Abstract
No study has analysed the temporal trends of the long-term results and clinical characteristics of patients with hepatocellular carcinoma (HCC) treated using radiofrequency ablation (RFA). Therefore, we examined temporal trends of characteristics of patients and treatment-naïve HCCs within the Milan criteria treated by RFA over 20 years. We retrospectively analysed 1099 patients with HCC within the Milan criteria treated with percutaneous RFA from January 2000 to December 2019. The overall survival (OS), recurrence-free survival (RFS), and factors affecting survival and local tumor progression were analysed using the Kaplan‒Meier method and Cox proportional hazards model. A trend test was performed to analyse the changing trends in participants and treatment outcomes. The overall and RFS of patients improved during the later period. In addition, viral hepatitis-related HCC incidence decreased, whereas that of alcohol- or non-alcoholic fatty liver disease-related HCC increased from the earlier to the later period (P for trend < 0.001). HBV antiviral therapy was increased and improved OS and RFS in patients treated using RFA. The outcomes after RFA over a 20-year period improved due to changes over time in target tumors and patients. The results could be useful for selecting patients who will benefit from RFA.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC), a type of liver cancer, presents a significant challenge in healthcare due to its prevalence and potential severity. Among the various stages of HCC, patients with very early-stage and early-stage tumors, characterized by single or 2–3 nodules each measuring less than 3 cm in diameter, have several therapeutic options1. These patients can benefit from radiofrequency ablation (RFA), surgical resection, or liver transplantation. Of these, RFA stands out as the most cost-effective therapeutic strategy for treating very early-stage HCC and 2–3 nodules < 3 cm in diameter2. Typically, RFA targets HCCs that fall within the Milan criteria (single HCC ≤ 5 cm in diameter, or up to three tumors, each ≤ 3 cm, without extrahepatic metastasis or macrovascular invasion). Previous studies have examined the long-term outcomes of RFA in early-stage HCC within the Milan criteria3,4,5,6,7,8. These studies have assessed parameters such as the overall survival (OS), recurrence rate, and recurrence-free survival (RFS) in patients who underwent RFA. Overall, these investigations have reported positive outcomes, demonstrating the effectiveness of RFA in achieving favorable long-term results for patients with HCC, particularly those with early-stage tumors. However, to the best of our knowledge, no study has conducted a comprehensive analysis of long-term outcomes of RFA over a 20-year period. Furthermore, none have explored the changes over time in the long-term outcomes and clinical characteristics of HCC patients treated with RFA. Over this extended period, medical practices and techniques for treating HCC patients have evolved. Therefore, the objective of our study is to investigate the long-term outcomes of RFA over a period exceeding 20 years and to evaluate the changes in various factors affecting the outcomes of RFA.
Results
Patient characteristics and temporal trends
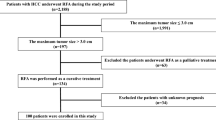
Between January 2000 and December 2019, 2274 patients with HCC underwent percutaneous RFA at Kyungpook National University Hospital. Among the 2,274 HCC cases treated by RFA, 1099 patients [median age: 61 (29–86) years; 823 men] were analysed (Fig. 1). Follow-up period ranged from 0.5 to 287 months (median, 75 months). Median overall survival was 75 months (6.2 years) and median recurrence-free survival was 32 months (2.7 years).The baseline characteristics of the patients and tumors are presented in Table 1. The average size of the 1330 tumors was 1.9 ± 0.7 cm (range: 1.0–4.8). RFA was mostly performed in a single session; however, 19 patients required two sessions within 2 weeks. Artificial ascites was induced during the procedure in 716 patients.
Tumor locations are described in Table 1 and depicted in Fig. 2. Table 2 shows the temporal trends of baseline characteristics and tumors over a 5-year interval observed throughout a 20-year period. Age, number of patients with antiviral therapy for HBV, number of patients with multiple tumors, and number of perivascular tumor cases increased significantly over time. In contrast, Child–Pugh score, proportion of viral hepatitis cases, tumor size, and the proportion of cases with high alpha-fetoprotein (AFP) (> 20 ng/mL) decreased significantly over time.
