshaw, J. M. A simple index of Crohn’s-disease activity. Lancet 1, 514 (1980).
Irvine, E. J. Usual therapy improves perianal Crohn’s disease as measured by a new disease activity index. McMaster IBD Study Group. J. Clin. Gastroenterol. 20, 27–32 (1995).
de Jong, M. J., Huibregtse, R., Masclee, A. A. M., Jonkers, D. & Pierik, M. J. Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: A systematic review. Clin. Gastroenterol. Hepatol. 16, 648-663.e643. https://doi.org/10.1016/j.cgh.2017.10.019 (2018).
Khanna, R. et al. A retrospective analysis: The development of patient reported outcome measures for the assessment of Crohn’s disease activity. Aliment. Pharmacol. Ther. 41, 77–86. https://doi.org/10.1111/apt.13001 (2015).
Herdman, M. et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 20, 1727–1736. https://doi.org/10.1007/s11136-011-9903-x (2011).
Brown, E. G., Wood, L. & Wood, S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 20, 109–117 https://doi.org/10.2165/00002018-199920020-00002 (1999).
Common Terminology Criteria for Adverse Events (CTCAE). U.S. Department of Health and Human Services, National Institutes of Health National Cancer Institute https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
Daperno, M. et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 60, 505–512 https://doi.org/10.1016/s0016-5107(04)01878-4 (2004).
Turner, D. et al. STRIDE-II: An update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. https://doi.org/10.1053/j.gastro.2020.12.031 (2021).
Peyrin-Biroulet, L. et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat-to-target. Am. J. Gastroenterol. 110, 1324–1338. https://doi.org/10.1038/ajg.2015.233 (2015).
Wu, F. et al. A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269. https://doi.org/10.1038/s41586-020-2008-3 (2020).
Sands, B. E. et al. Fistula healing in pivotal studies of ustekinumab in Crohn’s disease. Gastroenterology 152, S185–S185. https://doi.org/10.1016/s0016-5085(17)30930-7 (2017).
Sandborn, W. J. et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment. Pharmacol. Ther. 48, 65–77. https://doi.org/10.1111/apt.14794 (2018).
Johnson, A. M. et al. The real-world effectiveness and safety of ustekinumab in the treatment of Crohn’s disease: Results from the SUCCESS Consortium. Am. J. Gastroenterol. https://doi.org/10.14309/ajg.0000000000002047 (2022).
Schreiber, S. W. et al. P436 Efficacy and safety of intravenous ustekinumab re-induction therapy in Crohn’s disease patients with secondary loss of response to ustekinumab maintenance therapy: Week 16 results from the POWER trial. J. Crohn’s Colitis 17, i564–i566. https://doi.org/10.1093/ecco-jcc/jjac190.0566 (2023).
Meserve, J., Ma, C., Dulai, P. S., Jairath, V. & Singh, S. Effectiveness of reinduction and/or dose escalation of ustekinumab in Crohn’s disease: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 20, 2728-2740.e2721. https://doi.org/10.1016/j.cgh.2021.10.002 (2022).
Acknowledgements
This study was funded by an unrestricted research grant by Janssen-Cilag GmbH, Johnson & Johnson Platz 1, 41470 Neuss, Germany to D.C.B. Janssen-Cilag GmbH had no role in the design, conduction, analysis of the data, interpretation of the results or writing of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
D.C.B. designed and led the study, recruited patients, summarized the analysis of the data contributed in raw format by the CRO (Alimentiv Inc, London, ON, Canada), created all figures and tables for the main manuscript and wrote the first draft. All other authors recruited patients, validated and interpreted the analysis, edited the manuscript and approved its final version for submission.
Corresponding author
Ethics declarations
Competing interests
D.C.B. has over the past five years served on scientific advisory boards, speaker or moderator at CME events for, and/or received travel support from Janssen-Cilag, AbbVie, Pfizer, BMS, Falk Foundation, Takeda, Eli Lilly, Tillotts Pharma AG, and Galapagos. J.S. has received consulting fees from Abbvie, Bristol Myers Squibb, Dr Schär, Falk, Ferring, Fresenius Kabi, Immundiagnostik, Janssen, Medice, MSD, Pfizer, Pharmacosmos, Shire, Takeda, Thermofisher, Vifor, lecturing fees from Vifor Pharma, and is an Advisory Board member for Abbvie, Bristol Myers Squibb, Dr Schär, Ferring, Fresenius Kabi, Immundiagnostik, Janssen, MSD, NPS, Pharmacosmos, Takeda, Vifor and Shield. T.O. was the recipient of an unrestricted research grant from Biogen, Germany. Over the past five years T.O. has served on scientific advisory boards, and/or served as speaker or moderator at conferences and CME events for, and/or received travel support from, AbbVie, Amgen, Biogen, BMS, Celltrion, Janssen-Cilag, Lilly, MSD, Pfizer, Sandoz, Takeda, Tillots, Viatris and Vifor. A.L. has served on scientific advisory boards, and/or served as speaker or moderator for AbbVie, Biogen, BMS, Falk Foundation, Ferring, Hexal, Janssen, MSD, Nutrimmun, Pfizer, Takeda, and Tillotts Pharma. AS reports research funding from AbbVie and Celltrion. He has received lecture fees from AbbVie, Amgen, Astellas, Biogen, Celltrion, Janssen, Falk Foundation, Ferring, MSD, Recordati Pharma, Streamed-Up and Takeda and consulting fees from AbbVie, Astellas, Amgen, Biogen, CLS Behring, Consal, Eli Lilly, Galapagos, Hexal, Janssen, MSD, Norgine, Pfizer Pharma Takeda and Tillots Pharma. S.H. has received consulting and lecturing fees and has served on scientific advisory boards from Abbvie, Bristol Myers Squibb, Eli Lilly, Fresenius Kabi, Janssen, Galapagos, Pfizer, Pharmacosmos, Shield, Takeda. U.v.A. has received consulting and lecturing fees from Abbvie, Bristol Myers Squibb,EsoCap, Falk Foundation, Janssen, MSD, Pfizer, Pharmacosmos, Sanofi, Takeda, ViforPharma, and is an Advisory Board member for Abbvie, Bristol Myers Squibb, Janssen, MSD, Pharmacosmos, Takeda, Vifor , Pfizer and Sanofi. D.S., P.G. and A.F. declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baumgart, D.C., Stallmach, A., Grunert, P. et al. Induction and maintenance of mucosal healing in Crohn’s disease with ustekinumab in clinical practice across all care levels in Germany (MUCUS). Sci Rep 14, 20502 (2024). https://doi.org/10.1038/s41598-024-70241-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70241-9
Keywords
This article is cited by
-
Ustekinumab
Reactions Weekly (2024)