Tumor distribution. Liver segmentation on a computed tomography (CT) scan (a). The probabilities are categorized by location, with green for tumors in the subscapular region, purple for tumor adjacent to vessels, and red for all other locations on the axial CT scan (c). The probabilities of tumor existence are illustrated with multi-colored and multi-sized spheres, with brighter color and larger size representing higher probability, which are rendered on the 3D view (b,d). IVC inferior vena cava, LPV left portal vein, PV portal vein, MHV middle hepatic vein, RHV right hepatic vein, LHV left hepatic vein, PV8 portal vein for segment 8, 3D three-dimensional.
The proportion of underlying liver disease in patients with HCC also changed over time (Fig. 3a). Viral hepatitis, including hepatitis B and C, accounted for more than 70% of all cases over 20 years. The top cause throughout the study period was chronic HBV infection. However, the proportion of viral hepatitis decreased over the 5-year interval observed throughout a 20-year period (P for trend < 0.001). The number of patients with alcoholic liver disease steadily increased (P for trend < 0.001); that of patients with non-alcoholic fatty liver disease increased from 2.2 to 6.0% of the underlying diseases (P for trend = 0.02). Among HBV-related HCC cases, the number of cases of antiviral therapy before RFA increased rapidly during 2005‒2009 and 2010–2014 (Fig. 3b). Additionally, the number of cases of antiviral therapy before or after the procedure increased rapidly during 2000‒2004 and 2005‒2009 (Fig. 3c).
Temporal trend. Temporal trend of underlying liver disease in hepatocellular carcinoma (HCC) (a) and antiviral therapy (b, at the time of radiofrequency ablation; c, overall period) for hepatitis B according to the 5-year interval in the study patients. HBV hepatitis B virus, HCV hepatitis C virus, NAFLD non-alcoholic fatty liver disease.
RFA outcomes
The treatment outcomes are described in Supplementary Table S1. The procedure failed in 24 patients owing to either incomplete ablation or mistargeting. HCC most often recurred as intrahepatic recurrence, followed by local recurrence and extrahepatic recurrence. Procedure-related complications occurred in 47 patients (4.3%). One patient died 5 days after discharge and 8 days after RFA. The patient presented with acute kidney injury due to infection at the time of re-admission.
Hydrodissection using artificial ascites
Artificial ascites was induced more frequently in patients with subcapsular (odds ratio [OR] 3.35; 95% confidence interval [CI] 2.54–4.43, P < 0.001) and subphrenic (OR 4.31; 95% CI 3.16–5.59, P < 0.001) tumors. Additionally, artificial ascites was induced more frequently for tumors in segments VII or VIII than for those in segments V or VI and for those in segment IV than for those in segments II or III (Supplementary Table S2).
Overall and recurrence-free survival changes according to time and HBV antiviral therapy
The 1-, 3-, 5-, 10-, 15-, and 20-year OS rate was 95.9%, 78.8%, 66.0%, 44.6%, 33.8%, and 28.0%, and the RFS rate was 78.3%, 46.5%, 35.6%. 17.3%, 10.0%, and 8.0%, respectively. Based on the similarity in baseline characteristics, we grouped cohorts 1 and 2, and cohorts 3 and 4, into two groups (Supplementary Table S3). We compared survival between cohorts 1‒2 and 3‒4. OS and RFS rates were higher in cohort 3‒4 than in cohorts 1‒2 (P < 0.001). The 1-, 3-, 5-, 10-, 15-, and 20-year OS rate was 95.1%, 72.0%, 55.3%, 32.2%, 23.4%, and 19.4%, and RFS rate was 73.1%, 34.1%, 20.1%, 10.6%, 4.9%, and 3.9% in cohort 1–2. The 1-, 3-, 5-, 10-, and 15-year OS rate was 96.2%, 81.0%, 69.4%, 49.4%, and 43.2% and RFS rate was 79.9%, 50.4%, 40.5%, 19.1%, and 14.8% in cohort 3–4 (Fig. 4a,b).
Overall survival (OS) and recurrence-free survival (RFS). OS and RFS of all patients and patients with viral hepatitis B with or without antiviral therapy. OS (a) and RFS (b) of patients who underwent radiofrequency ablation in 2000–2004, 2005–2009, 2010–2014, and 2015–2019, respectively. OS (c) and RFS (d) of patients with viral hepatitis B who underwent radiofrequency ablation according to the antiviral therapy, respectively.
We analysed the survival of patients with HBV according to antiviral agent administration. We divided the cohort according to the time when entecavir became widely used under national medical insurance coverage (early 2007). OS and RFS improved in the subsequent period (2007–2019), and patients who received antiviral therapy in this period showed better OS and RFS (Fig. 4c,d).
Prognostic factors affecting OS and RFS in entire and hepatitis B patients
Prognostic factors affecting OS and RFS were assessed for both the entire patient cohort (Table 3) and those with hepatitis B (Supplementary Table S4). Age > 61 years, Child–Pugh class B, cirrhosis, non-viral etiology, and large tumor size (> 2 cm) were independent factors predicting poor OS. In the multivariable analysis, age > 61 years, Child–Pugh class B, cirrhosis, non-viral etiology, and large tumor size (> 2 cm) were independent prognostic factors for poor OS. Regarding RFS, age > 61 years, Child–Pugh class B, cirrhosis, non-viral etiology, > 1 tumor, large tumor size (> 2 cm), and high AFP (> 20 ng/mL) predicted poor RFS. In the multivariable analysis, age > 61 years, Child–Pugh class B, cirrhosis, non-viral etiology, > 1 tumor, large tumor size (> 3 cm), and high AFP (> 20 ng/mL) were independent predictors of poor RFS.
In hepatitis B patients, the independent factors predicting poor OS and RFS were similar across the entire patients. Antiviral therapy for HBV was found to be an independent factor that predicts better OS and RFS, as demonstrated in Table 3.
Prognostic factors for LTP
We analysed 1330 tumors to identify factors influencing LTP. LTP occurred in 159 cases at the first recurrence. Among patient and tumor factors, only large tumor size (> 2 cm) was an independent factor predicting LTP in the univariable and multivariable analyses (Table 4). LTP rates were increased significantly in larger tumor size (Supplementary Fig. S1).
Discussion
In this study, we examined the temporal trends in patients and tumor characteristics and outcomes of the long-term results following RFA of treatment-naïve HCC within the Milan criteria. We also analysed the factors affecting survival or tumor progression. Our findings revealed that RFA demonstrated good OS and RFS, with manageable adverse events. Notably, we observed an increasing incidence of HCC with non-viral etiologies, including alcoholic and non-alcoholic fatty liver disease-related HCC, over time. Additionally, while tumor size decreased, the number of tumors increased over 20-year study period. Nevertheless, tumor location did not independent predict survival and local tumor progression. The number of cases of hepatitis B antiviral therapy increased the overall and recurrence-free survival rates. To the best of our knowledge, no study has reported temporal trends in the clinical characteristics and long-term outcomes of patients who underwent RFA for treatment-naïve HCC.
A significant and noteworthy finding was the steep increase in cases of HBV antiviral therapy between 1999 and 2013 in South Korea9. This was likely due to the introduction of potent antiviral agents like entecavir tenofovir, which were widely prescribed for chronic hepatitis B in Korea by National Insurance Coverage Program since 2007 and 2013, respectively. The same program has prescribed these two antiviral agents for HBV-related HCC since late 2017. These changes in potent antiviral agents and insurance policies have benefited patients with HBV-related HCC who were treated with RFA. Treatment with oral antiviral agent is associated with favorable recurrence and survival outcomes in patients with HCC after curative treatment10,11,12,13, suggesting that viral etiology of hepatitis may serve as a favorable predictor for survival in our study.
Other changes in tumors included decreased size, more cases of 2–3 tumors or perivascular tumors, and fewer cases of high AFP (> 20 ng/mL). These changes are identifiable owing to advances in imaging technology during diagnosis and treatment, wide application of liver MRI, and introduction of real-time virtual sonography during RFA. These technological improvements likely led to the identification of smaller initial or recurrent nodules, potentially explaining the improved survival of HCC patients. Moreover, real-time virtual sonography with contrast-enhanced ultrasonography has reduced mistargeting rates associated with conventional ultrasonography during RFA for small and inconspicuous HCC, leading to higher success rates and improved long-term outcomes.
We also focused on the effect of tumor location on treatment outcomes. Tumor location was not a significant factor affecting survival or local recurrence. Follow-up proceeded only if no viable portion was detected initially. Additional ablation was performed in a second session if suspicious areas remained. Thus, we classified LTP as a recurrence rather than progression. Subcapsular HCC treatment using RFA is challenging because of higher risks of technical failure leading to LTP and complications. Achieving full coverage of an exophytic lesion in subcapsular HCC is difficult. However, several studies have shown no significant differences in OS, LTP, or complication rates between subcapsular and non-subcapsular HCC groups14,15. We showed that tumor location was not an independent predictor of OS, RFS, or LTP. The pitfalls of treating subcapsular or subphrenic HCC were overcome using artificial ascites and avoiding direct puncture. We extended the ablated zone by indirectly targeting the non-tumor tissue adjacent to the tumor to provide space for advancing the needle if direct puncture was inevitable. Perivascular location was not an independent predictor of OS, RFS, or LTP possibly because meticulous, multiple ablations with a consequent longer time might have overcome this effect irrespective of the heat-sink effect.
While our study provides valuable insights, it has some limitations to consider. Firstly, it is a retrospective single-center study which may have introduced selection bias. The results may not be reproducible at other centers with different methods, patient populations, and expertise levels. Secondly, the retrospective nature of the study may have resulted in underestimated data on antiviral therapy and adverse events. Antiviral therapy was based on medical and prescription records, but patients may have received such therapy from other hospitals or clinics which was not captured in the study. Similarly, only severe adverse events might have been described, thereby underestimating complications considered clinically insignificant, such as low-grade pain or fever. Thirdly, patients enrolled in clinical trials (HEAT and OPTIMA studies) were excluded from the analysis16,17. These clinical trials had an inclusion criterion of tumor size ≥ 3 cm; therefore, relatively larger HCC tumor sizes were excluded in this study. Finally, the analysis of OS, RFS, and associated factors only considered in the initial treatment, even though they could have been more influenced by subsequent treatments after HCC recurrence. Therefore, subsequent recurrence data should be collected in future studies to provide a more comprehensive picture of the treatment outcomes.
In conclusion, our 20-year retrospective study demonstrates favorable outcomes for RFA as a treatment for early-stage HCC within the Milan criteria. Over time, we observed an increase in non-viral HCC cases, more patients receiving antiviral therapy for hepatitis B, and a decrease in target tumor size. Age, liver function, viral hepatitis, and tumor size were identified as significant predictors of survival, while tumor size emerged as a predictor of local tumor progression. These changes over time in treatment approaches could contribute to improved outcomes for HCC patients undergoing RFA. Given the comprehensive nature of our analysis, these findings may aid in selecting patients who would benefit most from RFA treatment. However, it is essential to acknowledge the limitations of our study and consider subsequent recurrence data in future studies for a more complete understanding of treatment outcomes.
Materials and methods
Study design
This retrospective study received a priori approval from the Institutional Review Board of Kyungpook National University Hospital (approval number 2016-06-021). The study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki. The Institutional Review Board of Kyungpook National University Hospital waived the requirement for written informed consent due to the nature of the study.
Patients
The inclusion criteria were patients who underwent percutaneous RFA and included those with single HCC ≤ 5 cm in diameter, multinodular HCC (≤ 3 in number, each ≤ 3 cm in diameter), absence of portal vein thrombosis, Child–Pugh class A or B, no distant metastasis, and no history of HCC treatment. The exclusion criteria were RFA performed as combination therapy; patients lost to follow-up; and patients enrolled in clinical trials. We divided the 1099 patients into four cohorts by time period.
Cirrhosis was considered if there was evidence of portal hypertension, such as splenomegaly, ascites, gastroesophageal varices, or thrombocytopenia (platelet count < 150,000/µL)18. Use of antiviral agent was defined as oral antiviral agent administration for > 6 months.
We observed patients until death or last follow-up. OS was defined as the time from the date of RFA to death from any cause. RFS was measured from the date of RFA to the first date of recurrence determined using computed tomography (CT) or magnetic resonance imaging (MRI) or death from any cause.
Tumor analysis
HCC diagnosis was based on the most recent imaging criteria available at the time of patient inclusion1,19,20. HCC diagnosis was based on tumors > 1 cm in patients with chronic liver disease with typical features on dynamic CT or MRI, or histopathological evidence from a liver biopsy.
Tumor characteristics were analysed in all 1330 tumors or the representative 1099 tumors. Representative tumors were the largest in patients with multiple tumors. Subcapsular HCC was defined as abutting the liver capsule, with distance from the liver capsule to the tumor margin of < 0.1 cm on axial or coronal CT or MRI14. Perivascular HCC was defined as a tumor that abuts portal or hepatic vein branches > 3 mm in diameter21,22. Subphrenic HCC was defined as a tumor located within 0.1 cm from the diaphragm23.
To investigate tumor distribution, the liver was segmented according to the Couinaud classification24 and sub-segmented into 78 regions on CT images (Supplementary Table S5); the liver region was manually labeled using a 3D slicer (https://www.slicer.org/).
HCC recurrence was defined as a tumor sized ≥ 1 cm, with typical findings of contrast enhancement in the arterial phase, followed by contrast washout in the venous or delayed phase on CT or MRI1,19,20. Local tumor progression (LTP) was defined as a nodule with enhancement and washout at the edge of the ablation zone after at least one contrast-enhanced study demonstrating adequate ablation and no viable portion in the target tumor25.
RFA
RFA was performed percutaneously under ultrasound guidance (Prosound Alpha 10, Aloka, Japan & Ascendus, Hitachi, Japan) by two operators who had performed the procedure more than 100 times in the past 2 years. Procedures were performed under conscious sedation induced using an intravenous infusion of 5 mg midazolam. For pain control, we infused 2 mg fentanyl citrate diluted in 50 mL normal saline. Vital signs were monitored during the procedure.
We infused approximately 1 L of 5% dextrose solution to induce artificial ascites for tumors in the hepatic dome and those adjacent the peritoneum or other organs. We used tilting chairs to optimize visualization following infusion.
RFA was performed using a 17-gauge cooled-tip electrode (Cool-Tip, RF Ablation System, Covidien, Mansfield, MA, USA). Radiofrequency energy was delivered at a power of at least 80 W for 6–12 min at each application, according to the manufacturer’s manual. For HCC tumors > 3 cm, we used a cluster electrode or multiple electrodes with a switching controller. After ablation, the electrode tract was cauterized during electrode withdrawal to prevent bleeding and seeding of cancer cells.
Patients underwent abdominal CT and laboratory tests on the following day to confirm the ablation zone and the absence of complications. Patients underwent contrast-enhanced dynamic CT or liver MRI (Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid, Primovist®, Bayer HealthCare, Berlin, Germany) and laboratory testing every 3‒6 months for 5 years, and every 6 months thereafter.
Statistical analysis
All statistical analyses were performed using R (version 3.4.4; R Foundation for Statistical Computing, Vienna, Austria) and SPSS (version 20, IBM, Chicago, IL, USA). Categorical variables were compared using Pearson’s Chi-square tests, and continuous variables were compared using Student’s t-test or Mann‒Whitney U test. The trend change in characteristics of each cohort was analysed using the Cochran–Armitage trend test. Survival rates were determined using the Kaplan‒Meier method, and curves were compared using the log-rank test. Prognostic factors were assessed using Cox proportional hazards model. Factors that may be associated with survival and recurrence were selected based on previous studies4,7,14,26. Statistically significant variables with P-values < 0.05 were included, along with clinically significant factors, for multivariable analysis. The P-value for trend was analysed using the regression method and Jonckheere-Terpstra test. Statistical significance was set at P < 0.05.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 69(1) 182–236 (2018).
Cucchetti, A. et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J. Hepatol. 59(2), 300–307 (2013).
Choi, D. et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: Long-term results and prognostic factors in a large single-institution series. Eur. Radiol. 17(3), 684–692 (2007).
Kim, Y. S. et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J. Hepatol. 58(1), 89–97 (2013).
Lencioni, R. et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: Long-term results of percutaneous image-guided radiofrequency ablation. Radiology 234(3), 961–967 (2005).
Peng, Z. W. et al. Radiofrequency ablation as first-line treatment for small solitary hepatocellular carcinoma: Long-term results. Eur. J. Surg. Oncol.: J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 36(11), 1054–1060 (2010).
Shiina, S. et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 107(4), 569–577 (2012).
Lee, D. H. et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: Long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270(3), 900–909 (2014).
Choi, J., Han, S., Kim, N. & Lim, Y. S. Increasing burden of liver cancer despite extensive use of antiviral agents in a hepatitis B virus-endemic population. Hepatology 66(5), 1454–1463 (2017).
Wu, C. Y. et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 308(18), 1906–1914 (2012).
Yin, J. et al. Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma: A two-stage longitudinal clinical study. J. Clin. Oncol. 31(29), 3647–3655 (2013).
Huang, G. et al. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels: A randomized controlled trial. Ann. Surg. 268(6), 943–954 (2018).
Sohn, W. et al. Effect of oral antiviral treatment on long-term outcomes of radiofrequency ablation therapy for hepatitis B virus-related hepatocellular carcinoma. Oncotarget 7(30), 47794–47807 (2016).
Kang, T. W. et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: A propensity score matched study. Radiology 280(1), 300–312 (2016).
Sartori, S. et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: A prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology 248(2), 670–679 (2008).
Tak, W. Y. et al. Phase III HEAT study adding lyso-thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin. Cancer Res. 24(1), 73–83 (2018).
Lencioni, R. & Cioni, D. RFA plus lyso-thermosensitive liposomal doxorubicin: In search of the optimal approach to cure intermediate-size hepatocellular carcinoma. Hepat. Oncol. 3(3), 193–200 (2016).
Afdhal, N. et al. Thrombocytopenia associated with chronic liver disease. J. Hepatol. 48(6), 1000–1007 (2008).
Bruix, J. & Sherman, M. practice guidelines committee, american association for the study of liver diseases. Management of hepatocellular carcinoma. Hepatology 42(5), 1208–1236 (2005).
European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 56(4), 908–943 (2012).
Lu, D. S. et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J. Vasc. Interv. Radiol. 14(10), 1267–1274 (2003).
Kang, T. W. et al. Perivascular versus nonperivascular small HCC treated with percutaneous RF ablation: Retrospective comparison of long-term therapeutic outcomes. Radiology 270(3), 888–899 (2014).
Kim, N. et al. Stereotactic body radiation therapy versus radiofrequency ablation in Asian patients with hepatocellular carcinoma. J. Hepatol. 73(1), 121–129 (2020).
Couinaud, C., Delmas, A. & Patel, J. Le Foie: Etudes Anatomiques et Chirurgicales (Masson, 1957).
Ahmed, M. et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria—a 10-year update. Radiology 273(1), 241–260 (2014).
Lee, M. W. et al. Updated 10-year outcomes of percutaneous radiofrequency ablation as first-line therapy for single hepatocellular carcinoma < 3 cm: Emphasis on association of local tumor progression and overall survival. Eur. Radiol. 30(4), 2391–2400 (2020).
Funding
This work was supported by the National Research Foundation (NRF) grants funded by the Korean government [grant numbers NRF-2019R1F1A1060878 and NRF-2022R1F1A1071584 (Ministry of Science and ICT)].
Author information
Authors and Affiliations
Contributions
Conceptualization: W.Y.T.; Formal analysis: S.Y.J.; Investigation: S.Y.P., Y.O.K., Y.R.L., H.K.R., J.G.C., S.K., W.K.L., A.J.J.; Writing—original draft: S.Y.J.; Writing—review and editing: W.Y.T. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jang, S.Y., Park, S.Y., Kweon, Y.O. et al. Temporal trends and long-term outcomes of radiofrequency ablation for hepatocellular carcinoma within the Milan criteria. Sci Rep 14, 19815 (2024). https://doi.org/10.1038/s41598-024-70494-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70494-4